Effect of Cocoa Beverage and Dark Chocolate Consumption on Blood Pressure in Those with Normal and Elevated Blood Pressure: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Literature Search Strategy
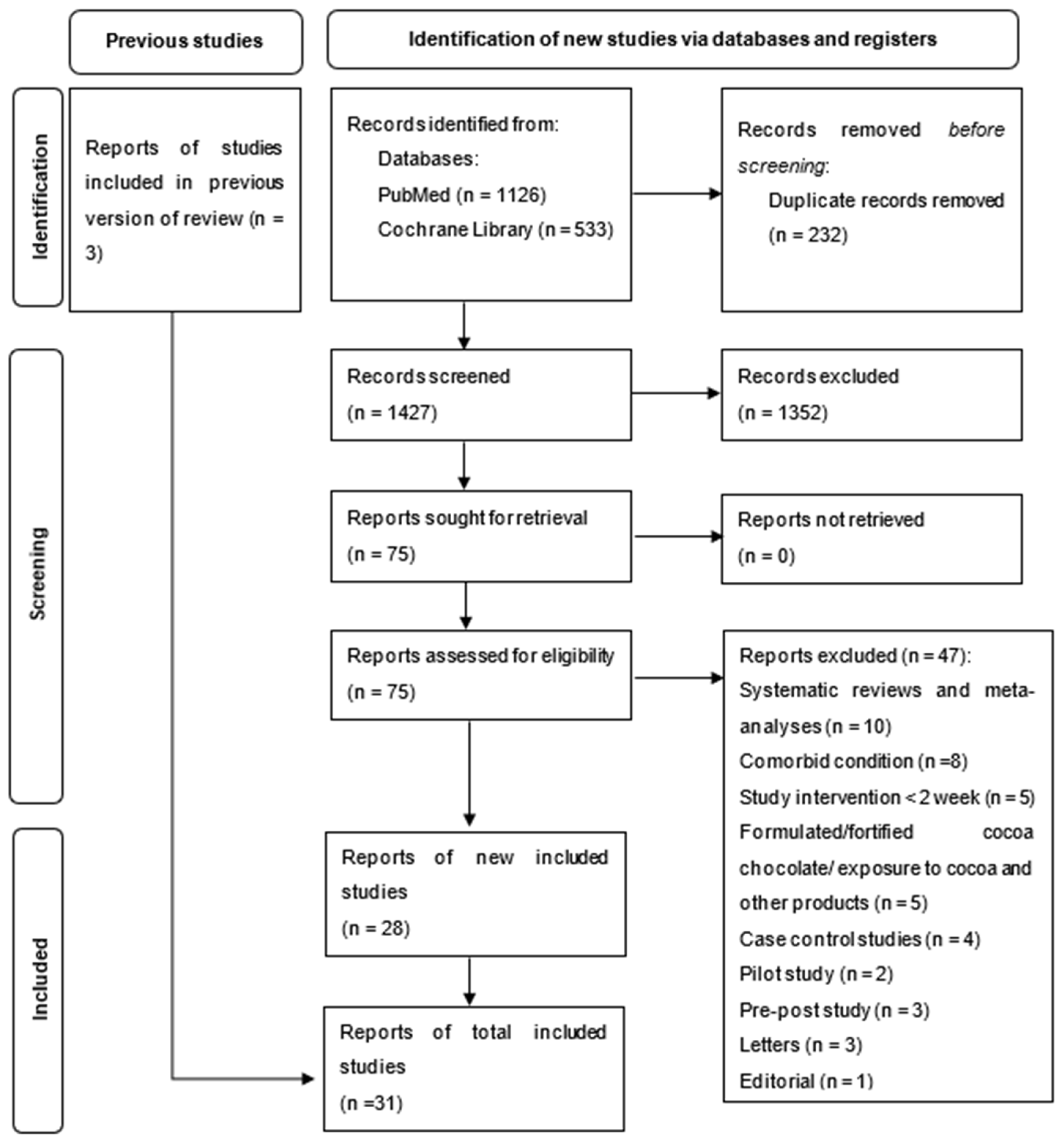
2.2. Selection of Studies
2.2.1. Eligibility Criteria
2.2.2. Exclusion Criteria
2.2.3. Data Extraction
2.2.4. Meta-Analysis
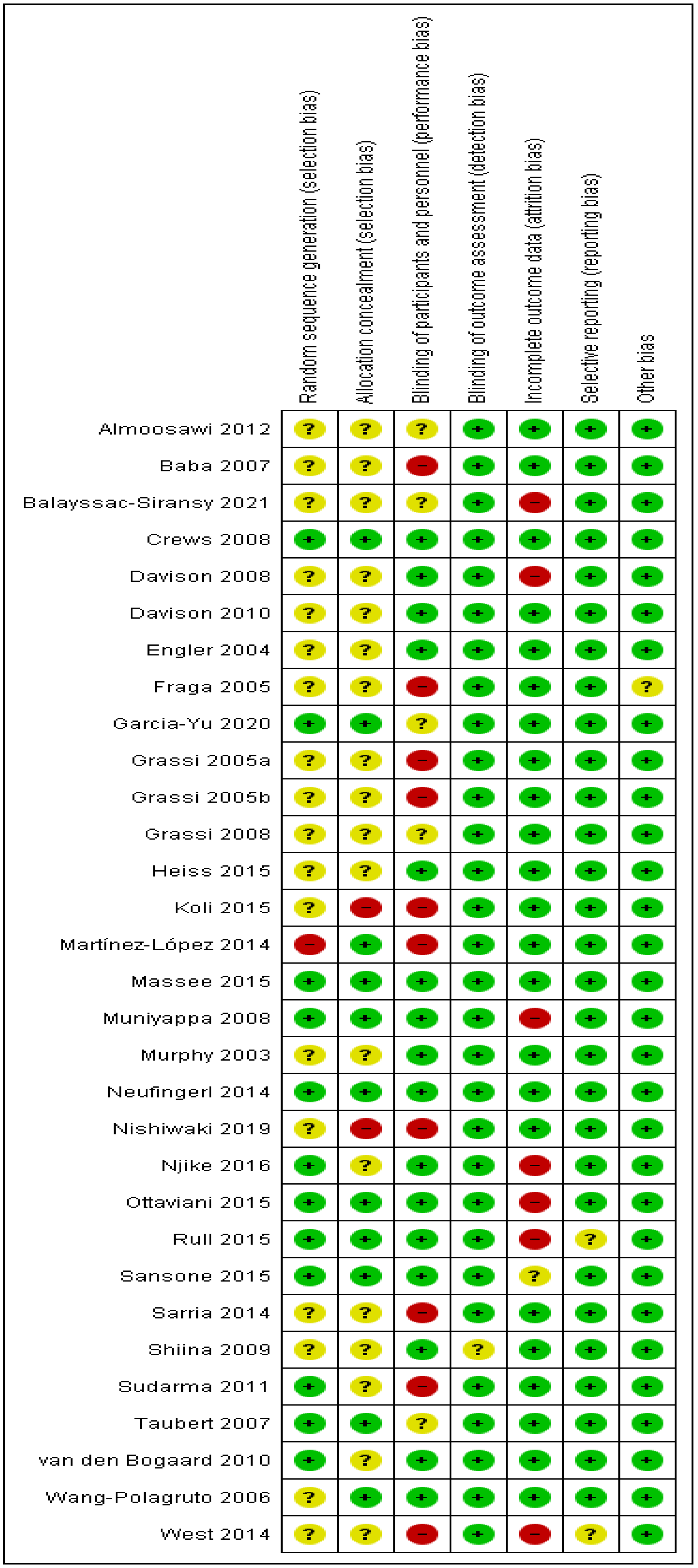
2.2.5. Risk of Bias
3. Results
3.1. Risk of Bias Assessment
3.2. Meta-Analysis
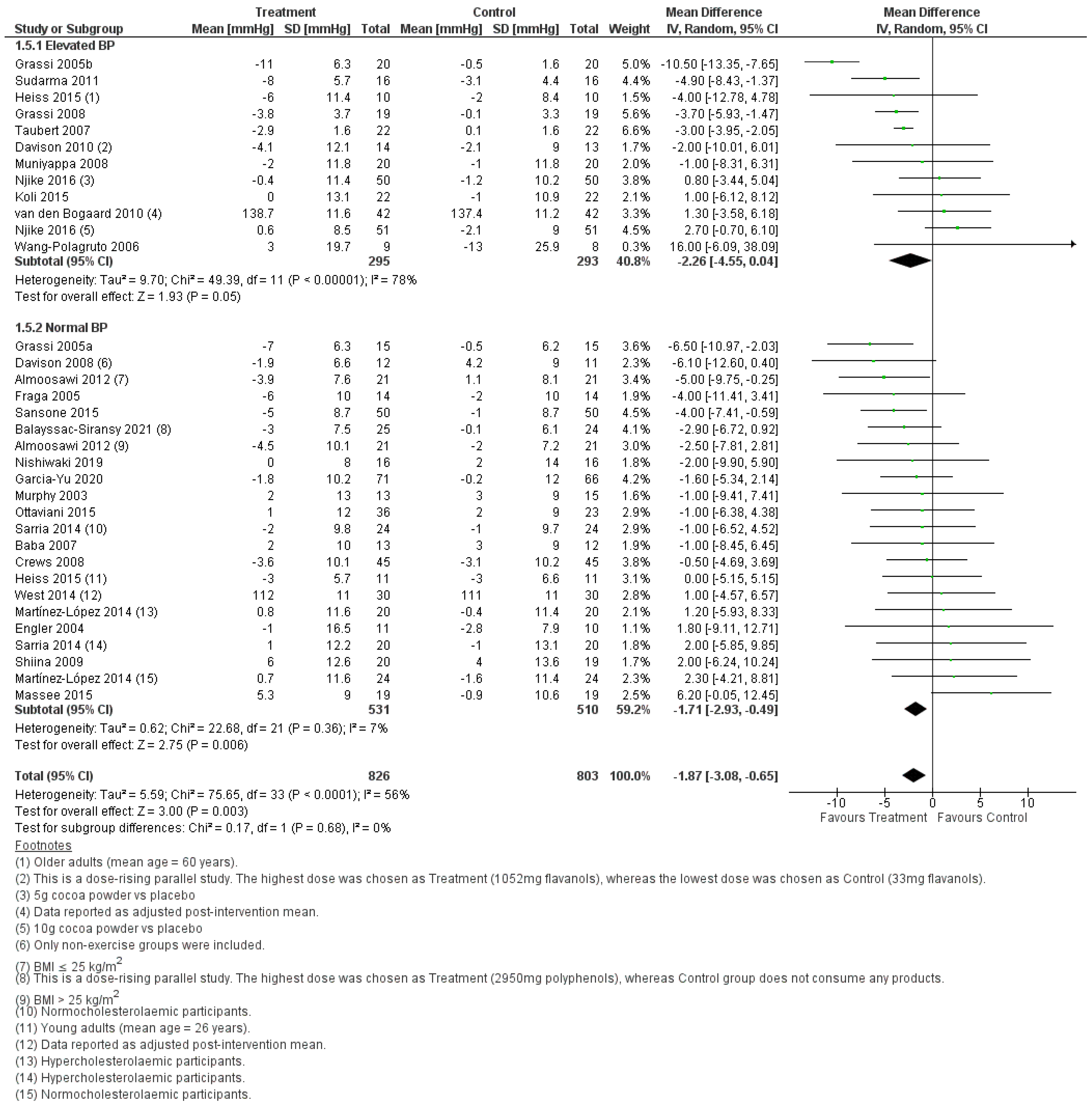
3.2.1. Effect of Cocoa Consumption on Resting BP
3.2.2. Effect of Cocoa Consumption on 24-h BP
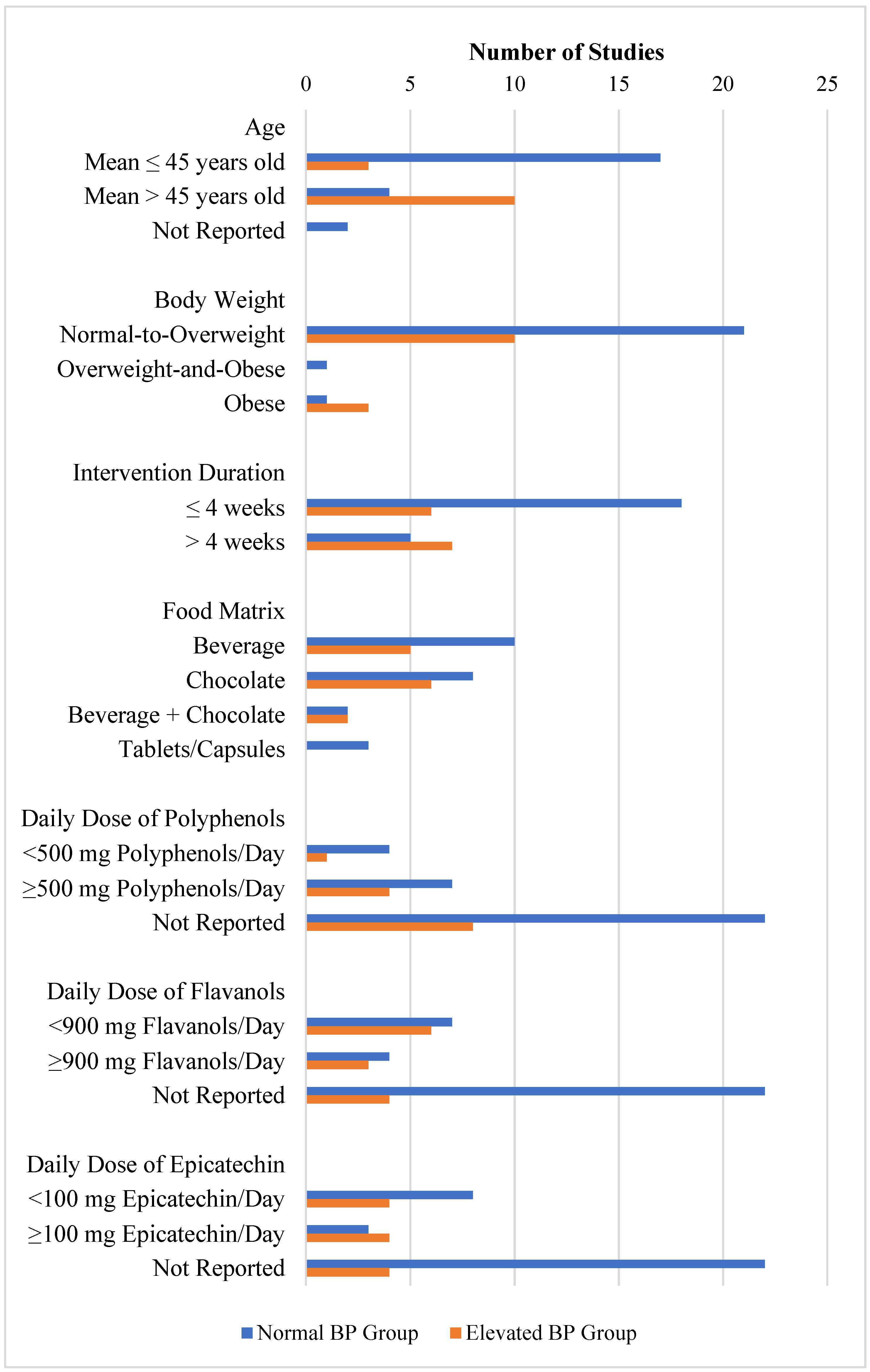
3.2.3. Effect of Cocoa Consumption on BP based on Intervention Duration
3.2.4. Effect of Food Matrices
3.2.5. Effect of Daily Dose of Polyphenols
3.2.6. Effect of Daily Dose of Flavanols
3.2.7. Effect of Daily Dose of Epicatechin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 24 October 2021).
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, K.; Bidel, Z.; Nazarzadeh, M.; Copland, E.; Canoy, D.; Ramakrishnan, R.; Pinho-Gomes, A.-C.; Woodward, M.; Adler, A.; Agodoa, L.; et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: An individual participant-level data meta-analysis. Lancet 2021, 397, 1625–1636. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Crozier, S.J.; Preston, A.G.; Hurst, J.W.; Payne, M.J.; Mann, J.; Hainly, L.; Miller, D.L. Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products. Chem. Cent. J. 2011, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Galleano, M.; Oteiza, P.I.; Fraga, C.G. Cocoa, chocolate, and cardiovascular disease. J. Cardiovasc. Pharmacol. 2009, 54, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Ludovici, V.; Barthelmes, J.; Nagele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Jayeola, O.C.; Oyagbemi, A.A.; Okunlola, O.I.; Olubamiwa, O.; Omobowale, T.O.; Ajibade, T.O.; Bolaji-Alabi, F.B.; Ogunpolu, B.S.; Falayi, O.O.; Saba, A.B.; et al. Effect of cocoa powder on hypertension and antioxidant status in uninephrectomized hypertensive rats. Vet. World 2019, 13, 695–705. [Google Scholar] [CrossRef]
- Kluknavsky, M.; Balis, P.; Puzserova, A.; Radosinska, J.; Berenyiova, A.; Drobna, M.; Lukac, S.; Muchova, J.; Bernatova, I. (-)-Epicatechin Prevents Blood Pressure Increase and Reduces Locomotor Hyperactivity in Young Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2016, 2016, 6949020. [Google Scholar] [CrossRef] [Green Version]
- Cienfuegos-Jovellanos, E.; Quinones Mdel, M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J. Agric. Food Chem. 2009, 57, 6156–6162. [Google Scholar] [CrossRef]
- Statista. Global Cocoa Bean Production in 2018/19 and 2020/21, by Country. Available online: https://www.statista.com/statistics/263855/cocoa-bean-production-worldwide-by-region/ (accessed on 25 April 2022).
- Hollenberg, N.K.; Fisher, N.D.; McCullough, M.L. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J. Am. Soc. Hypertens. 2009, 3, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Statista. Per Capita Chocolate Consumption Worldwide in 2017, by Country (in Kilograms). Available online: https://www.statista.com/statistics/819288/worldwide-chocolate-consumption-by-country/ (accessed on 25 April 2022).
- Miller, K.B.; Hurst, W.J.; Flannigan, N.; Ou, B.; Lee, C.Y.; Smith, N.; Stuart, D.A. Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J. Agric. Food Chem. 2009, 57, 9169–9180. [Google Scholar] [CrossRef]
- Vlachojannis, J.; Erne, P.; Zimmermann, B.; Chrubasik-Hausmann, S. The impact of cocoa flavanols on cardiovascular health. Phytother. Res. 2016, 30, 1641–1657. [Google Scholar] [CrossRef]
- Ried, K.; Fakler, P.; Stocks, N.P. Effect of cocoa on blood pressure. Cochrane Database Syst. Rev. 2017, 4, CD008893. [Google Scholar] [CrossRef]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Follmann, D.; Elliott, P.; Suh, I.L.; Cutler, J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1992, 45, 769–773. [Google Scholar] [CrossRef]
- Higgins, J.P. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int. J. Epidemiol. 2008, 37, 1158–1160. [Google Scholar] [CrossRef] [Green Version]
- Almoosawi, S.; Tsang, C.; Ostertag, L.M.; Fyfe, L.; Al-Dujaili, E.A.S. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: A randomized clinical trial. Food Funct. 2012, 3, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007, 85, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Balayssac-Siransy, E.; Ouattara, S.; Boka, K.J.M.; Ahiboh, H.; Yeo, T.A.; Yapo, P.D.; Kondo, A.L.; Toure, W.C.; Ede, K.F.; Dah, C.S.; et al. Dose-effect relation between regular consumption of 100% cocoa powder and blood pressure in young, healthy black Africans. Physiol. Rep. 2021, 9, e15070. [Google Scholar] [CrossRef] [PubMed]
- Crews, W.D., Jr.; Harrison, D.W.; Wright, J.W. A double-blind, placebo-controlled, randomized trial of the effects of dark chocolate and cocoa on variables associated with neuropsychological functioning and cardiovascular health: Clinical findings from a sample of healthy, cognitively intact older adults. Am. J. Clin. Nutr. 2008, 87, 872–880. [Google Scholar]
- Davison, K.; Berry, N.M.; Misan, G.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Dose-related effects of flavanol-rich cocoa on blood pressure. J. Hum. Hypertens. 2010, 24, 568–576. [Google Scholar] [CrossRef]
- Davison, K.; Coates, A.; Buckley, J.; Howe, P. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. 2008, 32, 1289–1296. [Google Scholar] [CrossRef] [Green Version]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.-K.; Milbury, P.; Paul, S.M.; Blumberg, J. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef]
- Fraga, C.G.; Actis-Goretta, L.; Ottaviani, J.I.; Carrasquedo, F.; Lotito, S.B.; Lazarus, S.; Schmitz, H.H.; Keen, C.L. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin. Dev. Immunol. 2005, 12, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Rodriguez-Sanchez, E.; Agudo-Conde, C.; Gonzalez-Sanchez, J.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Effects of Cocoa-Rich Chocolate on Blood Pressure, Cardiovascular Risk Factors, and Arterial Stiffness in Postmenopausal Women: A Randomized Clinical Trial. Nutrients 2020, 12, 1758. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Heiss, C.; Sansone, R.; Karimi, H.; Krabbe, M.; Schuler, D.; Rodriguez-Mateos, A.; Kraemer, T.; Cortese-Krott, M.M.; Kuhnle, G.G.; Spencer, J.P.; et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: A randomized, controlled, double-masked trial. Age 2015, 37, 9794. [Google Scholar] [CrossRef] [Green Version]
- Koli, R.; Kohler, K.; Tonteri, E.; Peltonen, J.; Tikkanen, H.; Fogelholm, M. Dark chocolate and reduced snack consumption in mildly hypertensive adults: An intervention study. Nutr. J. 2015, 14, 84. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lopez, S.; Sarria, B.; Sierra-Cinos, J.L.; Goya, L.; Mateos, R.; Bravo, L. Realistic intake of a flavanol-rich soluble cocoa product increases HDL-cholesterol without inducing anthropometric changes in healthy and moderately hypercholesterolemic subjects. Food Funct. 2014, 5, 364–374. [Google Scholar] [CrossRef]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Front. Pharmacol. 2015, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar] [CrossRef] [Green Version]
- Muniyappa, R.; Hall, G.; Kolodziej, T.L.; Karne, R.J.; Crandon, S.K.; Quon, M.J. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am. J. Clin. Nutr. 2008, 88, 1685–1696. [Google Scholar] [CrossRef]
- Neufingerl, N.; Zebregs, Y.E.; Schuring, E.A.; Trautwein, E.A. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Nishiwaki, M.; Nakano, Y.; Matsumoto, N. Effects of regular high-cocoa chocolate intake on arterial stiffness and metabolic characteristics during exercise. Nutrition 2019, 60, 53–58. [Google Scholar] [CrossRef]
- Njike, V.Y.; Hamburg, N.; Kellogg, M.; Annapureddy, A.; Vita, J. Dose and response to cocoa (DARC): A randomized double-blind controlled trial. Clin. Trials Regul. Sci. Cardiol. 2016, 23–24, 9–15. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Balz, M.; Kimball, J.; Ensunsa, J.L.; Fong, R.; Momma, T.Y.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. Safety and efficacy of cocoa flavanol intake in healthy adults: A randomized, controlled, double-masked trial. Am. J. Clin. Nutr. 2015, 102, 1425–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rull, G.; Mohd-Zain, Z.N.; Shiel, J.; Lundberg, M.H.; Collier, D.J.; Johnston, A.; Warner, T.D.; Corder, R. Effects of high flavanol dark chocolate on cardiovascular function and platelet aggregation. Vasc. Pharmacol. 2015, 71, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.; Spencer, J.P.; Schroeter, H.; Merx, M.W.; et al. Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: A randomised, controlled, double-masked trial: The Flaviola Health Study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef]
- Sarria, B.; Martinez-Lopez, S.; Sierra-Cinos, J.L.; Garcia-Diz, L.; Mateos, R.; Bravo, L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. Br. J. Nutr. 2014, 111, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Shiina, Y.; Funabashi, N.; Lee, K.; Murayama, T.; Nakamura, K.; Wakatsuki, Y.; Daimon, M.; Komuro, I. Acute effect of oral flavonoid-rich dark chocolate intake on coronary circulation, as compared with non-flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adults. Int. J. Cardiol. 2009, 131, 424–429. [Google Scholar] [CrossRef]
- Sudarma, V.; Sukmaniah, S.; Siregar, P. Effect of dark chocolate on nitric oxide serum levels and blood pressure in prehypertension subjects. Acta Med. Indones 2011, 43, 224–228. [Google Scholar]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Van den Bogaard, B.; Draijer, R.; Westerhof, B.E.; van den Meiracker, A.H.; van Montfrans, G.A.; van den Born, B.J. Effects on peripheral and central blood pressure of cocoa with natural or high-dose theobromine: A randomized, double-blind crossover trial. Hypertension 2010, 56, 839–846. [Google Scholar] [CrossRef] [Green Version]
- Wang-Polagruto, J.F.; Villablanca, A.C.; Polagruto, J.A.; Lee, L.; Holt, R.R.; Schrader, H.R.; Ensunsa, J.L.; Steinberg, F.M.; Schmitz, H.H.; Keen, C.L. Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women. J. Cardiovasc. Pharmacol. Ther. 2006, 47, S177–S186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, S.G.; McIntyre, M.D.; Piotrowski, M.J.; Poupin, N.; Miller, D.L.; Preston, A.G.; Wagner, P.; Groves, L.F.; Skulas-Ray, A.C. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br. J. Nutr. 2014, 111, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desch, S.; Schmidt, J.; Kobler, D.; Sonnabend, M.; Eitel, I.; Sareban, M.; Rahimi, K.; Schuler, G.; Thiele, H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am. J. Hypertens. 2010, 23, 97–103. [Google Scholar] [CrossRef]
- Febrianto, N.A.; Wang, S.; Zhu, F. Chemical and biological properties of cocoa beans affected by processing: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–32. [Google Scholar] [CrossRef]
- Fraga, C.G.; Litterio, M.C.; Prince, P.D.; Calabró, V.; Piotrkowski, B.; Galleano, M. Cocoa flavanols: Effects on vascular nitric oxide and blood pressure. J. Clin. Biochem. Nutr. 2010, 48, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Roura, E.; Andres-Lacueva, C.; Estruch, R.; Mata-Bilbao, M.L.; Izquierdo-Pulido, M.; Waterhouse, A.L.; Lamuela-Raventos, R.M. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann. Nutr. Metab. 2007, 51, 493–498. [Google Scholar] [CrossRef]
- Njike, V.Y.; Faridi, Z.; Shuval, K.; Dutta, S.; Kay, C.D.; West, S.G.; Kris-Etherton, P.M.; Katz, D.L. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int. J. Cardiol. 2011, 149, 83–88. [Google Scholar] [CrossRef]
- Wang, Y.; Feltham, B.A.; Suh, M.; Jones, P.J.H. Cocoa flavanols and blood pressure reduction: Is there enough evidence to support a health claim in the United States? Trends Food Sci. Technol. 2019, 83, 203–210. [Google Scholar] [CrossRef]

| Authors, Year | Country of Study | Age (Years) | BMI (kg/m2) | Sample Size | Study Design | Baseline BP | Intervention Duration (Weeks) | Control | Intervention | Serves/Day | Daily Dose of Polyphenol (mg) | Daily Dose of Flavanol (mg) | Daily Dose of Epicatechin (mg) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [27] | UK | — | ≥25, <25 | 42 | RSBPCT | Normal BP | 4 | Placebo | Dark chocolate | — | 500 | — | 17 | ↓ SBP (lean and overweight group) ↓ DBP (overweight group) |
| [28] | Japan | 38 | 22.1 | 25 | RPT | Normal BP | 12 | Sugar | Cocoa powder + sugar beverage | 2 | — | 512 | 377 | ≠∆ SBP and DBP |
| [29] | Côte d’Ivoire | 18–30 | 18.5–29.9 | 124 | RSBPT | Normal BP | 3 | No product | Cocoa beverage | — | 590/1475/2950 | — | — | ↓ SBP (2950 mg polyphenol compared to 590 mg polyphenol) |
| [30] | USA | ≥60 | 25 | 90 | RDBPCPT | Normal BP | 6 | Placebo | Chocolate bar and artificially sweetened cocoa beverage | 1 | — | — | — | ≠∆ SBP and DBP |
| [31] | Australia | 53–57 | 29.3 | 52 | RDBPT | Elevated BP | 6 | LF cocoa beverage (33 mg flavanol, 0 mg epicatechin) | Cocoa beverage | 1 | — | 372/712/1052 | 69/138/208 | ↓ SBP and DBP (1052 mg flavanol compared to 33 mg flavanol) |
| [32] | Australia | 18–65 | 33.5 | 49 | RDBPCPT | Normal BP | 12 | LF cocoa drink (36 mg flavanol) | Cocoa beverage | 2 | — | 902 | — | ↓ DBP (902 mg flavanol compared to 36 mg flavanol) ≠∆ SBP |
| [33] | USA | 32 | — | 21 | RDBPCT | Normal BP | 2 | Low flavanoid chocolate (trace amount epicatechin) | Chocolate bar | — | — | — | 46 | ≠∆ SBP and DBP |
| [34] | USA | 18 | 24.1 | 28 | RCT | Normal BP | 2 | Cocoa butter chocolate | Flavanol-containing milk chocolate | — | — | 168 | 39 | ↓ DBP and ≠∆ SBP (flavanol-containing milk chocolate) and ≠∆ SBP and DBP (cocoa butter chocolate) |
| [35] | Spain | 57 | 25.7 | 140 | RSBCPT | Normal BP | 26 | No product | Chocolate | 1 | 65.5 | — | 26.1 | ≠∆ SBP and DBP |
| [36] | Italy | 44.8 | <30 | 19 | RSBCT | Elevated BP | 2 | White chocolate | Chocolate | 2 | 1008 | — | 110.9 | ↓ BP (dark but not white chocolate) |
| [37] | Italy | 33.9 | 22.6 | 15 | RCT | Normal BP | 2 | White chocolate | Dark Chocolate | — | 500 | — | — | ↓ SBP (dark chocolate compared to white chocolate) |
| [38] | Italy | 43.7 | 18–27 | 20 | RCT | Elevated BP | 2 | White chocolate | Dark Chocolate | — | — | 88 | 66 | ↓ SBP and DBP (dark but not white chocolate) |
| [39] | Germany | young < 35; elderly 50–80 | young 24.9; elderly 26.5 | 42 | RDBPT | Normal BP | 2 | Placebo | Cocoa beverage | 2 | — | 900 | 128 | Young: ≠∆ SBP, ↓ DBP (treatment compared to control) Elderly: ↓ SBP & DBP (treatment compared to control) |
| [40] | Finland | 45.8 | 27.7 | 22 | RCCT | Elevated BP | 8 | Reduced snack intake | Replaced snack intake with dark chocolate | — | — | 603 | — | ≠∆ SBP and DBP |
| [41] | Spain | 25.9 | 23 (normocholesterolemic); 24 (hypercholesterolemic) | 24 | NCCFT | Normal BP | 4 | Milk | Dairy-based cocoa drink | 2 | — | 45.3 | 18.9 | ≠∆ SBP and DBP |
| [42] | USA | 24.3 | 23 | 40 | RDBPCT | Normal BP | 4 | Placebo tablet | Cocoa tablet | — | — | 250 | — | ≠∆ SBP and DBP |
| [43] | Australia | 40 | 26 ±4 | 28 | RDBPT | Normal BP | 4 | Placebo | Cocoa tablet | — | — | 234 | — | ≠∆ SBP and DBP |
| [44] | USA | 51 | 33.2 | 20 | RDBPCCT | Elevated BP | 2 | Placebo | Flavanol-rich cocoa drink | 2 | 902 | — | 174 | ≠∆ SBP and DBP |
| [45] | Netherlands | 40–70 | 18.8–30.8 | 143 | RDBPCFFPT | Normal BP | 4 | Placebo | Cocoa beverage | — | — | 325 | — | ≠∆ SBP and DBP |
| [46] | Japan | 20.2 | — | 32 | RCPT | Normal BP | 4 | No product | Chocolate | 2 | — | 508 | — | ≠∆ SBP and DBP |
| [47] | USA | 53.6 | <40 | 122 | RDBCMLSPT | Elevated BP | 8 | Placebo | Chocolate and cocoa beverage | — | — | 257/514 | 24/46 | ≠∆ SBP and DBP (both high and low dose compared to placebo) |
| [48] | USA | 30–55 | 24 | 59 | RDBPT | Normal BP | 12 | Placebo | Cocoa extract capsule | 2 | — | 1000 (week 0–1), followed by 1500 (week 1–3) and 2000 (week 3–12) | 110 (week0–1), followed by 165 (week 1–3) and 220 (week 3–12) | ≠∆ SBP and DBP |
| [49] | UK | 55.4 | 26.6 | 32 | RDBPCCT | Elevated BP | 6 | LF dark chocolate (88 mg flavanol) | HF dark cholate | 2 | — | 1064 | — | ≠∆ SBP and DBP |
| [50] | Germany | 35–60 | 23–27 | 100 | RDBCPT | Normal BP | 4 | Control placebo beverage + theobromine + caffeine | Fruit-flavored cocoa beverage | 2 | — | 900 | 128 | ↓ SBP and DBP (cocoa treatment compared to placebo) |
| [51] | Spain | 25–36 | <30 | 24 | RCCT | Normal BP | 4 | Milk | Dairy-based cocoa drink | 2 | 416 | — | — | ≠∆ SBP and DBP |
| [52] | Japan | 29.7 | 22.6 ± 2.0/22.6 ± 1.9 | 39 | RSBPT | Normal BP | 2 | White Chocolate | Dark chocolate | 1 | 550 | — | — | ≠∆ SBP and DBP |
| [53] | Indonesia | 25–44 | 18.5–24.9 | 32 | RPT | Elevated BP | 2 | White Chocolate | Dark chocolate | — | — | — | — | ↓ SBP and DBP (dark chocolate not white chocolate) |
| [54] | Germany | 64 | >27.5 or <18.5 | 44 | RSBCPT | Elevated BP | 18 | White Chocolate | Dark chocolate | 1 | 30 | — | 5.1 | ↓ SBP and DBP (dark not white chocolate) |
| [55] | Netherlands | 40–70 | 25.9 | 41 | RDBPCCT | Normal BP | 3 | Placebo | Flavanol-rich milk-based cocoa drink | — | — | 106 | — | ↑ 24-h ASBP (cocoa flavanol not placebo). ≠∆ PSBP (between cocoa flavanol and placebo after 2 h) ↓ CSBP |
| [56] | USA | 57.7 (HF), 55.4 (LF) | 24.9 (HF), 25.3 (LF) | 32 | RDBPT | Normal BP | 6 | LF cocoa powder (43 mg flavanol) + sucrose | HF cocoa powder + sucrose | — | — | 446 | — | ↓ SBP and DBP (control but not high dose) |
| [57] | USA | 40–64 | 25–37 | 30 | RDBPCCT | Normal BP | 4 | LF chocolate (43 mg flavanol) and sugarless cocoa-free beverage | Dark chocolate + cocoa beverage | — | — | 814 | — | ↑ SBP and DBP (both high dose and control) |
| Subgroups | WMD (95% CI) mmHg | p-Value | I2 (%) | n | Subgroup Differences (p-Value) |
|---|---|---|---|---|---|
| Intervention Duration | |||||
| Resting Systolic BP | |||||
| ≤4 weeks | −2.35 [−4.09, −0.61] | 0.008 | 60 | 22 | |
| >4 weeks | −0.81 [−2.55, 0.92] | 0.36 | 44 | 12 | |
| Overall | −1.87 [−3.08, −0.65] | 0.003 | 56 | 34 | 0.22 |
| Resting Diastolic BP | |||||
| ≤4 weeks | −2.14 [−3.21, −1.06] | <0.001 | 35 | 22 | |
| >4 weeks | −0.38 [−1.52, 0.75] | 0.51 | 43 | 12 | |
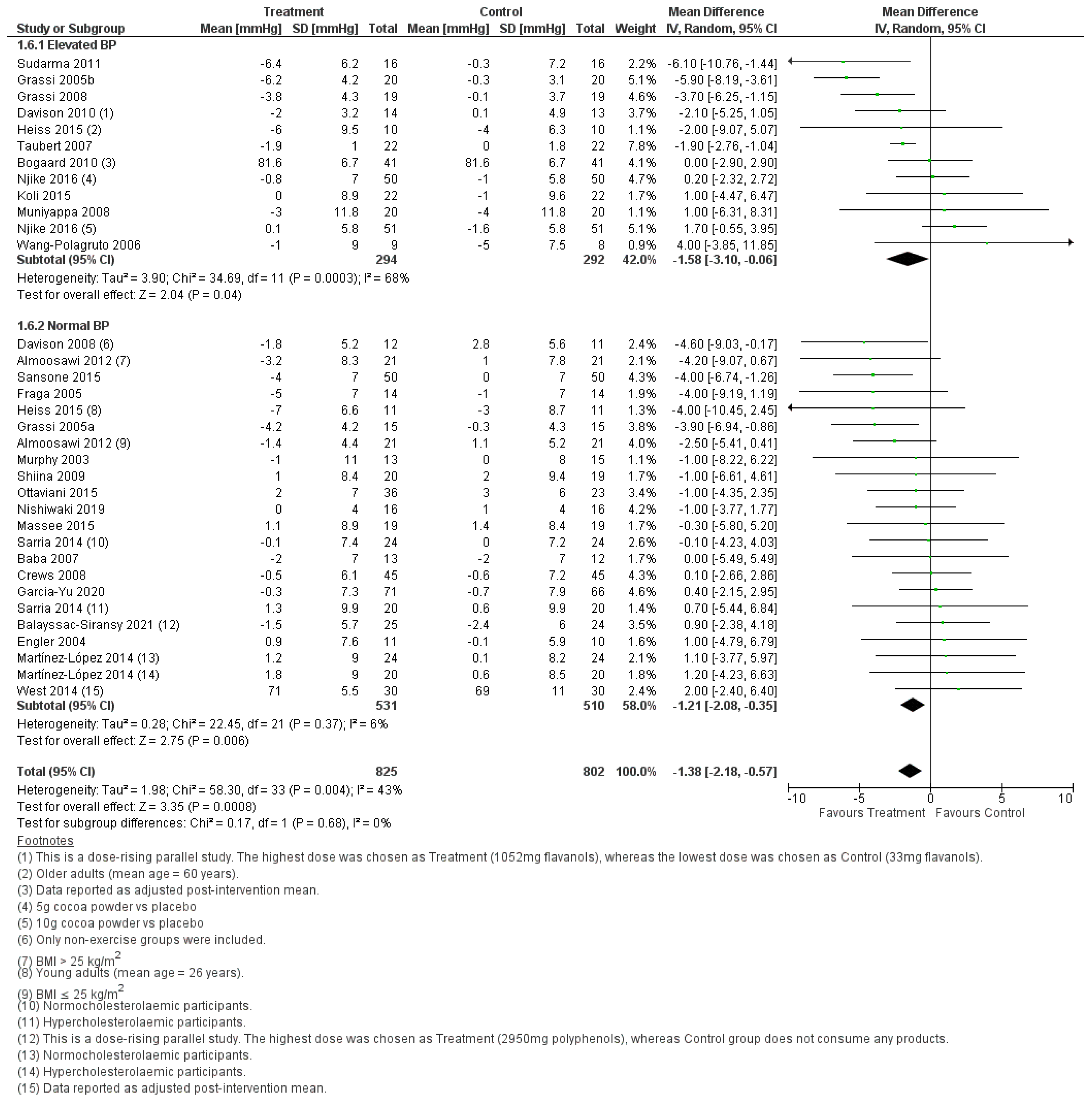
| Overall | −1.38 [−2.18, −0.57] | <0.001 | 43 | 36 | 0.03 |
| 24-h Systolic BP | |||||
| ≤4 weeks | −4.07 [−8.21, 0.07] | 0.05 | 85 | 7 | |
| >4 weeks | −0.82 [−2.52, 0.87] | 0.34 | 0 | 6 | |
| Overall | −2.64 [−5.02, −0.26] | 0.03 | 75 | 13 | 0.15 |
| 24-h Diastolic BP | |||||
| ≤4 weeks | −4.03 [−6.96, −1.11] | 0.007 | 77 | 7 | |
| >4 weeks | −1.53 [−0.47, 0.58] | 0.38 | 0 | 6 | |
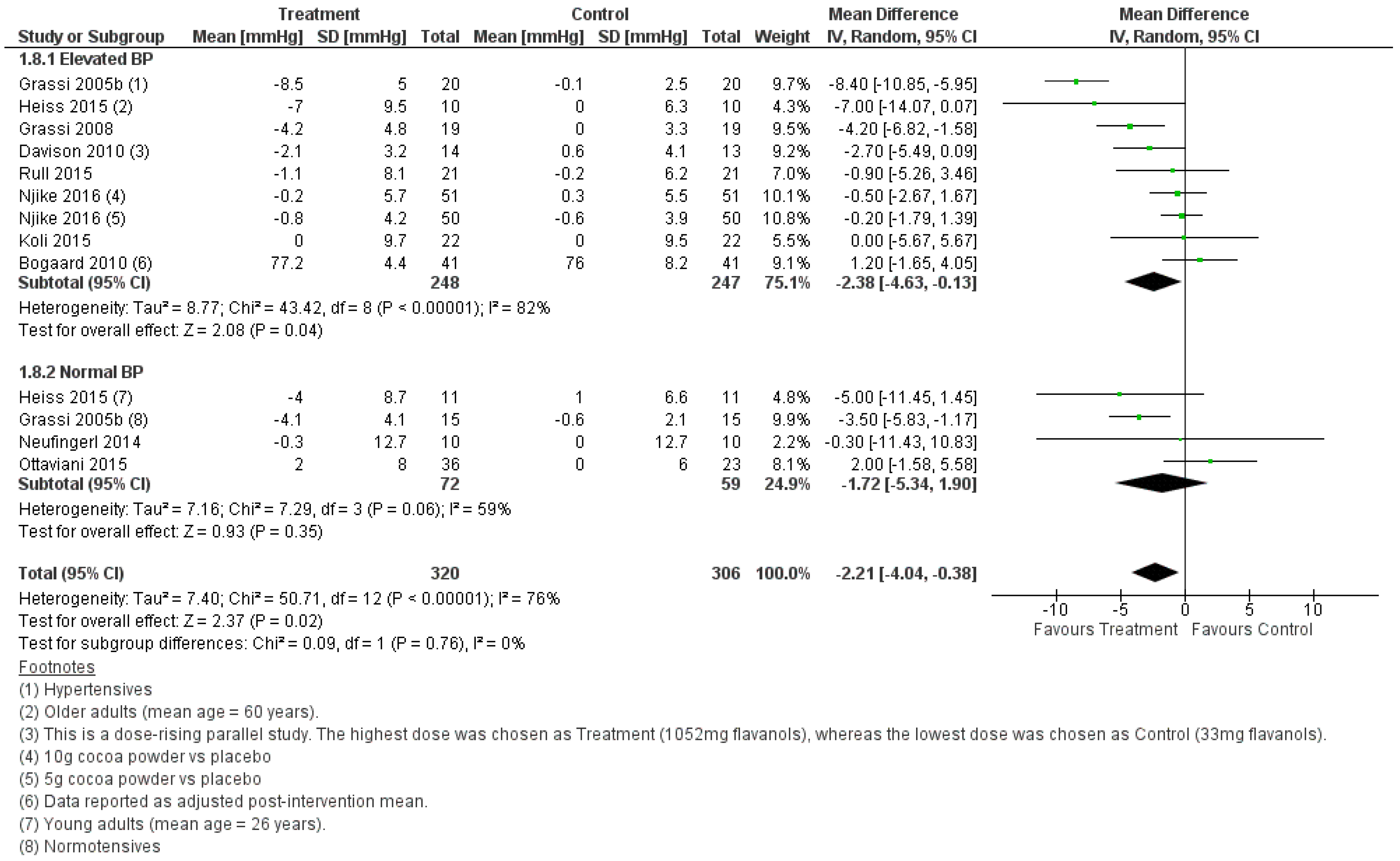
| Overall | −2.21 [−4.04, −0.38] | 0.02 | 76 | 13 | 0.02 a |
| Food Matrices | |||||
| Resting Systolic BP | |||||
| Beverage | −1.54 [−3.08, 0.01] | 0.05 | 0 | 14 | |
| Chocolate | −3.94 [−5.71, −2.18] | <0.001 | 63 | 13 | |
| Beverage + Chocolate | 1.22 [−0.86, 3.30] | 0.25 | 0 | 4 | |
| Tablets/Capsules | 1.52 [−3.35, 6.39] | 0.54 | 40 | 3 | |
| Overall | −1.87 [−3.08, −0.65] | <0.001 | 56 | 34 | 0.002 |
| Resting Diastolic BP | |||||
| Beverage | −1.06 [−2.26, 0.15] | 0.03 | 7 | 14 | |
| Chocolate | −2.59 [−3.78, −1.40] | <0.001 | 50 | 13 | |
| Beverage + Chocolate | 0.90 [−0.46, 2.26] | 0.20 | 0 | 4 | |
| Tablets/Capsules | −0.84 [−3.50, 1.83] | 0.54 | 0 | 3 | |
| Overall | −1.38 [−2.18, −0.57] | <0.001 | 43 | 34 | 0.003 |
| 24-h Systolic BP | |||||
| Beverage | −1.36 [−5.17, 2.44] | 0.48 | 49 | 5 | |
| Chocolate | −5.03 [−8.36, −1.70] | 0.003 | 72 | 5 | |
| Beverage + Chocolate | −0.32 [−2.52, 1.88] | 0.78 | 0 | 2 | |
| Tablets/Capsules | 1.00 [−4.44, 6.44] | 0.72 | — | 1 | |
| Overall | −2.64 [−5.02, −0.26] | 0.03 | 75 | 13 | 0.10 |
| 24-h Diastolic BP | |||||
| Beverage | −2.16 [−5.13, 0.81] | 0.15 | 48 | 5 | |
| Chocolate | −3.92 [−6.69, −1.15] | 0.006 | 74 | 5 | |
| Beverage + Chocolate | −0.30 [−1.59, 0.98] | 0.64 | 0 | 2 | |
| Tablets/Capsules | 2.00 [−1.58, 5.58] | 0.27 | — | 1 | |
| Overall | −2.21 [−4.04, −0.38] | 0.02 | 76 | 13 | 0.04 |
| Daily Dose of Polyphenols | |||||
| Resting Systolic BP | |||||
| <500 mg | −0.55 [−3.50, 2.39] | 0.71 | 61 | 5 | |
| ≥500 mg | −2.44 [−4.19, −0.70] | 0.006 | 24 | 10 | |
| Overall | −1.95 [−3.31, −0.59] | 0.005 | 37 | 15 | 0.28 |
| Resting Diastolic BP | |||||
| <500 mg | −1.49 [−2.34, −0.65] | <0.001 | 2 | 5 | |
| ≥500 mg | −1.57 [−2.97, −0.17] | 0.03 | 27 | 10 | |
| Overall | −1.40 [−2.27, −0.53] | 0.002 | 15 | 15 | 0.93 |
| Sensitivity Analysis: Daily Dose of Polyphenols b | |||||
| Resting Systolic BP | |||||
| <500 mg | 0.78 [−2.74, 4.29] | 0.66 | 38 | 4 | |
| ≥500 mg | −2.44 [−4.19, −0.70] | 0.006 | 24 | 10 | |
| Overall | −1.50 [−3.20, 0.19] | 0.08 | 38 | 14 | 0.11 |
| Resting Diastolic BP | |||||
| <500 mg | 0.24 [−1.49, 2.13] | 0.81 | 0 | 4 | |
| ≥500 mg | −1.57 [−2.97, −0.17] | 0.03 | 27 | 10 | |
| Overall | −1.14 [−2.25, −0.04] | 0.04 | 16 | 15 | 0.14 |
| Daily Dose of Flavanols | |||||
| Resting Systolic BP | |||||
| <900 mg | −0.39 [−3.95, 3.17] | 0.83 | 79 | 12 | |
| ≥900 mg | −2.88 [−5.06, −0.70] | 0.01 | 0 | 6 | |
| Overall | −1.31 [−3.72, 1.10] | 0.29 | 69 | 18 | 0.24 |
| Resting Diastolic BP | |||||
| <900 mg | −0.37 [−2.18, 1.44] | 0.69 | 64 | 12 | |
| ≥900 mg | −2.88 [−4.43, −1.33] | <0.001 | 0 | 6 | |
| Overall | −1.22 [−2.58, 0.14] | 0.08 | 56 | 18 | 0.04 |
| 24-h Systolic BP | |||||
| <900 mg | −2.56 [−6.43, 1.31] | 0.20 | 86 | 7 | |
| ≥900 mg | −2.10 [−4.71, 0.52] | 0.12 | 0 | 5 | |
| Overall | −2.42 [−5.06, 0.22] | 0.07 | 76 | 12 | 0.85 |
| 24-h Diastolic BP | |||||
| <900 mg | −1.94 [−4.68, 0.80] | 0.17 | 85 | 7 | |
| ≥900 mg | −1.94 [−4.71, 0.84] | 0.17 | 49 | 5 | |
| Overall | −2.01 [−3.97, −0.04] | 0.05 | 77 | 12 | 1.00 |
| Sensitivity Analysis: Daily Dose of Flavanols c | |||||
| Resting Systolic BP | |||||
| <900 mg | 1.14 [−0.60, 2.88] | 0.20 | 0 | 11 | |
| ≥900 mg | −2.88 [−5.06, −0.70] | 0.01 | 0 | 6 | |
| Overall | −0.43 [−1.82, 0.97] | 0.55 | 3 | 17 | 0.005 |
| Resting Diastolic BP | |||||
| <900 mg | 0.43 [−0.67, 1.52] | 0.44 | 0 | 11 | |
| ≥900 mg | −2.88 [−4.43, −1.33] | <0.001 | 0 | 6 | |
| Overall | −0.75 [−1.84, 0.34] | 0.18 | 25 | 17 | <0.001 |
| 24-h Systolic BP | |||||
| <900 mg | 0.66 [−0.94, 2.27] | 0.42 | 0 | 5 | |
| ≥900 mg | −2.10 [−4.71, 0.52] | 0.12 | 0 | 5 | |
| Overall | −0.10 [−1.48, 1.27] | 0.88 | 1 | 10 | 0.08 |
| 24-h Diastolic BP | |||||
| <900 mg | −0.05 [−1.19, 1.09] | 0.93 | 0 | 5 | |
| ≥900 mg | −1.94 [−4.71, 0.84] | 0.17 | 49 | 5 | |
| Overall | −0.58 [−1.74, 0.59] | 0.28 | 18 | 10 | 0.22 |
| Daily Dose of Epicatechin | |||||
| Resting Systolic BP | |||||
| <100 mg | −1.47 [−4.52, 1.58] | 0.35 | 78 | 12 | |
| ≥100 mg | −3.01 [−4.58, −1.44] | <0.001 | 0 | 7 | |
| Overall | −1.89 [−3.81, 0.02] | 0.05 | 66 | 19 | 0.38 |
| Resting Diastolic BP | |||||
| <100 mg | −0.98 [−2.67, 0.71] | 0.26 | 65 | 12 | |
| ≥100 mg | −2.82 [−4.18, −1.45] | <0.001 | 0 | 7 | |
| Overall | −1.53 [−2.76, −0.31] | 0.01 | 55 | 19 | 0.10 |
| 24-h Systolic BP | |||||
| <100 mg | −2.97 [−7.50, 1.56] | 0.20 | 91 | 5 | |
| ≥100 mg | −3.66 [−5.68, −1.63] | <0.001 | 0 | 5 | |
| Overall | −2.99 [−5.77, −0.22] | 0.03 | 81 | 10 | 0.79 |
| 24-h Diastolic BP | |||||
| <100 mg | −2.27 [−5.38, 0.84] | 0.15 | 90 | 5 | |
| ≥100 mg | −2.79 [−5.54, −0.04] | 0.05 | 59 | 5 | |
| Overall | −2.53 [−4.61, −0.45] | 0.02 | 82 | 10 | 0.81 |
| Sensitivity Analysis: Daily Dose of Epicatechin c | |||||
| Resting Systolic BP | |||||
| <100 mg | −0.23 [−1.82, 1.35] | 0.77 | 4 | 11 | |
| ≥100 mg | −3.01 [−4.58, −1.44] | <0.001 | 0 | 7 | |
| Overall | −1.42 [−2.66, −0.18] | 0.03 | 13 | 18 | 0.01 |
| Resting Diastolic BP | |||||
| <100 mg | −0.12 [−1.19, 0.96] | 0.83 | 7 | 12 | |
| ≥100 mg | −2.82 [−4.18, −1.45] | <0.001 | 0 | 8 | |
| Overall | −1.13 [−2.17, −0.10] | 0.03 | 30 | 20 | 0.002 |
| 24-h Systolic BP | |||||
| <100 mg | 0.66 [−1.43, 2.76] | 0.53 | 34 | 3 | |
| ≥100 mg | −3.66 [−5.68, −1.63] | <0.001 | 0 | 5 | |
| Overall | −1.37 [−3.61, 0.87] | 0.23 | 60 | 8 | 0.004 |
| 24-h Diastolic BP | |||||
| <100 mg | −0.05 [−1.22, 1.12] | 0.93 | 0 | 3 | |
| ≥100 mg | −2.79 [−5.54, −0.04] | 0.05 | 59 | 5 | |
| Overall | −1.31 [−2.98, 0.35] | 0.12 | 60 | 8 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoah, I.; Lim, J.J.; Osei, E.O.; Arthur, M.; Tawiah, P.; Oduro, I.N.; Aduama-Larbi, M.S.; Lowor, S.T.; Rush, E. Effect of Cocoa Beverage and Dark Chocolate Consumption on Blood Pressure in Those with Normal and Elevated Blood Pressure: A Systematic Review and Meta-Analysis. Foods 2022, 11, 1962. https://doi.org/10.3390/foods11131962
Amoah I, Lim JJ, Osei EO, Arthur M, Tawiah P, Oduro IN, Aduama-Larbi MS, Lowor ST, Rush E. Effect of Cocoa Beverage and Dark Chocolate Consumption on Blood Pressure in Those with Normal and Elevated Blood Pressure: A Systematic Review and Meta-Analysis. Foods. 2022; 11(13):1962. https://doi.org/10.3390/foods11131962
Chicago/Turabian StyleAmoah, Isaac, Jia Jiet Lim, Emmanuel Ofori Osei, Michael Arthur, Phyllis Tawiah, Ibok Nsa Oduro, Margaret Saka Aduama-Larbi, Samuel Tetteh Lowor, and Elaine Rush. 2022. "Effect of Cocoa Beverage and Dark Chocolate Consumption on Blood Pressure in Those with Normal and Elevated Blood Pressure: A Systematic Review and Meta-Analysis" Foods 11, no. 13: 1962. https://doi.org/10.3390/foods11131962
APA StyleAmoah, I., Lim, J. J., Osei, E. O., Arthur, M., Tawiah, P., Oduro, I. N., Aduama-Larbi, M. S., Lowor, S. T., & Rush, E. (2022). Effect of Cocoa Beverage and Dark Chocolate Consumption on Blood Pressure in Those with Normal and Elevated Blood Pressure: A Systematic Review and Meta-Analysis. Foods, 11(13), 1962. https://doi.org/10.3390/foods11131962











