Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Cell Culture and Cell Viability
2.4. Animal and Experimental Design
2.5. Measurements of Nitrite (NO) Production
2.6. Immunoblotting Analysis
2.7. Measurements of Cytokines Production
2.8. Measurements of NK Cell Cytotoxicity
2.9. Isolation of Peripheral Blood Mononuclear Cells, Mesenteric Lymph Node Cells, Peritoneal Exudate Cells, and Peyer’s Patch Cells
2.10. Flow Cytometry Analysis
2.11. Statistical Analysis
3. Results
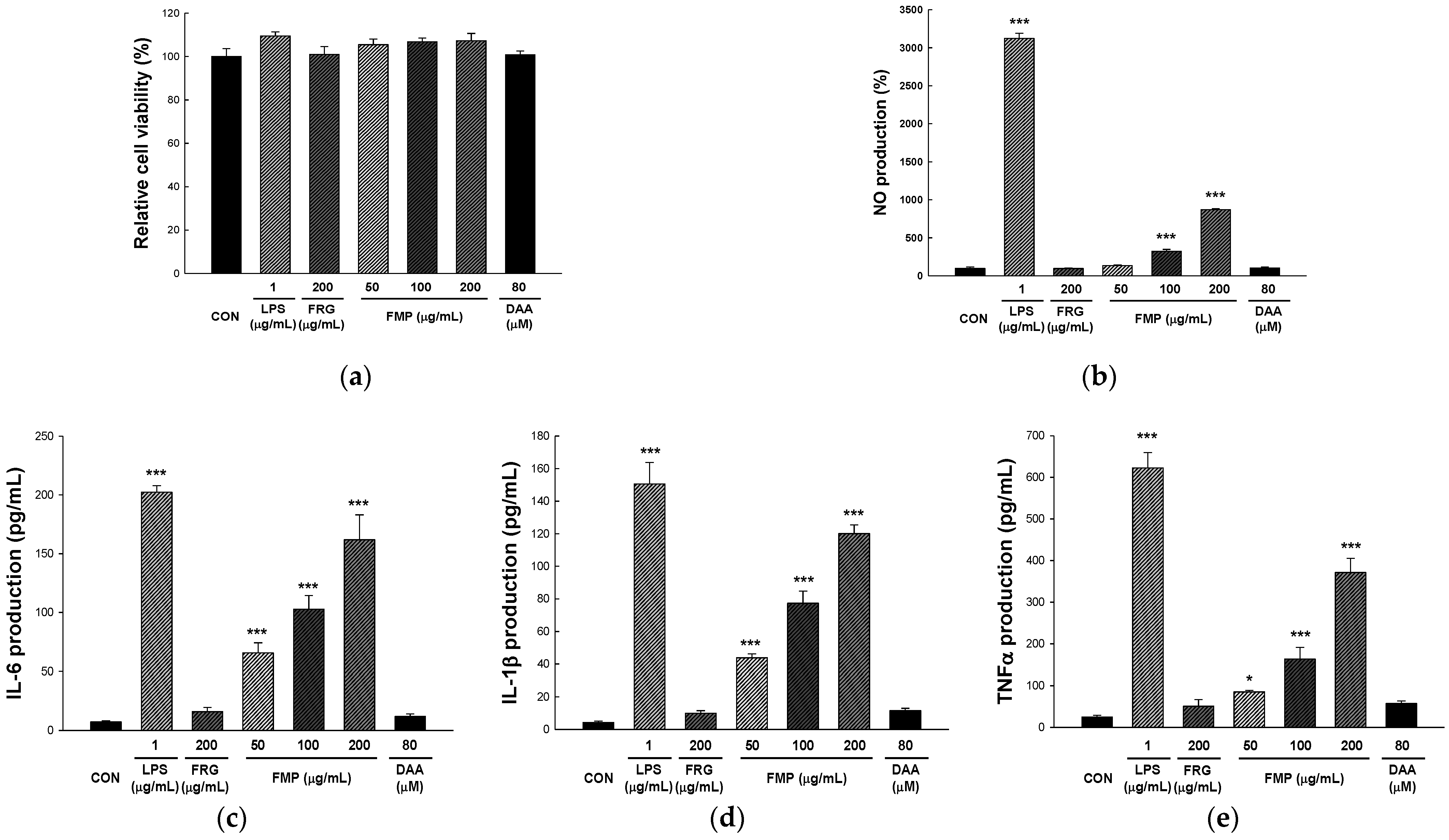
3.1. Effect of FMP on RAW 264.7 Cell Viability
3.2. Effect of FMP on NO Production in RAW 264.7 Cells
3.3. Effect of FMP on Cytokine Production in RAW 264.7 Cells
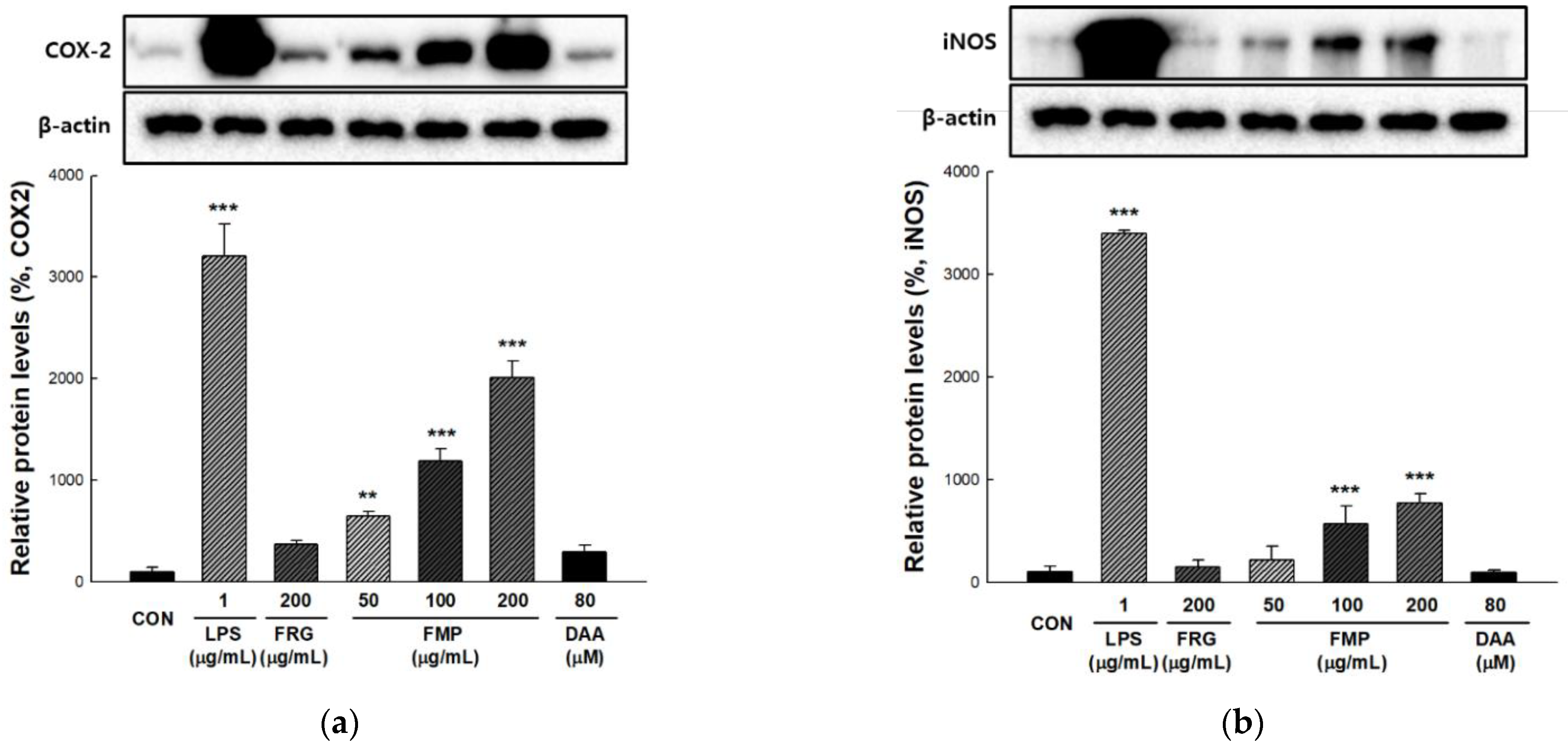
3.4. Effect of FMP on COX-2 and iNOS Protein Expression in RAW 264.7 Cells
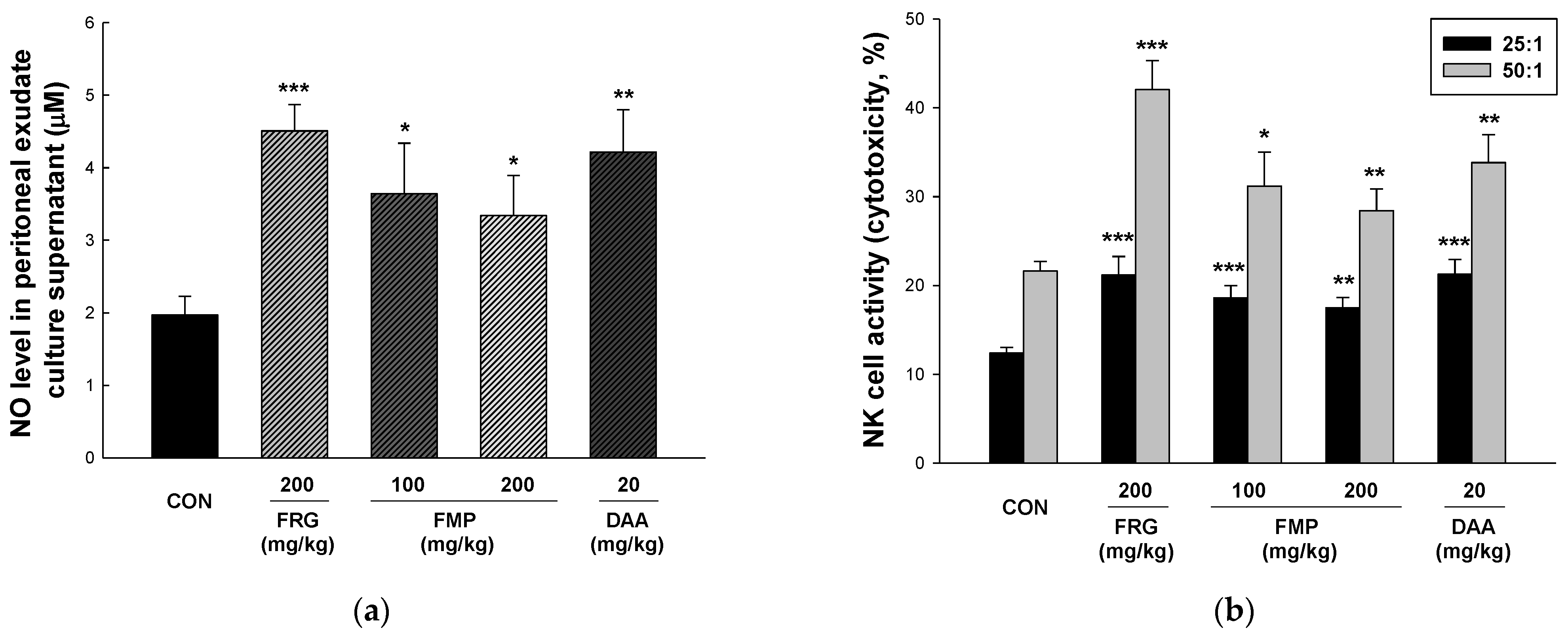
3.5. Effect of FMP on NO Production in Peritoneal Exudate Cells
3.6. Effect of FMP on NK Cytotoxicity Activity
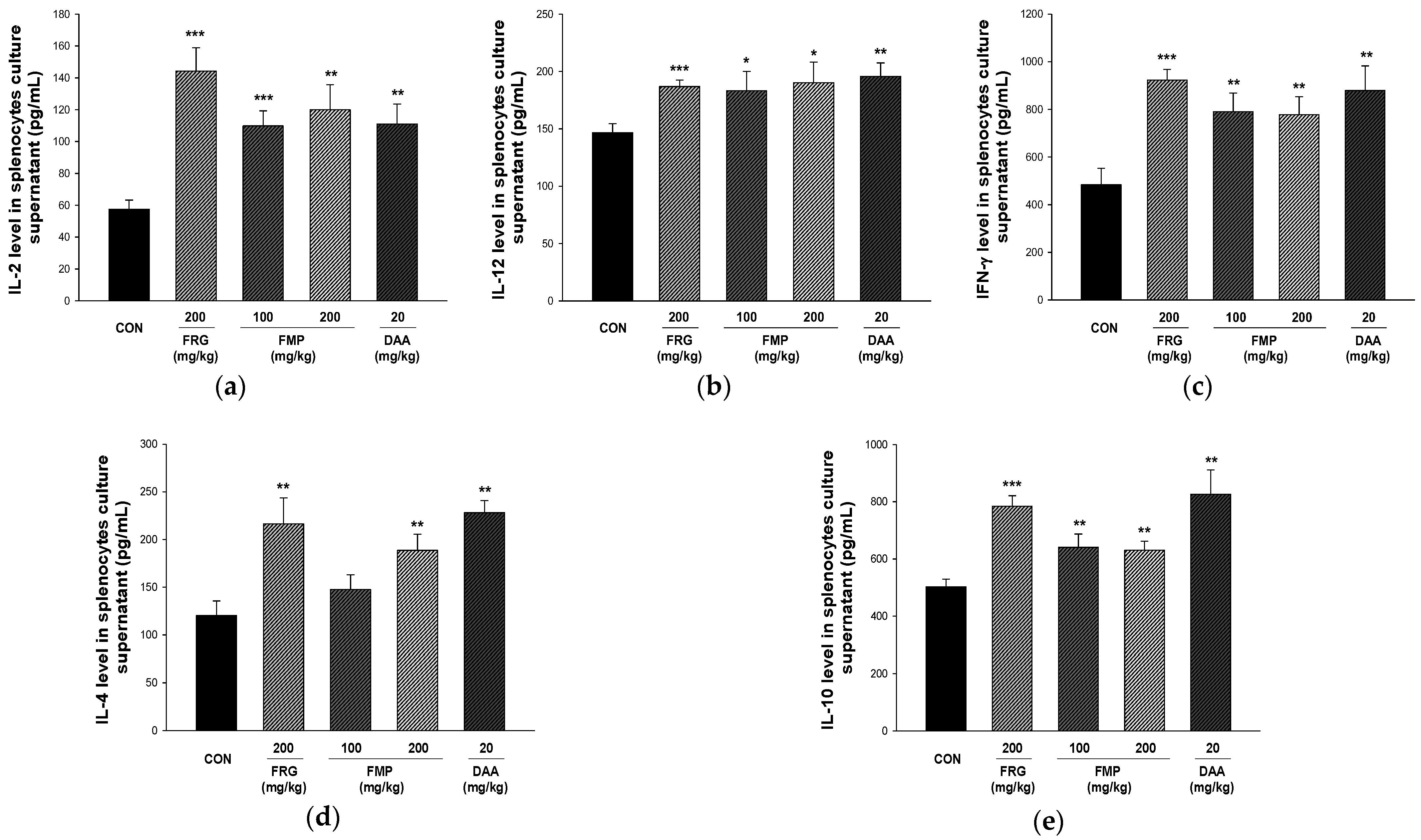
3.7. Effect of FMP on Cytokine Production in Cultured Splenocytes
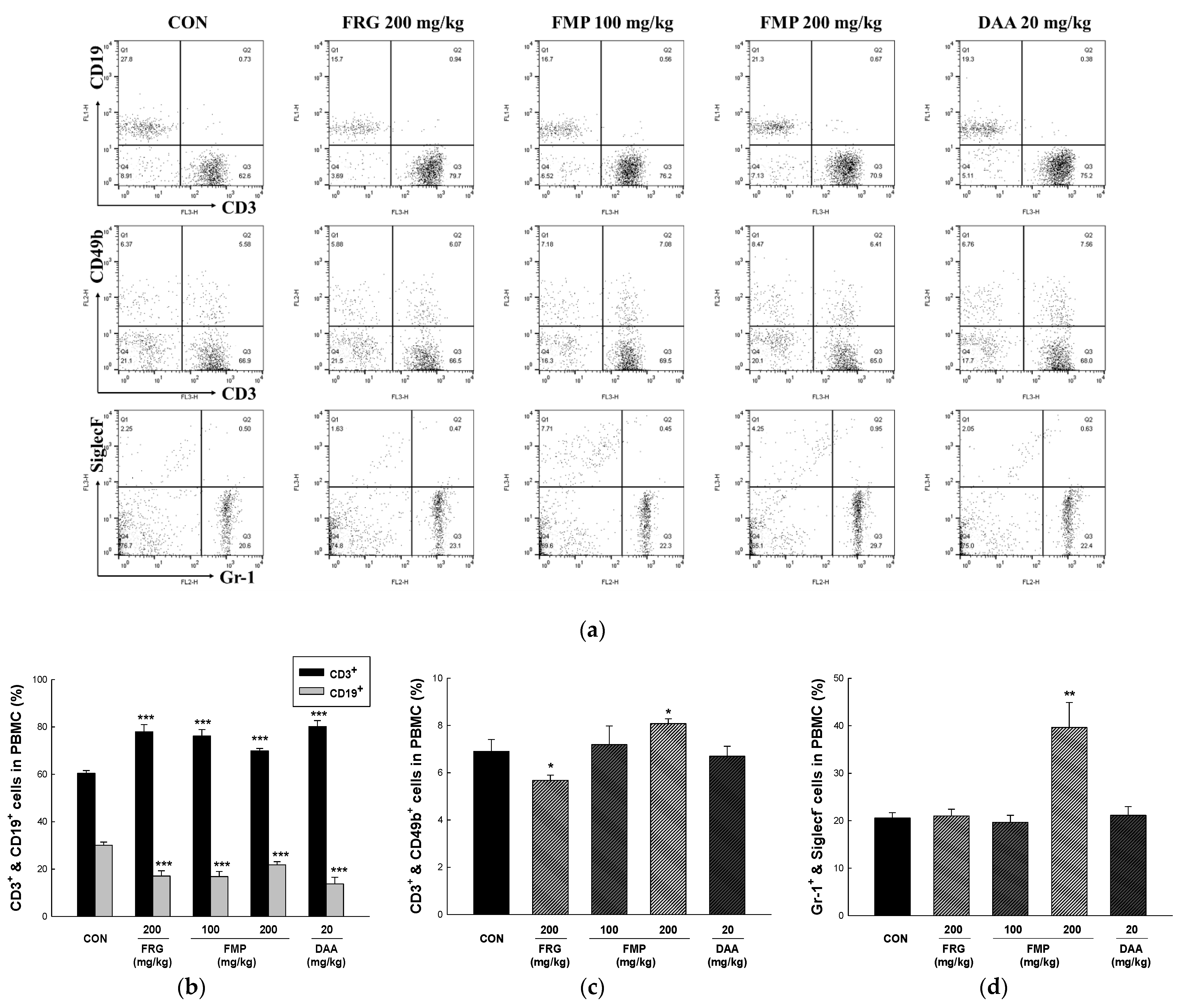
3.8. Effects of FMP on Absolute Number of Immune Cell Subtypes in Spleen, Mesenteric Lymph Nodes, Peritoneal Exudate Cells and Peyer’s Patch Cells
3.9. Effects of FMP on Populations of Peripheral Blood Mononuclear Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farzana, M.; Shahriar, S.; Jeba, F.R.; Tabassum, T.; Araf, Y.; Ullah, M.; Tasnim, J.; Chakraborty, A.; Naima, T.A.; Marma, K.K.S.; et al. Functional food: Complementary to fight against COVID-19. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Nejati, M.; Dehghan, P.; Hashempour-Baltork, F.; Alizadeh, A.M.; Farshi, P.; Khosravi-Darani, K. Potential dietary interventions for COVID-19 infection based on the Gut-Immune axis: An update review on bioactive component of macronutrients. Int. J. Prev. Med. 2021, 12, 105. [Google Scholar] [PubMed]
- Yang, J.; Gadi, R.; Paulino, R.; Thomson, T. Total phenolics, ascorbic acid, and antioxidant capacity of noni (Morinda citrifolia L.) juice and powder as affected by illumination during storage. Food Chem. 2010, 122, 627–632. [Google Scholar] [CrossRef]
- Chan-Blanco, Y.; Vaillant, F.; Perez, A.M.; Reynes, M.; Brillouet, J.M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Ahmad, A.N.; Daud, Z.A.M.; Ismail, A. Review on potential therapeutic effect of Morinda citrifolia L. Curr. Opin. Food Sci. 2016, 8, 62–67. [Google Scholar] [CrossRef]
- Ali, M.; Kenganora, M.; Manjula, S.N. Health benefits of Morinda citrifolia (Noni): A review. Pharmacogn. J. 2016, 8, 321–334. [Google Scholar] [CrossRef]
- Jayaraman, S.K.; Manoharan, M.S.; Illanchezian, S. Antibacterial, antifungal and tumor cell suppression potential of Morinda citrifolia fruit extracts. Int. J. Integr. Biol. 2008, 3, 44–49. [Google Scholar]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Liu, S.N.; Han, Y.; Zhou, Z.J. Lactic acid bacteria in traditional fermented Chinese foods. Food Res. Int. 2011, 44, 643–651. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, P.; Hu, B.; Xu, L.; Liu, S.; Yu, H.; Guo, Y.; Xie, Y.; Yao, W.; Qian, H. Correlation analysis reveals the intensified fermentation via Lactobacillus plantarum improved the flavor of fermented noni juice. Food Biosci. 2021, 43, 101234. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, R.; Yang, R.; Cai, K.; Li, C.; Li, W. Changesin phenols, polysaccharides and volatile profiles of noni (Morinda citrifolia L.) juice during fermentation. Molecules 2021, 26, 2604. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Kwon, H.Y.; La, I.J.; Jo, Y.H.; Han, X.; Men, X.; Lee, S.J.; Kim, Y.D.; Seong, G.S.; Lee, O.H. Development and validation of an analytical method for deacetylasperulosidic acid, asperulosidic acid, scopolin, asperuloside and scopoletin in fermented Morinda citrifolia L. (Noni). Separations 2021, 8, 80. [Google Scholar] [CrossRef]
- Potterat, O.; Von Felten, R.; Dalsgaard, P.W.; Hamburger, M. Identification of TLC markers and quantification by HPLC-MS of various constituents in noni fruit powder and commercial noni-derived products. J. Agric. Food Chem. 2007, 55, 7489–7494. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, H.J.; Oh, Y.J.; Kim, M.J.; Kim, S.H.; Jeong, T.S.; Baek, N.I. Iridoid glycosides isolated fromOldenlandia diffusa inhibit LDL-oxidation. Arch. Pharm. Res. 2005, 28, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Nishigori, C.; Takeuchi, T.; Oka, S.; Morimoto, K.; Utani, A.; Miyachi, Y. 4-Nitroquinoline 1-oxide forms 8-hydroxydeoxyguanosine in human fibroblasts through reactive oxygen species. Toxicol. Sci. 2006, 91, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.L.; Chen, M.; Su, C.X.; West, B.J. In vivo antioxidant activity of deacetylasperulosidic Acid in noni. J. Anal. Methods Chem. 2013, 2013, 804504. [Google Scholar] [CrossRef]
- Oh, J.S.; Seong, G.S.; Kim, Y.D.; Choung, S.Y. Deacetylasperulosidic acid ameliorates pruritus, immune imbalance, and skin barrier dysfunction in 2, 4-dinitrochlorobenzene-induced atopic dermatitis NC/Nga mice. Int. J. Mol. Sci. 2021, 23, 226. [Google Scholar] [CrossRef]
- Oh, J.S.; Seong, G.S.; Kim, Y.D.; Choung, S.Y. Effects of deacetylasperulosidic acid on atopic dermatitis through modulating immune balance and skin barrier function in HaCaT, HMC-1, and EOL-1 Cells. Molecules 2021, 26, 3298. [Google Scholar] [CrossRef]
- Choi, S.I.; Han, X.; Men, X.; Lee, S.J.; Kim, Y.D.; La, I.J.; Seong, G.S.; Lee, O.H. Enhancement of immune activities of fermented Morinda citrifolia L. (Noni) and six marker compounds. J. Food Hyg. Saf. 2022, 37, 29–37. [Google Scholar] [CrossRef]
- Howell, M.; Shepherd, M. The immune system. Anaesth. Intensive Care Med. 2021, 22, 518–521. [Google Scholar] [CrossRef]
- Moser, M.; Leo, O. Key concepts in immunology. Vaccine 2010, 28, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Neutrophils, eosinophils, mast cells, NK cells. Cell Death Differ. 2019, 26, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Striz, I.; Brabcova, E.; Kolesar, L.; Sekerkova, A. Cytokine networking of innate immunity cells: A potential target of therapy. Clin. Sci. 2014, 126, 593–612. [Google Scholar] [CrossRef]
- Langers, I.; Renoux, V.M.; Thiry, M.; Delvenne, P.; Jacobs, N. Natural killer cells: Role in local tumor growth and metastasis. Biol. Targets Ther. 2012, 6, 73–82. [Google Scholar]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef]
- Getz, G.S. Bridging the innate and adaptive immune systems. J. Lipid Res. 2005, 46, 619–622. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, M.; Yao, X.; Yu, L. Purification, structural characterization, and antioxidant activity of the COP-W1 polysaccharide from Codonopsis tangshen Oliv. Carbohydr. Polym. 2020, 236, 116020. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, J.; Xiao, C.; Harqin, C.; Ma, M.; Long, T.; Li, Z.; Yang, Y.; Liu, J.; Zhao, L. Structural characterization of a low-molecular-weight polysaccharide from Angelica pubescens Maxim. f. biserrata Shan et Yuan root and evaluation of its antioxidant activity. Carbohydr. Polym. 2020, 236, 116047. [Google Scholar]
- Bezerra, I.D.L.; Caillot, A.R.C.; Palhares, L.C.G.F.; Santana-Filho, A.P.; Chavante, S.F.; Sassaki, G.L. Structural characterization of polysaccharides from Cabernet Franc, Cabernet Sauvignon and Sauvignon Blanc wines: Anti-inflammatory activity in LPS stimulated RAW 264.7 cells. Carbohydr. Polym. 2018, 186, 91–99. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, L.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.S.; de Oliveira, D.; Hotza, D. Properties and applications of Morinda citrifolia (noni): A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Cha, H.; Choung, M.; Choi, R.; Choi, D.; Youn, A.R. Antioxidant activities of acidic ethanol extract and the anthocyanin rich fraction from Aronia melanocarpa. Korean J. Food Cook Sci. 2014, 30, 573–578. [Google Scholar] [CrossRef][Green Version]
- Beda, N.; Nedospasov, A. A spectrophotometric assay for nitrate in an excess of nitrite. Nitric Oxide 2005, 13, 93–97. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Ray, A.; Dittel, B.N. Isolation of mouse peritoneal cavity cells. J. Vis. Exp. 2010, 35, e1488. [Google Scholar] [CrossRef]
- Cassado, A.D.A.; D’Império Lima, M.R.; Bortoluci, K.R. Revisiting mouse peritoneal macrophages: Heterogeneity, development, and function. Front. Immunol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Strowig, T.; Brilot, F.; Münz, C. Noncytotoxic functions of NK cells: Direct pathogen restriction and assistance to adaptive immunity. J. Immunol. 2008, 180, 7785–7791. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Th1/th2 cells. Inflamm. Bowel Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Choi, Y.E.; Han, X.; Men, X.; Lee, S.J.; Park, B.W.; Kim, J.J.; Kim, S.H.; Lee, O.H. Fermented Platycodon grandiflorum extract alleviates TNF-α/IFN-γ-induced inflammatory response in HaCaT cells and modulates immune balance on 1-chloro-2, 4-dinitrobenzene-induced atopic dermatitis in NC/Nga mice. J. Funct. Foods 2021, 85, 104617. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Hong, H.D. New technology-immunoregulatory actions of polysaccharides from natural plant resources. Bull. Food Technol. 2011, 24, 390–409. [Google Scholar]
- Dols-Lafargue, M.; Gindreau, E.; Le Marrec, C.; Chambat, G.; Heyraud, A.; Lonvaud-Funel, A. Changes in red wine soluble polysaccharide composition induced by malolactic fermentation. J. Agric. Food Chem. 2007, 55, 9592–9599. [Google Scholar] [CrossRef]
- Mercier, F.E.; Ragu, C.; Scadden, D.T. The bone marrow at the crossroads of blood and immunity. Nat. Rev. Immunol. 2012, 12, 49–60. [Google Scholar] [CrossRef]
- Chakravarti, B.; Abraham, G.N. Aging and T-cell-mediated immunity. Mech. Ageing Dev. 1999, 108, 183–206. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Pahl, M.V.; Crum, A.; Norris, K. Effect of uremia on structure and function of immune system. J. Ren. Nutr. 2012, 22, 149–156. [Google Scholar] [CrossRef]
- Warrington, R.; Watson, W.; Kim, H.L.; Antonetti, F.R. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2011, 7, S1. [Google Scholar] [CrossRef]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.A.; Beltrán, C.J.; Aguirre, I.M.; Silva, P.; Peralta, A.L.; Malinarich, F.; Hermoso, M.A. Toll-like receptors are key participants in innate immune responses. Biol. Res. 2007, 40, 97–112. [Google Scholar] [CrossRef]
- Booth, J.S.; Nichani, A.K.; Benjamin, P.; Dar, A.; Krieg, A.M.; Babiuk, L.A.; Mutwiri, G.K. Innate immune responses induced by classes of CpG oligodeoxynucleotides in ovine lymph node and blood mononuclear cells. Vet. Immunol. Immunopathol. 2007, 115, 24–34. [Google Scholar] [CrossRef]
- Harizi, H. The immunobiology of prostanoid receptor signaling in connecting innate and adaptive immunity. BioMed Res. Int. 2013, 2013, 683405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, H.; Shao, Y.; Liu, J.; Li, J.; Xing, M. Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 2017, 8, 98103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- León, B.; Ardavín, C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol. Cell Biol. 2008, 86, 320–324. [Google Scholar] [CrossRef]
- Shibuya, K.; Robinson, D.; Zonin, F.; Hartley, S.B.; Macatonia, S.E.; Somoza, C.; Hunter, C.A.; Murphy, K.M.; O’Garra, A. IL-1α and TNF-α are required for IL-12-induced development of Th1 cells producing high levels of IFN-γ in BALB/c but not C57BL/6 mice. J. Immunol. 1998, 160, 1708–1716. [Google Scholar]
- Kenny, T.P.; Keen, C.L.; Schmitz, H.H.; Gershwin, M.E. Immune effects of cocoa procyanidin oligomers on peripheral blood mononuclear cells. Exp. Biol. Med. 2007, 232, 293–300. [Google Scholar]
| Cell Phenotypes | CON | FRG 200 mg/kg | FMP 100 mg/kg | FMP 200 mg/kg | DAA 20 mg/kg | |
|---|---|---|---|---|---|---|
| Spleen | Total spleen cells (×104 cells) | 345.33 ± 34.75 | 394.00 ± 22.69 | 440.00 ± 11.67 ** | 456.33 ± 57.59 | 456.33 ± 85.97 |
| CD3+ (×104 cells) | 174.82 ± 26.52 | 215.58 ± 19.08 | 266.53 ± 11.73 ** | 313.72 ± 56.22 * | 267.24 ± 69.10 | |
| CD19+ (×104 cells) | 142.87 ± 19.25 | 145.40 ± 13.64 | 137.25 ± 6.09 | 114.41 ± 21.20 | 140.59 ± 47.50 | |
| CD3+ /CD49b+ (×104 cells) | 13.12 ± 1.04 | 14.92 ± 1.17 | 17.68 ± 1.78 * | 13.74 ± 3.51 | 16.41 ± 3.16 | |
| CD4+ (×104 cells) | 102.16 ± 14.34 | 136.44 ± 13.67 | 149.06 ± 6.35 ** | 179.72 ± 32.57 * | 147.05 ± 39.22 | |
| CD8+ (×104 cells) | 65.62 ± 12.92 | 70.09 ± 9.07 | 83.14 ± 4.98 | 97.15 ± 13.13 | 85.91 ± 25.44 | |
| CD4+/CD25+ (×104 cells) | 13.95 ± 1.71 | 20.51 ± 1.47 * | 22.15 ± 0.87 *** | 27.89 ± 6.87 | 22.87 ± 6.64 | |
| CD49b+/NKG2D+ (×104 cells) | 11.54 ± 1.34 | 17.48 ± 2.19 * | 16.35 ± 1.01 ** | 18.42 ± 2.89 * | 16.89 ± 3.82 | |
| CD23+/B220+ (×104 cells) | 28.83 ± 11.07 | 99.87 ± 11.08 *** | 64.78 ± 5.08 ** | 88.25 ± 16.04 ** | 63.77 ± 15.91 | |
| MLN | Total mesenteric lymph node cells (×104 cells) | 259.33 ± 20.75 | 274.33 ± 23.71 | 435.33 ± 34.44 *** | 401.00 ± 44.54 ** | 291.33 ± 22.63 |
| CD4+ (×104 cells) | 135.75 ± 15.14 | 161.18 ± 14.89 | 226.88 ± 24.11 ** | 221.95 ± 35.36 * | 154.40 ± 19.31 | |
| CD8+ (×104 cells) | 48.64 ± 4.13 | 48.79 ± 8.72 | 83.99 ± 10.02 ** | 81.43 ± 14.45 * | 59.20 ± 8.07 | |
| CD4+/CD25+ (×104 cells) | 14.09 ± 1.50 | 23.93 ± 5.20 | 27.27 ± 2.56 *** | 26.19 ± 3.86 ** | 16.78 ± 2.17 | |
| PEC | Total peritoneal exudate cells (×104 cells) | 163.67 ± 18.97 | 238.33 ± 11.20 ** | 300.33 ± 23.56 *** | 307.33 ± 37.59 ** | 244.00 ± 32.19 * |
| CD3+ (×104 cells) | 19.06 ± 2.30 | 40.56 ± 6.50 ** | 29.65 ± 4.74 * | 25.06 ± 2.39 | 28.06 ± 4.04 | |
| CD19+ (×104 cells) | 92.18 ± 33.63 | 131.74 ± 14.53 | 197.16 ± 22.82 ** | 162.16 ± 26.51 | 145.54 ± 34.46 | |
| CD3+/CD49+ (×104 cells) | 6.07 ± 3.86 | 6.39 ± 0.06 | 5.70 ± 1.24 | 10.95 ± 2.38 | 5.62 ± 0.85 | |
| CD206+/CD11b+ (×104 cells) | 11.03 ± 1.29 | 13.66 ± 2.24 | 18.23 ± 1.29 ** | 22.10 ± 11.42 | 9.57 ± 0.96 | |
| CD11c+/F4/80+ (×104 cells) | 5.73 ± 1.33 | 10.81 ± 1.01 ** | 13.04 ± 1.60 ** | 13.08 ± 1.15 *** | 14.01 ± 3.98 | |
| PP | Total Peyer’s patch cells (×104 cells) | 267.33 ± 37.43 | 288.00 ± 10.26 | 342.67 ± 61.27 | 360.33 ± 21.52 * | 478.67 ± 106.69 |
| CD4+ (×104 cells) | 54.91 ± 8.97 | 68.73 ± 1.24 | 69.53 ± 16.77 | 86.52 ± 7.05 ** | 122.70 ± 40.84 | |
| CD8+ (×104 cells) | 12.61 ± 3.80 | 12.55 ± 0.36 | 14.73 ± 4.09 | 15.50 ± 3.99 | 26.66 ± 10.94 | |
| CD49+/NKG2D+ (×104 cells) | 4.84 ± 1.24 | 3.69 ± 0.24 | 11.87 ± 3.63 | 8.86 ± 0.97 ** | 17.45 ± 6.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-I.; La, I.-J.; Han, X.; Men, X.; Lee, S.-J.; Oh, G.; Kwon, H.-Y.; Kim, Y.-D.; Seong, G.-S.; Kim, S.-H.; et al. Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice. Foods 2022, 11, 1925. https://doi.org/10.3390/foods11131925
Choi S-I, La I-J, Han X, Men X, Lee S-J, Oh G, Kwon H-Y, Kim Y-D, Seong G-S, Kim S-H, et al. Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice. Foods. 2022; 11(13):1925. https://doi.org/10.3390/foods11131925
Chicago/Turabian StyleChoi, Sun-Il, Im-Joung La, Xionggao Han, Xiao Men, Se-Jeong Lee, Geon Oh, Hee-Yeon Kwon, Yong-Deok Kim, Geum-Su Seong, Seung-Hyung Kim, and et al. 2022. "Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice" Foods 11, no. 13: 1925. https://doi.org/10.3390/foods11131925
APA StyleChoi, S.-I., La, I.-J., Han, X., Men, X., Lee, S.-J., Oh, G., Kwon, H.-Y., Kim, Y.-D., Seong, G.-S., Kim, S.-H., & Lee, O.-H. (2022). Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice. Foods, 11(13), 1925. https://doi.org/10.3390/foods11131925