Native Non-Saccharomyces Yeasts as a Tool to Produce Distinctive and Diverse Tamjanika Grape Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Wine Production
2.3. Standard Physicochemical Analyses
2.4. Determination of Wine Volatile Compounds Composition (HS-SPME-GC-MS)
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussion
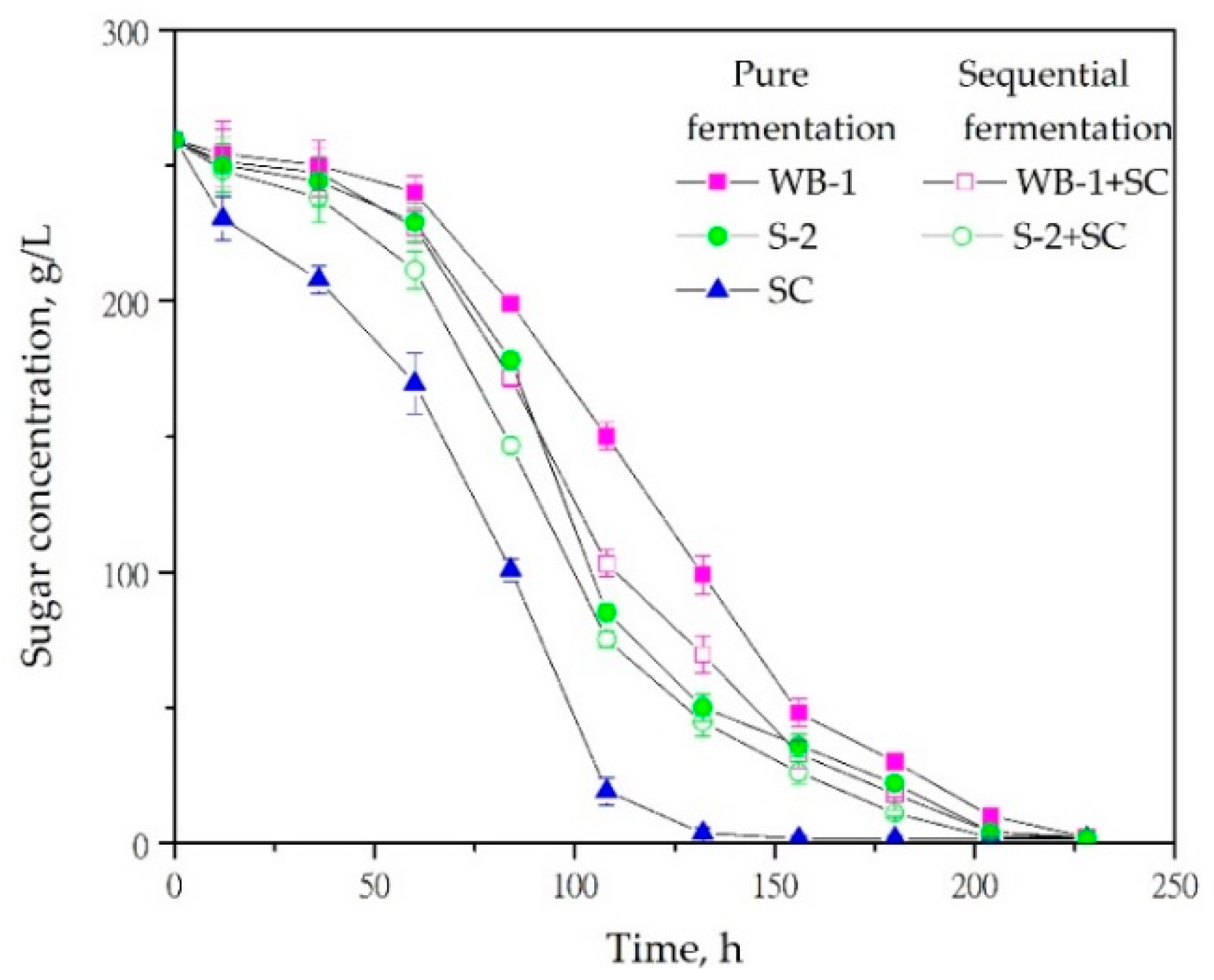
3.1. Fermentation Kinetics
3.2. Standard Quality Parameter of Wine
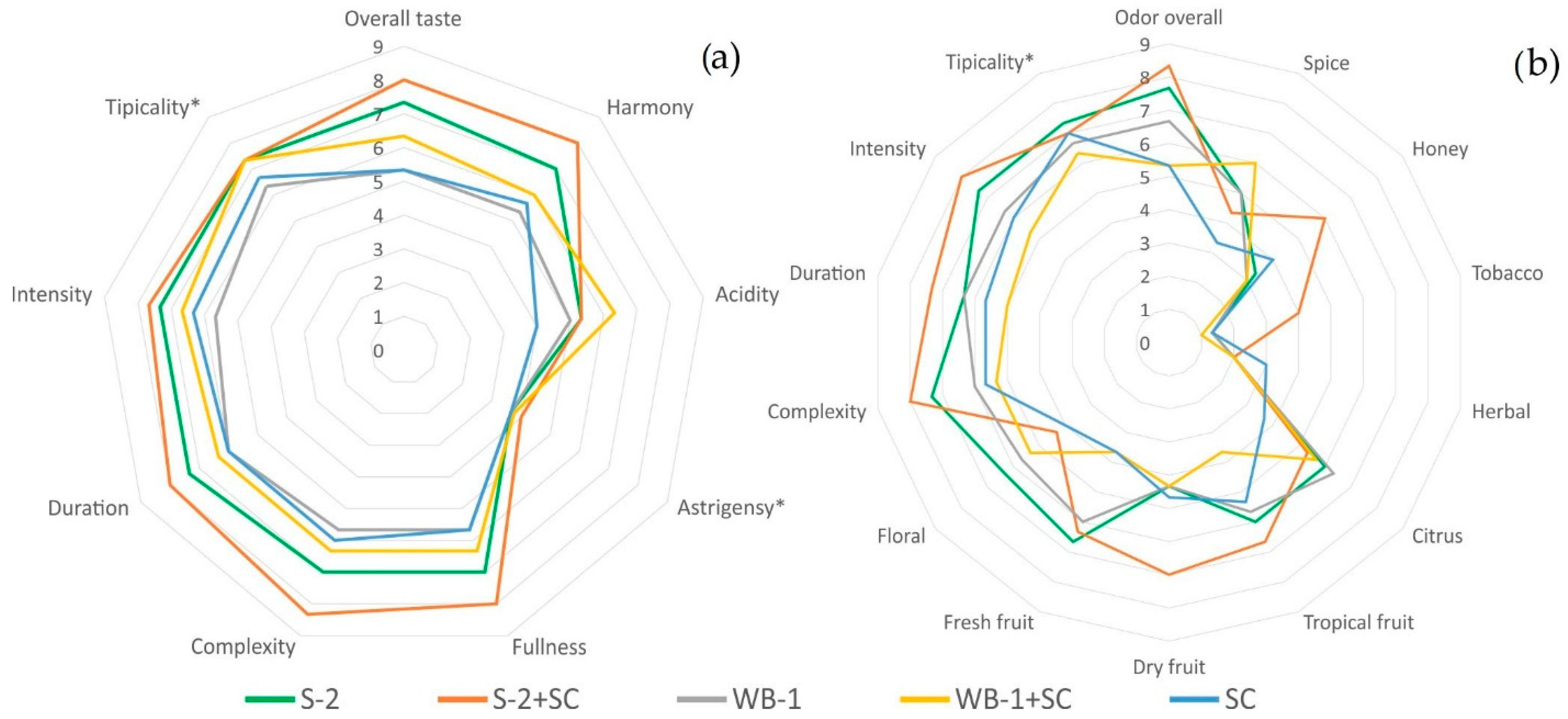
3.3. Sensory Evaluation
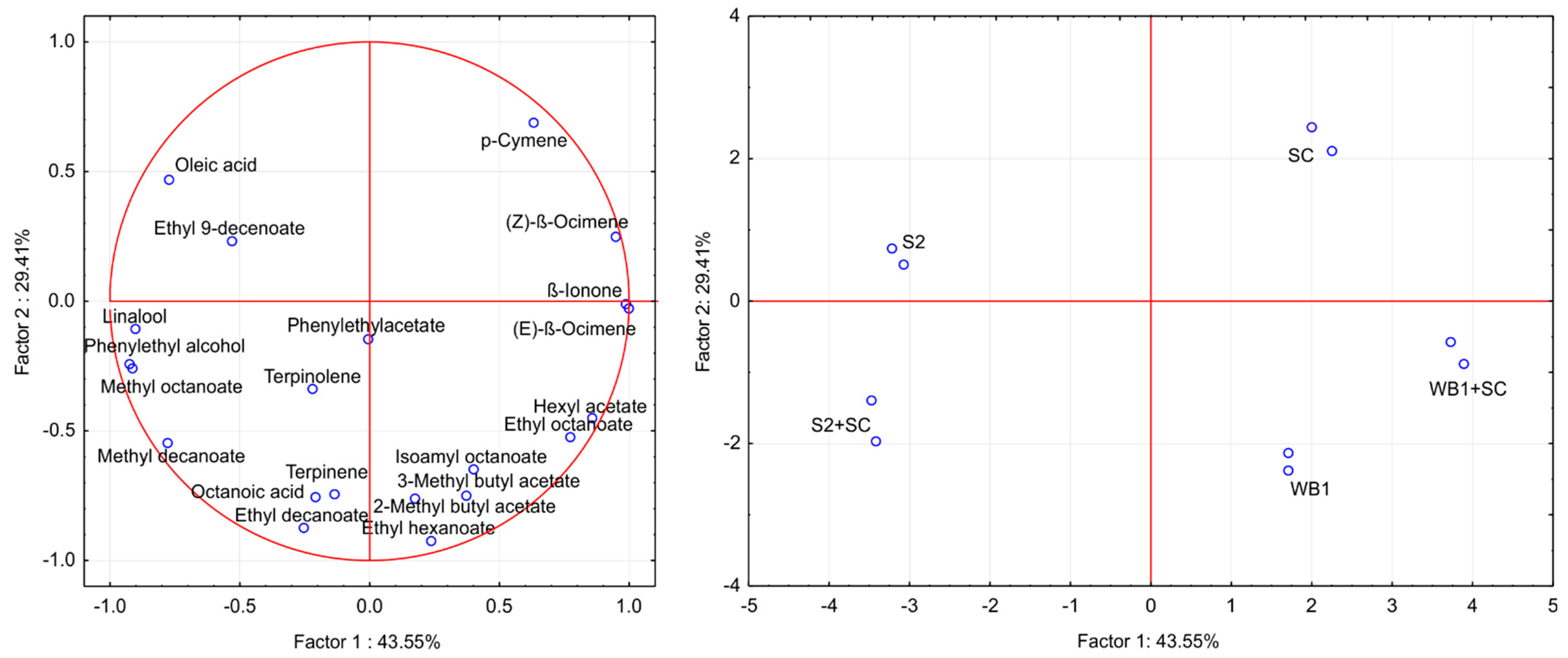
3.4. Volatile Composition of Wines
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yurkov, A.M. Yeasts of the Soil—Obscure but Precious. Yeast 2018, 35, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Laaksonen, O.; Li, P.; Gu, Q.; Yang, B. Use of Non-Saccharomyces Yeasts in Berry Wine Production: Inspiration from Their Applications in Winemaking. J. Agric. Food Chem. 2022, 70, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial Terroir and Food Innovation: The Case of Yeast Biodiversity in Wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.; Loira, I.; del Fresno, J.; González, C.; Suárez-Lepe, J. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretorius, I.S. Tasting the Terroir of Wine Yeast Innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garofalo, C.; Russo, P.; Beneduce, L.; Massa, S.; Spano, G.; Capozzi, V. Non-Saccharomyces Biodiversity in Wine and the ‘Microbial Terroir’: A Survey on Nero Di Troia Wine from the Apulian Region, Italy. Ann. Microbiol. 2016, 66, 143–150. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [Green Version]
- Fleet, G. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Andorrà, I.; Berradre, M.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of Mixed Culture Fermentations on Yeast Populations and Aroma Profile. LWT 2012, 49, 8–13. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The Oenological Potential of Hanseniaspora uvarum in Simultaneous and Sequential Co-Fermentation with Saccharomyces cerevisiae for Industrial Wine Production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mančić, S.; Stamenković Stojanović, S.; Danilović, B.; Djordjević, N.; Malićanin, M.; Lazić, M.; Karabegović, I. Oenological Characterization of Native Hanseniaspora uvarum Strains. Fermentation 2022, 8, 92. [Google Scholar] [CrossRef]
- Du Plessis, H.; Du Toit, M.; Nieuwoudt, H.; Van der Rijst, M.; Hoff, J.; Jolly, N. Modulation of Wine Flavor Using Hanseniaspora uvarum in Combination with Different Saccharomyces cerevisiae, Lactic Acid Bacteria Strains and Malolactic Fermentation Strategies. Fermentation 2019, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Albertin, W.; Setati, M.E.; Miot-Sertier, C.; Mostert, T.T.; Colonna-Ceccaldi, B.; Coulon, J.; Girard, P.; Moine, V.; Pillet, M.; Salin, F.; et al. Hanseniaspora uvarum from Winemaking Environments Show Spatial and Temporal Genetic Clustering. Front. Microbiol. 2016, 6, 1569. [Google Scholar] [CrossRef]
- Zilelidou, E.A.; Nisiotou, A. Understanding Wine through Yeast Interactions. Microorganisms 2021, 9, 1620. [Google Scholar] [CrossRef]
- Reisman, H.B. Problems in Scale-Up of Biotechnology Production Processes. Crit. Rev. Biotechnol. 1993, 13, 195–253. [Google Scholar] [CrossRef]
- Mančić, S.; Danilović, B.; Malićanin, M.; Stamenković Stojanović, S.; Nikolić, N.; Lazić, M.; Karabegović, I. Fermentative Potential of Native Yeast Candida famata for Prokupac Grape Must Fermentation. Agriculture 2021, 11, 358. [Google Scholar] [CrossRef]
- Malićanin, M.; Danilović, B.; Stamenković Stojanović, S.; Cvetković, D.; Lazić, M.; Karabegović, I.; Savić, D. Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine. Horticulturae 2022, 8, 212. [Google Scholar] [CrossRef]
- Karabegović, I.; Malićanin, M.; Danilović, B.; Stanojević, J.; Stamenković Stojanović, S.; Nikolić, N.; Lazić, M. Potential of Non-Saccharomyces Yeast for Improving the Aroma and Sensory Profile of Prokupac Red Wine. OENO One 2021, 55, 181–195. [Google Scholar] [CrossRef]
- Lakićević, S.H.; Karabegović, I.T.; Cvetković, D.J.; Lazić, M.L.; Jančić, R.; Popović-Djordjević, J.B. Insight into the Aroma Profile and Sensory Characteristics of ‘Prokupac’ Red Wine Aromatised with Medicinal Herbs. Horticulturae 2022, 8, 277. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Biogeographical Regionalization of Wine Yeast Communities in Greece and Environmental Drivers of Species Distribution at a Local Scale. Front. Microbiol. 2021, 12, 705001. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Fernández, M.; Úbeda, J.F.; Briones, A.I. Typing of Non-Saccharomyces Yeasts with Enzymatic Activities of Interest in Wine-Making. Int. J. Food Microbiol. 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine Aromatic Compound Production and Fermentative Behaviour within Different Non-Saccharomyces Species and Clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Maicas, S. Advances in Wine Fermentation. Fermentation 2021, 7, 187. [Google Scholar] [CrossRef]
- Blanco, P.; Castrillo, D.; Graña, M.J.; Lorenzo, M.J.; Soto, E. Evaluation of Autochthonous Non-Saccharomyces Yeasts by Sequential Fermentation for Wine Differentiation in Galicia (NW Spain). Fermentation 2021, 7, 183. [Google Scholar] [CrossRef]
- Suzzi, G.; Schirone, M.; Sergi, M.; Marianella, R.M.; Fasoli, G.; Aguzzi, I.; Tofalo, R. Multistarter from Organic Viticulture for Red Wine Montepulciano d’Abruzzo Production. Front. Microbiol. 2012, 3, 135. [Google Scholar] [CrossRef] [Green Version]
- Nisiotou, A.; Mallouchos, A.; Tassou, C.; Banilas, G. Indigenous Yeast Interactions in Dual-Starter Fermentations May Improve the Varietal Expression of Moschofilero Wine. Front. Microbiol. 2019, 10, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation Behaviour and Metabolic Interactions of Multistarter Wine Yeast Fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Jood, I.; Hoff, J.W.; Setati, M.E. Evaluating Fermentation Characteristics of Kazachstania Spp. and Their Potential Influence on Wine Quality. World J. Microbiol. Biotechnol. 2017, 33, 129. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora Vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Navarro, Y.; Mas, A.; Torija, M.-J.; Beltran, G. A Rapid Method for Selecting Non-Saccharomyces Strains with a Low Ethanol Yield. Microorganisms 2020, 8, 658. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; De Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative Aptitude of Non-Saccharomyces Wine Yeast for Reduction in the Ethanol Content in Wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Varela, J.; Varela, C. Microbiological Strategies to Produce Beer and Wine with Reduced Ethanol Concentration. Curr. Opin. Biotechnol. 2019, 56, 88–96. [Google Scholar] [CrossRef]
- Binati, R.L.; Lemos Junior, W.J.F.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of Non-Saccharomyces Yeasts to Wine Volatile and Sensory Diversity: A Study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris Strains Isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Waldir, D.E.E. Perspectives and Uses of Non-Saccharomyces Yeasts in Fermented Beverages. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Lidia Solís-Oviedo, R., de la Cruz Pech-Canul, Á., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-495-4. [Google Scholar]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the Overall Quality of Wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Sainz, F.; Pardo, J.; Ruiz, A.; Expósito, D.; Armero, R.; Querol, A.; Guillamón, J.M. Use of Non-Conventional Yeasts to Increase Total Acidity in the Cava Base Wines. LWT 2022, 158, 113183. [Google Scholar] [CrossRef]
- Vilela, A. Use of Nonconventional Yeasts for Modulating Wine Acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Romano, P. Function of Yeast Species and Strains in Wine Flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Comi, G.; Romano, P.; Cocolin, L.; Fiore, C. Characterization of Kloeckera apiculata Strains from the Friuli Regionin Northern Italy. World J. Microbiol. Biotechnol. 2001, 17, 391–394. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile Profile Characterization of Croatian Commercial Sparkling Wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef]
- Shi, W.-K.; Wang, J.; Chen, F.-S.; Zhang, X.-Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on Wine Flavor and Quality through Simultaneous and Sequential Co-Fermentation with Saccharomyces cerevisiae. LWT 2019, 116, 108477. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative Analysis of Headspace Volatile Compounds Using Comprehensive Two-Dimensional Gas Chromatography and Their Contribution to the Aroma of Chardonnay Wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Sun, F.; Wang, W.; Liu, Y.; Wang, J.; Sun, J.; Mu, J.; Gao, Z. Effects of Spontaneous Fermentation on the Microorganisms Diversity and Volatile Compounds during ‘Marselan’ from Grape to Wine. LWT 2020, 134, 110193. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Q.; Tian, B.; Zhu, S.; Du, F.; Mao, R.; Li, S.; Liu, L.; Zhu, Y. Evaluation of Four Indigenous Non-Saccharomyces Yeasts Isolated from the Shangri-La Wine Region (China) for Their Fermentation Performances and Aroma Compositions in Synthetic Grape Juice Fermentation. J. Fungi 2022, 8, 146. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma Characterization Based on Aromatic Series Analysis in Table Grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.A.; Sánchez-Palomo, E.; Osorio Alises, M.; González Viñas, M.A. Chemical and Sensory Aroma Typicity of La Mancha Petit Verdot Wines. LWT 2022, 162, 113418. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 57, pp. 131–175. ISBN 978-0-12-002659-3. [Google Scholar]
- Del Caro, A.; Fanara, C.; Genovese, A.; Moio, L.; Piga, A.; Piombino, P. Free and Enzymatically Hydrolysed Volatile Compounds of Sweet Wines from Malvasia and Muscat Grapes (Vitis vinifera L.) Grown in Sardinia. S. Afr. J. Enol. Vitic. 2016, 33, 115–121. [Google Scholar] [CrossRef]
- Katarína, F.; Katarína, M.; Ivan, Š. Effect of Indigenous S. cerevisiae Strains on Higher Alcohols, Volatile Acids and Esters in Wine. Czech J. Food Sci. 2017, 35, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhong, T.; Heygi, F.; Wang, Z.; Du, M. Effects of Inoculation Protocols on Aroma Profiles and Quality of Plum Wine in Mixed Culture Fermentation of Metschnikowia pulcherrima with Saccharomyces cerevisiae. LWT 2022, 161, 113338. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhu, B.; Zhang, X.; Wang, H.; Yan, A.; Zhang, G.; Wang, X.; Xu, H. The Accumulation Profiles of Terpene Metabolites in Three Muscat Table Grape Cultivars through HS-SPME-GCMS. Sci. Data 2020, 7, 5. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. S. Afr. J. Enol. Vitic. 2017, 4, 49–58. [Google Scholar] [CrossRef]
- Benito, A.; Calderón, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical Impact of Metschnikowia pulcherrima in the Volatile Profile of Verdejo White Wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Pure Fermentation | Sequential Fermentation | |||
|---|---|---|---|---|---|
| Candida famata WB-1 | Hanseniaspora uvarum S-2 | Saccharomyces cerevisiae QA23 | Candida famata WB-1 | Hanseniaspora uvarum S-2 | |
| Ethanol, % v/v | 12.79 ± 0.30 a | 13.05 ± 0.09 a | 13.85 ± 0.10 b | 13.32 ± 0.17 c | 13.49 ± 0.20 c |
| Total extract, g/L | 21.2 ± 0.50 a | 24.5 ± 0.80 b | 18.5 ± 0.58 c | 19.60 ± 0.90 c | 21.4 ± 0.58 a |
| Total acids (as tartaric acid), g/L | 6.35 ± 0.13 a | 5.96 ± 0.32 ad | 4.92 ± 0.13 b | 4.39 ± 0.27 c | 5.62 ± 0.13 ad |
| Volatile acids (as acetic acid), g/L | 0.60 ± 0.06 a | 0.59 ± 0.05 a | 0.42 ± 0.03 b | 0.62 ± 0.04 a | 0.48 ± 0.02 c |
| Reducing sugar g/L | 2.03 ± 0.08 a | 3.81 ± 0.31 b | 2.38 ± 0.18 c | 1.35 ± 0.10 d | 1.79 ± 0.18 e |
| Free SO2, mg/L | 37 ± 2.10 a | 69 ± 1.90 b | 12 ± 1.10 c | 35 ± 1.80 ad | 33 ± 1.40 ad |
| Total SO2, mg/L | 87 ± 2.80 a | 105 ± 4.20 b | 71 ± 2.30 c | 98 ± 2.80 d | 102 ± 3.50 d |
| pH | 3.19 ± 0.02 a | 3.31 ± 0.05 b | 3.65 ± 0.04 c | 3.69 ± 0.09 c | 3.62 ± 0.11 c |
| Parameter | Aroma Descriptor | ODT, mg/L | Pure Fermentation | Sequential Fermentation | |||
|---|---|---|---|---|---|---|---|
| Candida famata WB-1 | Hanseniaspora uvarum S-2 | Saccharomyces cerevisiae QA23 | Candida famata WB-1 | Hanseniaspora uvarum S-2 | |||
| Higher alcohols | |||||||
| Isobutanol | Wine, solvent, bitter | 40 * | nd | nd | 0.11 ± 0.00 | nd | nd |
| 3-Methyl-1-butanol | Whiskey, malt, burnt | 30 * | 10.72 ± 0.35 a | 10.23 ± 0.14 a | 16.51 ± 1.21 b | 11.86 ± 0.23 c | 9.52 ± 0.31 d |
| 2-Methyl-1-butanol | Malt | 30 * | 3.83 ± 0.01 a | 4.14 ± 0.09 b | tr | 4.07 ± 0.02 b | 4.57 ± 0.03 c |
| 1-Pentanol | Bitter, almond, balsamic | 64 * | 30.14 ± 0.63 a | 38.09 ± 1.31 b | 21.31 ± 0.64 c | 47.74 ± 1.25 d | 35.91 ± 0.92 e |
| 4-Methyl-1-pentanol | Almond, toasted | 50 * | nd | nd | 0.01 ± 0.00 | nd | nd |
| 2,3-Butanediol | Butter, creamy | 668 * | 7.73 ± 0.01 a | nd | nd | 8.81 ± 0.08 b | nd |
| Phenylethyl alcohol | honey, spice, rose, lilac | 14 * | tr | 16.26 ± 0.60 a | tr | tr | 22.52 ± 0.71 b |
| Total higher alcohols | 52.42 ± 1.01 | 68.72 ± 2.14 | 37.94 ±1.85 | 72.48 ± 1.58 | 72.52 ± 1.97 | ||
| Acids | |||||||
| Hexanoic acid | Cheese, oily | 0.42 ** | tr | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.018 ± 0.02 a | tr |
| Octanoic acid | Sweet, cheese | 0.5 ** | 2.97 ± 0.00 a | 1.50 ± 0.09 b | 0.44 ± 0.00 c | 0.90 ± 0.01 d | 1.77 ± 0.04 e |
| Decanoic acid | Rancid, fat | 1 ** | tr | 0.01 ± 0.00 | nd | nd | tr |
| Oleic acid | Fat | 0.5 * | nd | 1.33 ± 0.02 a | 2.28 ± 0.12 b | nd | 2.19 ± 0.09 b |
| Total acids | 2.97 ± 0.00 | 2.86 ± 0.09 | 2.74 ± 0.12 | 0.92 ± 0.03 | 1.77 ± 0.13 | ||
| Esters | |||||||
| Ethyl lactate | Fruit, butter | 154 * | nd | nd | 0.01 ± 0.00 | nd | nd |
| Isopentyl acetate | Banana | 30 * | nd | nd | 1.72 ± 0.17 | nd | nd |
| Ethyl hexanoate | Apple peel, fruit | 0.014 * | 0.79 ± 0.00 a | 0.47 ± 0.04 b | 0.17 ± 0.00 c | 0.88 ± 0.04 d | 0.80 ± 0.02 a |
| Ethyl butanoate | Pineapple, apple, peach | 20 * | tr | nd | 0.01 ± 0.00 | tr | tr |
| 3-Methyl butyl acetate | Banana | 0.03 * | 0.04 ± 0.00 a | 0.03 ± 0.00 b | Nd | 0.06 ± 0.00 c | 0.04 ± 0.00 a |
| 2-Methyl butyl acetate | Fruity, fatty, pleasant | 0.05 ** | 0.83 ± 0.04 a | 0.36 ± 0.03 b | Nd | 0.33 ± 0.01 b | 0.22 ± 0.01 c |
| Hexyl acetate | Fruit, herb | 0.67 * | 2.78 ± 0.12 a | tr | Tr | 2.41 ± 0.04 b | tr |
| Ethyl levulinate | - | nd | 0.01 ± 0.00 a | Nd | nd | 0.01 ± 0.00 a | |
| Ethyl 3-furoate | - | nd | nd | 0.05 ± 0.00 | nd | nd | |
| Methyl heptanoate | - | nd | nd | nd | nd | 0.01 ± 0.00 | |
| Methyl octanoate | Orange | 0.2 * | tr | 0.64 ± 0.01 a | tr | tr | 0.99 ± 0.04 b |
| Diethyl succinate | Wine, fruit | 200 * | tr | 0.72 ± 0.05 a | 0.87 ± 0.04 b | 0.83 ± 0.05 b | 0.77 ± 0.02 a |
| Ethyl octanoate | Fruit, fat | 0.58 * | 2.25 ± 0.12 a | 1.52 ± 0.09 b | 1.43 ± 0.02 b | 2.77 ± 0.11 c | 1.96 ± 0.06 d |
| Isoamyl hexanoate | - | 0.60 ± 0.01 a | 0.40 ± 0.00 b | tr | tr | tr | |
| Phenylethylacetate | Rose, honey, tobacco | 0.25 ** | 0.97 ± 0.04 a | 0.87 ± 0.02 b | 0.55 ± 0.00 c | 0.45 ± 0.01 d | 0.35 ± 0.00 e |
| Ethyl nonanoate | tr | 0.48 ± 0.02 | nd | nd | tr | ||
| Methyl decanoate | Wine | 0.05 † | 0.05 ± 0.00 a | tr | tr | tr | 0.11 ± 0.00 b |
| Ethyl 9-decenoate | Green, fruity, fatty | 0.1 ** | tr | 0.24 ± 0.01 a | 0.05 ± 0.00 b | 0.06 ± 0.01 b | 0.05 ± 0.00 b |
| Ethyl decanoate | Grape | 0.2 ** | 1.79 ± 0.06 a | 1.83 ± 0.03 ab | 1.46 ± 0.11 c | 1.82 ± 0.10 ab | 1.89 ± 0.03 b |
| Isoamyl octanoate | - | 0.125 ** | 0.12 ± 0.01 a | 0.09 ± 0.00 b | 0.06 ± 0.00 c | 0.17 ± 0.01 d | 0.14 ± 0.01 a |
| Ethyl dodecanoate | Sweet, floral, soapy | 0.8 ** | 0.44 ± 0.02 a | 0.38 ± 0.02 b | 0.08 ± 0.00 c | 0.17 ± 0.00 d | 0.35 ± 0.02 b |
| Ethyl myristate | Sweet fruity, fatty, butter | 2 ** | tr | tr | 0.01 ± 0.00 | tr | tr |
| Ethyl palmitate | Wax, fatty | 2 ** | tr | 0.07 ± 0.00 a | tr | tr | 0.09 ± 0.00 b |
| Ethyl 9-octadecanoate | - | nd | 2.16 ± 0.09 a | nd | 0.91 ± 0.01 b | tr | |
| Total esters | 10.61 ± 0.42 | 12.00 ± 0.41 | 4.74 ± 0.34 | 10.86 ± 0.39 | 7.78 ± 0.61 | ||
| Terpenes | |||||||
| α-Terpinene | Lemon | 0.25 ‡ | tr | 0.01 ± 0.00 | tr | tr | tr |
| p-Cymene | Solvent, minty, citrus | 0.011 ‡ | 0.034 ± 0.00 a | 0.043 ± 0.01 b | 0.06 ± 0.00 c | 0.06 ± 0.00 c | 0.02 ± 0.00 d |
| Limonene | Lemon, orange | 0.015 ** | tr | 0.01 ± 0.00 | tr | tr | nd |
| (Z)-β-Ocimene | Citrus, herb, flower | 0.034 # | 0.055 ± 0.00 a | tr | 0.083 ± 0.00 b | 0.12 ± 0.01 c | tr |
| (E)-β-Ocimene | Sweet, herb | 0.034 # | 0.11 ± 0.01 a | tr | 0.072 ± 0.00 b | 0.167 ± 0.03 c | tr |
| p-Mentha-3,8-diene | - | tr | tr | 0.07 ± 0.00 | |||
| γ-Terpinene | Woody, citrus | 0.26 ¥ | 0.75 ± 0.03 a | 0.71 ± 0.02 a | nd | 0.45 ± 0.02 b | 0.48 ± 0.01 b |
| Terpinolene | Piney | 0.041 ¥ | 0.036 ± 0.00 a | 0.012 ± 0.00 b | 0.038 ± 0.00 a | 0.046 ± 0.00 c | 0.094 ± 0.00 d |
| Linalool | Flower, lavender | 0.025 * | tr | 0.12 ± 0.00 a | nd | tr | 0.08 ± 0.00 b |
| 1,3,8-p-Menthatriene | Turpentine | tr | nd | nd | 0.70 ± 0.04 | nd | |
| allo-Ocimene | - | 0.042 ± 0.00 a | nd | 0.047 ± 0.00 b | 0.167 ± 0.02 c | tr | |
| Nerol | Rose, flower | 0.3 * | tr | nd | 0.01 ± 0.00 | tr | nd |
| Citronellol | Sweet, citrus-like | 0.10 ** | tr | nd | nd | 0.01 ± 0.00 | nd |
| Total terpenes | 1.027 ± 0.04 | 0.905 ± 0.03 | 0.310 ± 0.00 | 1.79 ± 0.12 | 0.674 ± 0.01 | ||
| C13-norisoprenoid | |||||||
| β-Ionone | Balsamic, rose, violet | 7 × 10−6 ¥ | 0.0004 ± 0.00 a | tr | 0.0003 ± 0.00 b | 0.0008 ± 0.00 c | tr |
| Other | |||||||
| Dimethyl sulfate | - | 0.536 ± 0.14 | |||||
| Diethyl sulfate | nd | nd | 0.01 ± 0.00 | tr | nd | ||
| Benzaldehyde | Almond | 0.35 * | 0.07 ± 0.00 a | 0.05 ± 0.00 b | 0.15 ± 0.01 c | 0.07 ± 0.00 a | 0.06 ± 0.00 d |
| Levoglucosenone | - | nd | 0.01 ± 0.00 | tr | nd | tr | |
| 4-Vinylguaiacol | Spices, curry | 0.04 ‡ | 0.01 ± 0.00 | nd | nd | nd | nd |
| Compound | Pure Fermentation | Sequential Fermentation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Candida famata WB-1 | Hanseniaspora uvarum S-2 | Saccharomyces cerevisiae QA23 | Candida famata WB-1 | Hanseniaspora uvarum S-2 | ||||||
| OAV | ROC, % | OAV | ROC, % | OAV | ROC, % | OAV | ROC, % | OAV | ROC, % | |
| Higher alcohol | ||||||||||
| Phenylethyl alcohol | 0.00 | 0.00 | 1.16 | 1.40 | 0.00 | 0.00 | 0.00 | 0.00 | 1.61 | 1.59 |
| Acids | ||||||||||
| Octanoic acid | 5.94 | 4.97 | 3.00 | 3.62 | 0.88 | 1.92 | 1.80 | 1.47 | 3.54 | 3.49 |
| Oleic acid | 0.00 | 0.00 | 2.66 | 3.21 | 4.56 | 9.97 | 0.00 | 0.00 | 4.38 | 4.32 |
| Esters | ||||||||||
| Ethyl hexanoate | 56.43 | 47.20 | 33.57 | 40.49 | 12.14 | 26.54 | 62.86 | 51.24 | 57.14 | 56.35 |
| 3-Methyl butyl acetate | 1.33 | 1.12 | 1.00 | 1.21 | 0.00 | 0.00 | 2.00 | 1.63 | 1.33 | 1.31 |
| 2-Methyl butyl acetate | 16.60 | 13.89 | 7.20 | 8.68 | 0.00 | 0.00 | 6.60 | 5.38 | 2.40 | 2.37 |
| Hexyl acetate | 4.15 | 3.47 | 0.00 | 0.00 | 0.00 | 0.00 | 3.60 | 2.93 | 0.00 | 0.00 |
| Methyl octanoate | 0.00 | 0.00 | 3.20 | 3.86 | 0.00 | 0.00 | 0.00 | 0.00 | 4.95 | 4.88 |
| Ethyl octanoate | 3.88 | 3.25 | 2.62 | 3.16 | 2.47 | 5.39 | 4.78 | 3.89 | 3.38 | 3.33 |
| Phenylethylacetate | 3.88 | 3.25 | 3.48 | 4.20 | 2.20 | 4.81 | 1.80 | 1.47 | 0.60 | 0.59 |
| Methyl decanoate | 0.00 | 0.00 | 1.02 | 1.23 | 0.00 | 0.00 | 0.00 | 0.00 | 2.20 | 2.17 |
| Ethyl 9-decenoate | 0.00 | 0.00 | 2.40 | 2.89 | 0.50 | 1.09 | 0.60 | 0.49 | 0.50 | 0.49 |
| Ethyl decanoate | 8.95 | 7.49 | 9.15 | 11.04 | 7.30 | 15.95 | 9.10 | 7.42 | 9.10 | 8.97 |
| Isoamyl octanoate | 0.96 | 0.80 | 0.72 | 0.87 | 0.48 | 1.05 | 1.36 | 1.11 | 1.12 | 1.10 |
| Terpenes | ||||||||||
| p-Cymene | 3.09 | 2.59 | 3.91 | 4.71 | 5.45 | 11.92 | 5.45 | 4.45 | 1.82 | 1.79 |
| (Z)-β-Ocimene | 1.62 | 1.35 | 0.00 | 0.00 | 2.44 | 5.34 | 3.53 | 2.88 | 0.00 | 0.00 |
| (E)-β-Ocimene | 3.24 | 2.71 | 0.00 | 0.00 | 2.12 | 4.63 | 4.91 | 4.00 | 0.00 | 0.00 |
| γ-Terpinene | 2.88 | 2.41 | 2.73 | 3.29 | 0.00 | 0.00 | 1.73 | 1.41 | 1.85 | 1.82 |
| Terpinolene | 0.88 | 0.73 | 0.29 | 0.35 | 0.93 | 2.03 | 1.12 | 0.91 | 2.29 | 2.26 |
| Linalool | 0.00 | 0.00 | 4.80 | 5.79 | 0.00 | 0.00 | 0.00 | 0.00 | 3.20 | 3.16 |
| C13-norisoprenoid | ||||||||||
| β-Ionone | 5.71 | 4.78 | 0.00 | 0.00 | 4.29 | 9.37 | 11.43 | 9.32 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabegović, I.; Malićanin, M.; Popović, N.; Stamenković Stojanović, S.; Lazić, M.; Stanojević, J.; Danilović, B. Native Non-Saccharomyces Yeasts as a Tool to Produce Distinctive and Diverse Tamjanika Grape Wines. Foods 2022, 11, 1935. https://doi.org/10.3390/foods11131935
Karabegović I, Malićanin M, Popović N, Stamenković Stojanović S, Lazić M, Stanojević J, Danilović B. Native Non-Saccharomyces Yeasts as a Tool to Produce Distinctive and Diverse Tamjanika Grape Wines. Foods. 2022; 11(13):1935. https://doi.org/10.3390/foods11131935
Chicago/Turabian StyleKarabegović, Ivana, Marko Malićanin, Nikola Popović, Sandra Stamenković Stojanović, Miodrag Lazić, Jelena Stanojević, and Bojana Danilović. 2022. "Native Non-Saccharomyces Yeasts as a Tool to Produce Distinctive and Diverse Tamjanika Grape Wines" Foods 11, no. 13: 1935. https://doi.org/10.3390/foods11131935
APA StyleKarabegović, I., Malićanin, M., Popović, N., Stamenković Stojanović, S., Lazić, M., Stanojević, J., & Danilović, B. (2022). Native Non-Saccharomyces Yeasts as a Tool to Produce Distinctive and Diverse Tamjanika Grape Wines. Foods, 11(13), 1935. https://doi.org/10.3390/foods11131935








