Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacteria Strains
2.2. Reagents and Standards
2.3. Sample Preparation
2.4. Physicochemical Characteristics and Viable Cells Tests
2.4.1. Viable Cell Counts
2.4.2. pH, Total Acids (TA) and Soluble Solid Content (SSC)
2.4.3. UPLC Determination of Soluble Sugars and Organic Acids
2.4.4. UPLC Determination of Vitamin C (Vc) and Flavones
2.4.5. UPLC-MS-MS Determination of Limonin
2.5. HS-SPME-GC-MS Determination of Volatile Compounds
2.6. Antioxidant Activities Assay
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS Assay
2.6.3. FRAP Test
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Detection and Analysis on Physicochemical Characteristics of Fermented Orange Juices and Viable Count
3.1.1. Viable Count Detection
3.1.2. Variations in Soluble Solid Content (SSC) and Soluble Sugars
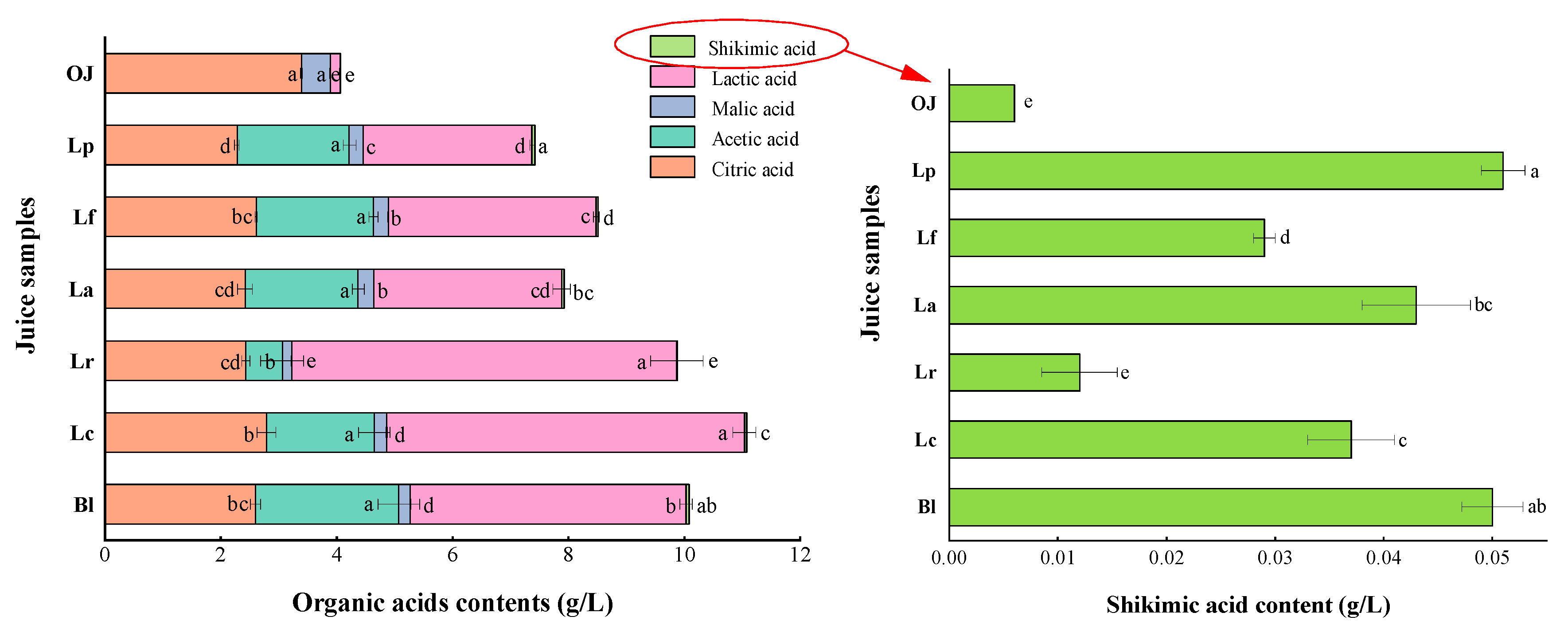
3.1.3. Variations in Total Acid (TA) and Organic Acids
3.1.4. Variations in Vitamin C (Vc), Limonin and Flavones
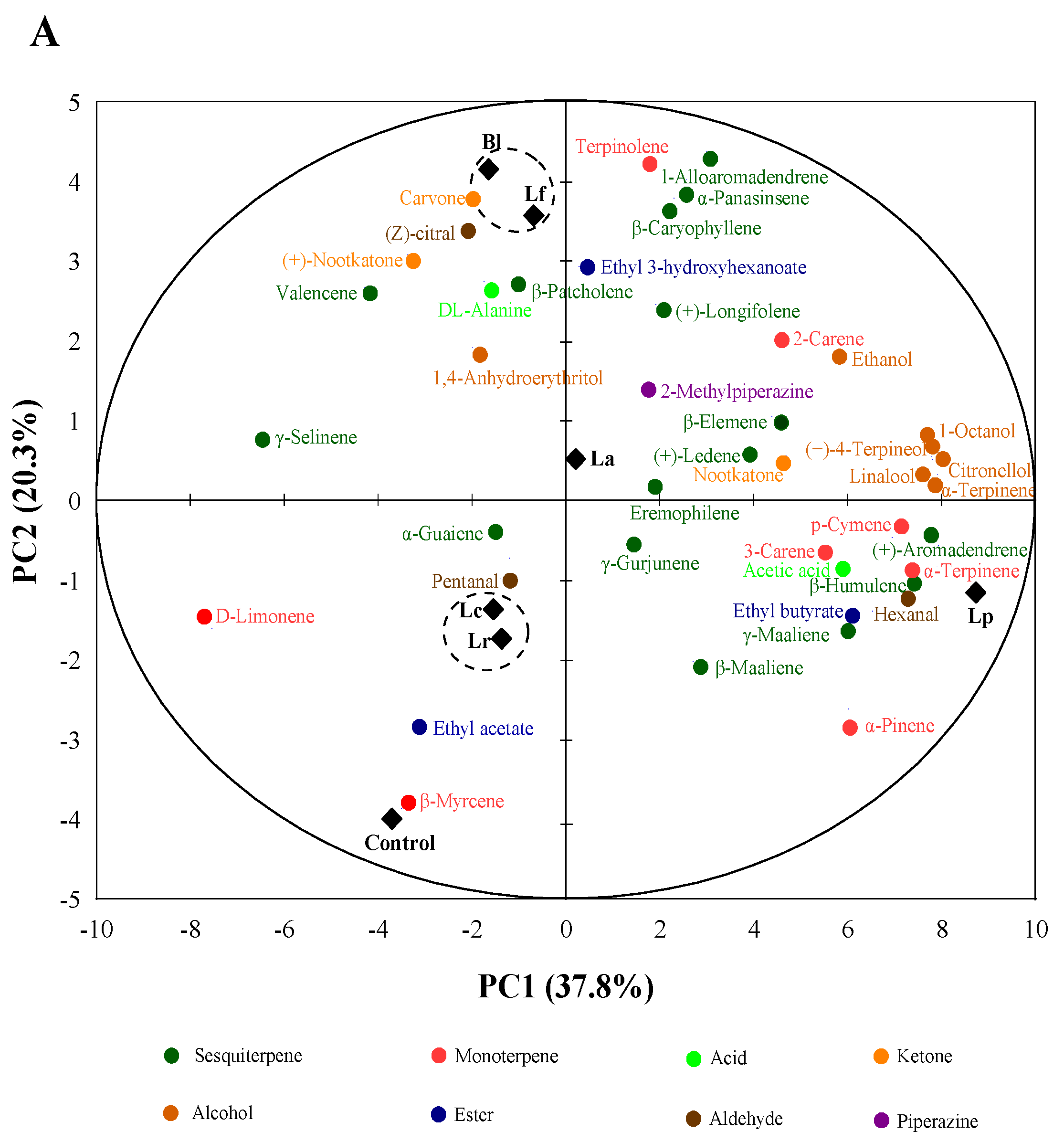
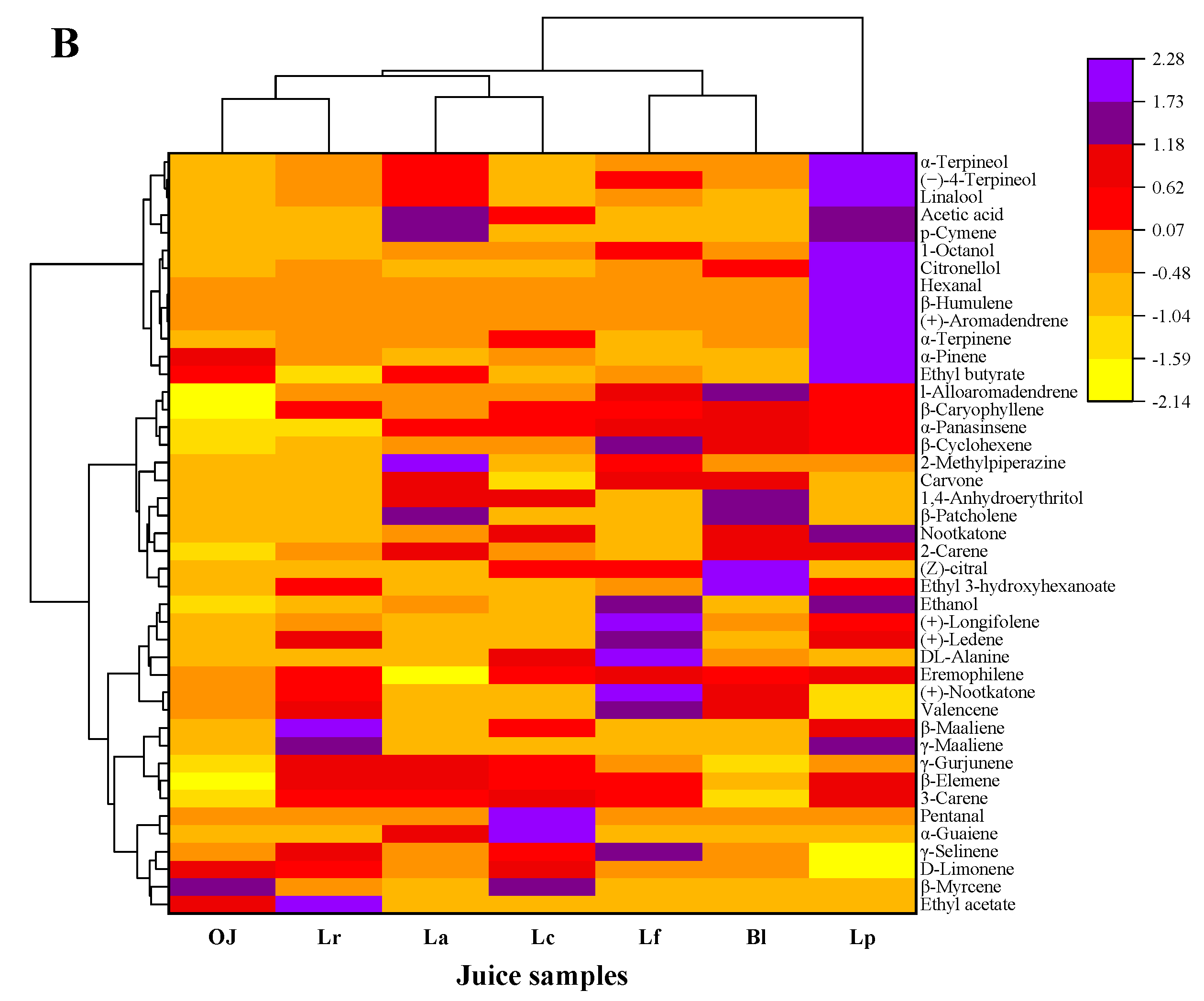
3.2. Detection and Analysis on the Volatile Compounds of Fermented Orange Juice
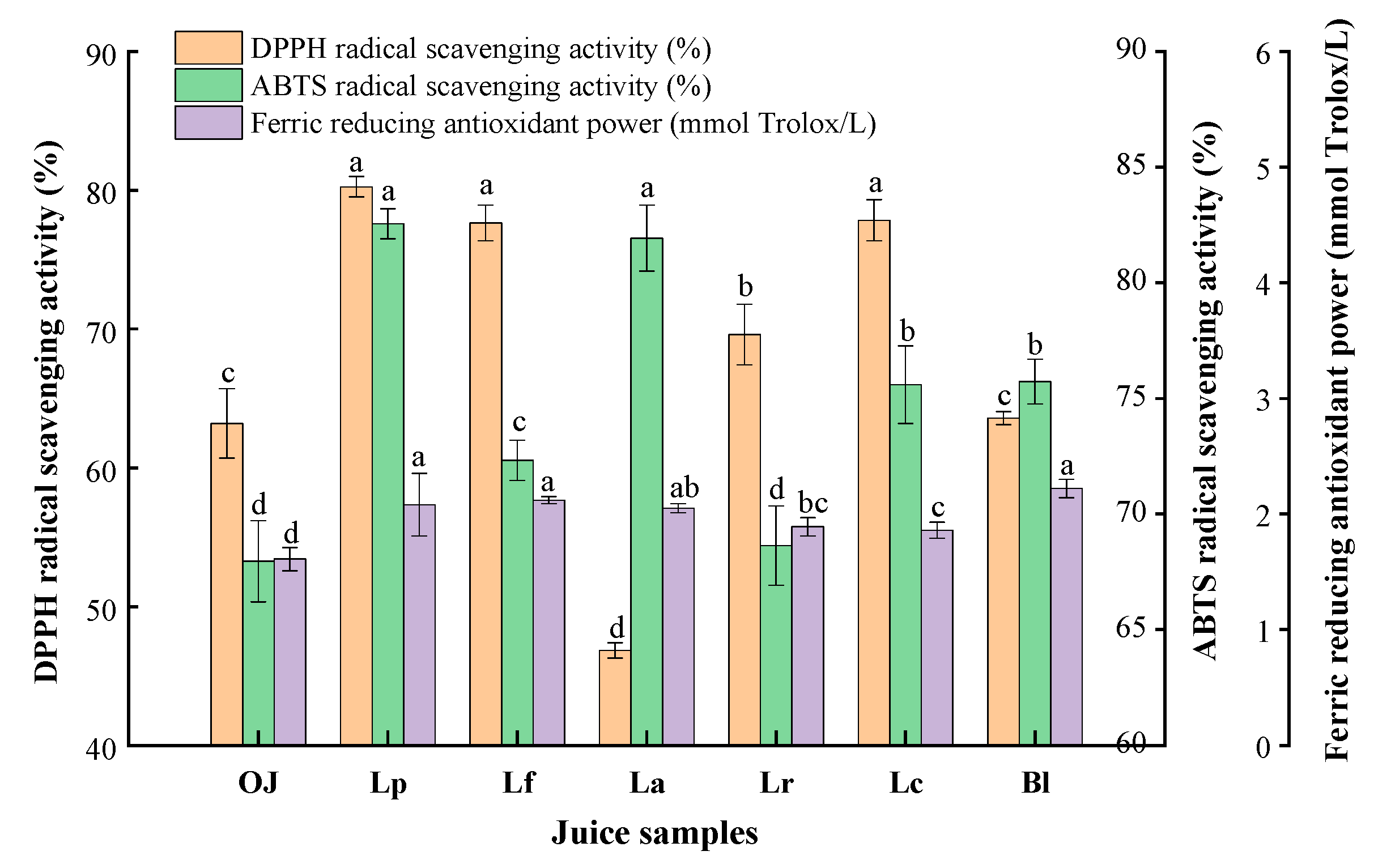
3.3. Detection and Analysis on Antioxidant Activity of the Fermented Orange Juice
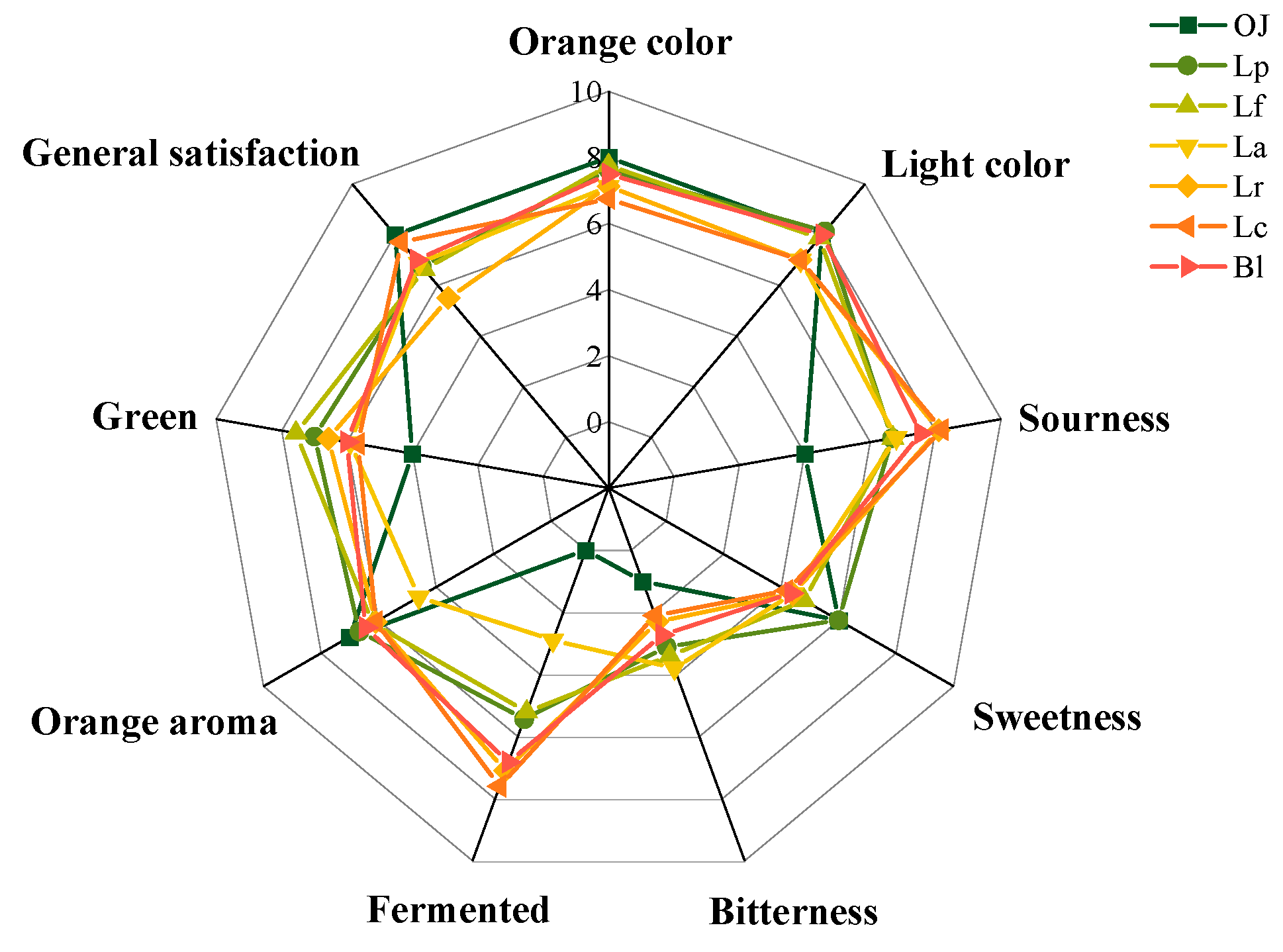
3.4. Analysis on the Sensory Properties of the Fermented Orange Juice
3.4.1. Sensory Evaluation of the Fermented Orange Juice
3.4.2. Analysis on the Aroma-Active Volatile Profiles Influencing Sensory Properties of Fermented Orange Juices
3.4.3. Analysis on the Key Factors Significantly Influencing Sensory Properties of Fermented Orange Juice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuasa, M.; Shimada, A.; Matsuzaki, A.; Eguchi, A.; Tominaga, M. Chemical composition and sensory properties of fermented citrus juice using probiotic lactic acid bacteria. Food Biosci. 2021, 39, 100810. [Google Scholar] [CrossRef]
- Hou, J.X.; Liang, L.; Su, M.Y.; Yang, T.M.; Mao, X.J.; Wang, Y.X. Variations in phenolic acids and antioxidant activity of navel orange at different growth stages. Food Chem. 2021, 360, 129980. [Google Scholar] [CrossRef]
- Guérin, M.; Silva, C.R.D.; Garcia, C.; Remize, F. Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 2020, 6, 115–135. [Google Scholar] [CrossRef]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Alexopoulos, A.; Plessas, S. Microbiological and chemical properties of chokeberry juice fermented by novel lactic acid bacteria with potential probiotic properties during fermentation at 4 ℃ for 4 weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Carafa, I.; Barp, L.; Caruso, M.; Licciardello, C.; Larcher, R.; Tuohy, K.; Martens, S. Effects of Lactobacillus spp. on the phytochemical composition of juices from two varieties of Citrus sinensis L. Osbeck: ‘Tarocco’ and ‘Washington navel’. LWT-Food Sci. Technol. 2020, 125, 109205. [Google Scholar] [CrossRef]
- Marrero, S.C.; Martínez-Rodríguez, A.; Pérez, S.E.M.; Moya, S.P. New trends and applications in fermented beverages. Fermented Beverages 2019, 5, 31–66. [Google Scholar]
- Cui, S.M.; Zhao, N.; Lu, W.W.; Zhao, F.; Zheng, S.Y.; Wang, W.J.; Chen, W. Effect of different Lactobacillus species on volatile and nonvolatile flavor compounds in juices fermentation. Food Sci. Nutri. 2019, 7, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lu, Y.Q.; Yu, H.Y.; Chen, Z.Y.; Tian, H.X. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Devaere, J.; Schouteten, J.J.; Gellynck, X.; de Winne, A.; Matemu, A.O.; Raes, K. Effect of lactic acid fermentation of watermelon juice on its sensory acceptability and volatile compounds. Food Chem. 2021, 358, 129809. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Niamah, A.K. Bacterial Viability, Antioxidant Stability, Antimutagenicity and Sensory Properties of Onion Types Fermentation by Using Probiotic Starter during Storage. Nutri. Food Sci. 2022. Available online: https://www.emerald.com/insight/content/doi/10.1108/NFS-07-2021-0204/full/html (accessed on 4 January 2022).
- de la Fuente, B.; Luz, C.; Puchol, C.; Meca, G.; Barba, F.J. Evaluation of fermentation assisted by Lactobacillus brevis POM, and Lactobacillus plantarum (TR-7, TR-71, TR-14) on antioxidant compounds and organic acids of an orange juice-milk based beverage. Food Chem. 2021, 343, 128414. [Google Scholar] [CrossRef] [PubMed]
- Standards and Specifications of Health Functional Foods. Available online: https://www.mfds.go.kr/files/upload/eng/7_Health%20Functioanl%20Food%20Code.pdf (accessed on 15 June 2004).
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.M.B.; Jafarpour, D.; Jouki, M. Improving bioactive properties of peach juice using Lactobacillus strains fermentation: Antagonistic and anti-adhesion effects, anti-inflammatory and antioxidant properties, and Maillard reaction inhibition. Food Chem. 2021, 365, 130501. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef]
- Wu, C.Y.; Li, T.L.; Qi, J.; Jiang, T.; Xu, H.D.; Lei, H.J. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT-Food Sci. Technol. 2020, 122, 109064. [Google Scholar] [CrossRef]
- Tian, H.X.; Shen, Y.B.; Yu, H.Y.; He, Y.J.; Chen, C. Effects of 4 Probiotic Strains in Coculture with Traditional Starters on the Flavor Profile of Yogurt. J. Food Sci. 2017, 82, 1693–1701. [Google Scholar] [CrossRef]
- Li, H.C.; Huang, J.T.; Wang, Y.Q.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: Chemical composition, antioxidant activity and sensorial properties. LWT-Food Sci. Technol. 2020, 131, 109803. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Sun, Y.J.; Xi, W.P.; Shen, Y.; Qiao, L.P.; Zhong, L.Z.; Ye, X.Q.; Zhou, Z.Q. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014, 145, 674–680. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Barba, F.J.; Nemati, Z.; Shokofti, S.S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Sevindik, O.; Guclu, G.; Agirman, B.; Selli, S.; Kadiroglu, P.; Bordiga, M.; Capanoglu, E.; Kelebek, H. Impacts of selected lactic acid bacteria strains on the aroma and bioactive compositions of fermented gilaburu (Viburnum opulus) juices. Food Chem. 2022, 378, 132079. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.N.; Tai, Y.N.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef]
- Menéndez, R.; Nauffal, D.; Cremades, M.J. Prognostic factors in restoration of pulmonary flow after submassive pulmonary embolism: A multiple regression analysis. Eur. Respir. J. 1998, 11, 560–564. [Google Scholar] [PubMed]
- Wang, J.; Gambetta, J.M.; Jeffery, D.W. Comprehensive study of volatile compounds in two Australian rosé wines: Aroma extract dilution analysis (AEDA) of extracts prepared using solvent-assisted flavor evaporation (SAFE) or headspace solid-phase extraction (HS-SPE). J. Agric. Food Chem. 2016, 64, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: Process optimisation and product stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef]
- Herrero, M.; Garcia, L.A.; Diaz, M. Organic acids in cider with simultaneous inoculation of yeast and malolactic bacteria: Effect of fermentation temperature. J. Inst. Brew. 1999, 105, 229–232. [Google Scholar] [CrossRef]
- Markkinen, N.; Laaksonen, O.; Nahku, R.; Kuldjärv, R.; Yang, B. Impact of lactic acid fermentation on acids, sugars, and phenolic compounds in black chokeberry and sea buckthorn juices. Food Chem. 2019, 286, 204–215. [Google Scholar] [CrossRef]
- Hugenholtz, J. Citrate metabolism in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 165–178. [Google Scholar] [CrossRef]
- Ghosh, S.; Chisti, Y.; Banerjee, U.C. Production of shikimic acid. Biotechnol. Adv. 2012, 30, 1425–1431. [Google Scholar] [CrossRef]
- Wei, M.; Wang, S.Y.; Gu, P.; Ouyang, X.Y.; Liu, S.X.; Li, Y.Q.; Zhang, B.L.; Zhu, B.Q. Comparison of physicochemical indexes, amino acids, phenolic compounds and volatile compounds in bog bilberry juice fermented by Lactobacillus plantarum under different pH conditions. J. Food Sci. Technol. 2018, 55, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Thumthanaruk, B.; Shetty, K. Changes in physicochemical, astringency, volatile compounds and antioxidant activity of fresh and concentrated cashew apple juice fermented with Lactobacillus plantarum. J. Food Sci. Technol. 2018, 55, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.S.; Zhang, Y.; Li, H.T.; Wu, C.H.; El-Seedi, H.R.; Ye, W.K.; Wang, Z.W.; Li, C.B.; Zhang, X.F.; Kai, G.Y. Limonoids from citrus: Chemistry, anti-tumor potential, and other bioactivities. J. Funct. Foods 2020, 75, 104213. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.F.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Gao, X.L.; Feng, T.; Liu, E.; Shan, P.; Zhang, Z.K.; Liao, L.; Ma, H.L. Ougan juice debittering using ultrasound-aided enzymatic hydrolysis: Impacts on aroma and taste. Food Chem. 2021, 345, 128767. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem. J. 2009, 91, 187–192. [Google Scholar] [CrossRef]
- Xu, W.; Song, M.; Chen, C.; Tong, H.; Liang, G.; Gmitter, F.G. Juice volatile composition differences between Valencia orange and its mutant Rohde Red Valencia are associated with carotenoid profile differences. Food Chem. 2018, 245, 223–232. [Google Scholar]
- Ricci, A.; Levante, A.; Cirlini, M.; Calani, L.; Bernini, V.; Del Rio, D.; Galaverna, G.; Neviani, E.; Lazzi, C. The influence of viable cells and cell-free extracts of Lactobacillus casei on volatile compounds and polyphenolic profile of elderberry juice. Front. Microbiol. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Rosenfeld, H.J.; Aaby, K.; Lea, P. Influence of temperature and plant density on sensory quality and volatile terpenoids of carrot (Daucus carota L.) root. J. Sci. Food Agric. 2002, 82, 1384–1390. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Ouyang, X.Y.; Laaksonen, O.; Liu, X.Y.; Shao, Y.; Zhao, H.F.; Zhang, B.L.; Zhu, B.Q. Effect of Lactobacillus acidophilus, Oenococcus oeni, and Lactobacillus brevis on composition of bog bilberry juice. Foods 2019, 8, 430. [Google Scholar] [CrossRef] [Green Version]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int. J. Food Microbiol. 2017, 248, 56–62. [Google Scholar] [CrossRef] [PubMed]
- San, A.T.; Joyce, D.C.; Hofman, P.J.; Macnish, A.J.; Webb, R.I.; Matovic, N.J.; Williams, C.M.; De Voss, J.J.; Wong, S.H.; Smyth, H.E. Stable isotope dilution assay (SIDA) and HS-SPME-GCMS quantification of key aroma volatiles for fruit and sap of Australian mango cultivars. Food Chem. 2017, 221, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellami, I.; Mall, V.; Schieberle, P. Changes in the key odorants and aroma profiles of hamlin and valencia orange juices not from concentrate (NFC) during chilled storage. J. Agric. Food Chem. 2018, 66, 7428–7440. [Google Scholar] [CrossRef]
- van Gemert, L.J. Compilations of Odour Threshold Values in Air, Water and Other Media. Oliemans Punter Parters BV. 2011. Available online: https://www.academia.edu/3443692/Compilations_of_odour_threshold_values_in_air_water_and_other_media_second_enlarged_and_revised_edition (accessed on 31 August 2011).
- Kwaw, E.; Ma, Y.K.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Lucius, A.; Meyer, T.; de Mejia, E.G. Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-Amylase and α-Glucosidase by highbush blueberry (Vaccinium corombosum). J. Agric. Food Chem. 2011, 59, 8923–8930. [Google Scholar] [CrossRef]
- Bai, L.; Maimaitiyiming, R.; Wang, L. Effects of four individual lactic acid bacteria on the physical and chemical and antioxidant properties of Kuqa apple juice during fermentation. J. Food Process Pres. 2021, 45, 2411–2502. [Google Scholar] [CrossRef]
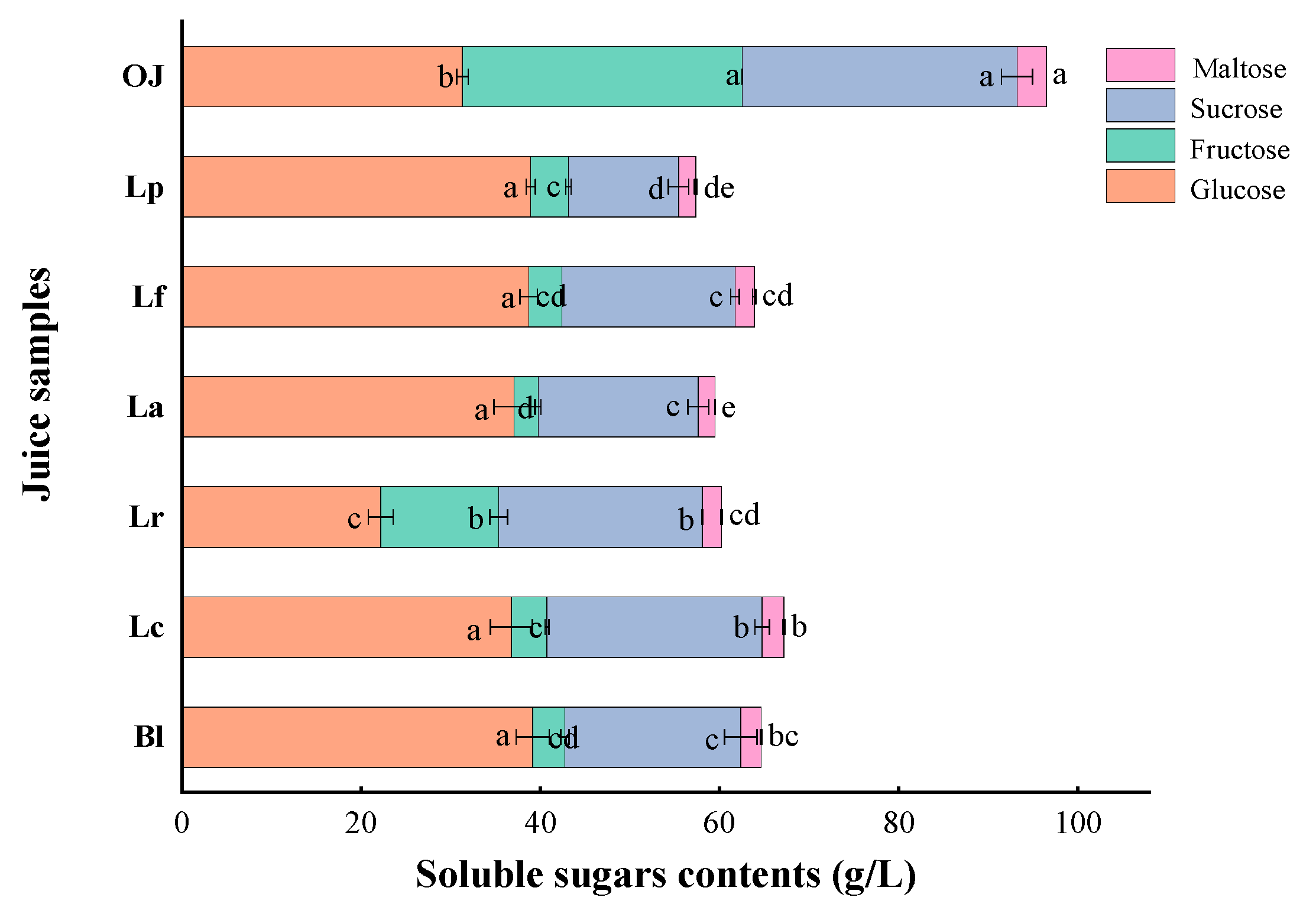

| Index | OJ | Lp | Lf | La | Lr | Lc | Bl |
|---|---|---|---|---|---|---|---|
| Viable counts (log CFU/mL) | - | 7.48 ± 0.24 b | 7.58 ± 0.59 ab | 7.42 ± 0.01 b | 7.71 ± 0.26 ab | 8.07 ± 0.04 a | 7.84 ± 0.32 ab |
| pH | 6.60 ± 0.39 a | 4.82 ± 0.02 b | 4.17 ± 0.02 cd | 4.23 ± 0.12 cd | 3.96 ± 0.12 d | 4.33 ± 0.02 c | 4.48 ± 0.14 c |
| SSC (°Brix) | 11.24 ± 0.25 a | 8.20 ± 0.30 c | 8.85 ± 0.07 b | 8.17 ± 0.31 c | 8.05 ± 0.07 c | 8.20 ± 0.30 c | 8.80 ± 0.26 b |
| TA (%) | 0.69 ± 0.01 c | 0.76 ± 0.03 bc | 0.79 ± 0.03 bc | 0.86 ± 0.03 b | 1.09 ± 0.07 a | 1.23 ± 0.01 a | 1.14 ± 0.00 a |
| SSC/TA ratio | 16.30 ± 0.51 a | 10.75 ± 0.34 b | 11.12 ± 0.00 b | 9.64 ± 1.31 b | 7.69 ± 0.12 c | 6.70 ± 0.86 c | 7.79 ± 0.83 c |
| No. | Compounds | Control | Fermented Orange Juices | |||||
|---|---|---|---|---|---|---|---|---|
| Lp | Lf | La | Lr | Lc | Bl | |||
| Esters | ||||||||
| 1 | Ethyl acetate | 0.32 ± 0.15 a | ND | ND | ND | 0.51 ± 0.00 a | ND | ND |
| 2 | Ethyl butyrate | 1.19 ± 0.51 b | 2.27 ± 0.18 a | 0.77 ± 0.13 bc | 1.17 ± 0.43 b | 0.19 ± 0.04 d | 0.52 ± 0.12 cd | 0.35 ± 0.02 cd |
| 3 | Ethyl 3-hydroxyhexanoate | ND | 0.86 ± 0.27 b | 0.43 ± 0.19 bc | 0.13 ± 0.05 c | 0.94 ± 0.31 b | 0.12 ± 0.00 c | 2.99 ± 0.31 a |
| Monoterpenes | ||||||||
| 4 | α-Pinene | 1.30 ± 0.37 b | 2.31 ± 0.28 a | 0.40 ± 0.07 cd | 0.35 ± 0.05 cd | 0.59 ± 0.08 cd | 0.67 ± 0.13 c | 0.28 ± 0.13 d |
| 5 | β-Myrcene | 8.73 ± 0.04 a | 5.01 ± 1.09 c | 4.69 ± 1.39 c | 5.07 ± 1.49 c | 5.97 ± 1.47 bc | 8.25 ± 0.70 ab | 4.16 ± 1.23 c |
| 6 | D-Limonene | 364.62 ± 6.87 a | 188.27 ± 15.61 d | 281.61 ± 30.12 c | 308.71 ± 9.52 bc | 336.9 ± 24.43 ab | 356.61 ± 28.95 a | 306.88 ± 5.90 bc |
| 7 | 2-Carene | ND | 0.43 ± 0.05 a | 0.09 ± 0.01 c | 0.43 ± 0.07 a | 0.21 ± 0.07 b | 0.17 ± 0.06 bc | 0.47 ± 0.05 a |
| 8 | 3-Carene | ND | 0.95 ± 0.14 a | 0.59 ± 0.08 c | 0.62 ± 0.06 bc | 0.69 ± 0.05 bc | 0.76 ± 0.00 b | ND |
| 9 | Terpinolene | ND | 0.27 ± 0.04 ab | 0.55 ± 0.31 a | 0.19 ± 0.09 ab | 0.13 ± 0.08 b | 0.23 ± 0.14 ab | 0.37 ± 0.29 ab |
| 10 | α-Terpinene | ND | 0.41 ± 0.26 a | ND | 0.05 ± 0.00 b | 0.09 ± 0.07 b | 0.12 ± 0.05 b | 0.07 ± 0.01 b |
| 11 | p-Cymene | ND | 0.94 ± 0.64 a | ND | 0.73 ± 0.12 a | ND | ND | ND |
| Sesquiterpenes | ||||||||
| 12 | Valencene | 82.43 ± 0.34 b | 55.03 ± 2.26 c | 108.91 ± 4.41 a | 62.57 ± 2.06 c | 96.63 ± 4.68 a | 60.27 ± 1.97 c | 107.12 ± 9.41 a |
| 13 | Eremophilene | 1.21 ± 0.02 c | 1.84 ± 0.00 a | 1.77 ± 0.12 ab | ND | 1.70 ± 0.11 ab | 1.49 ± 0.33 abc | 1.47 ± 0.09 bc |
| 14 | γ-Maaliene | ND | 7.46 ± 0.79 a | ND | ND | 5.90 ± 1.30 a | ND | ND |
| 15 | (+)-Ledene | ND | 1.98 ± 0.00 a | 2.31 ± 0.21 a | ND | 2.06 ± 0.19 a | ND | ND |
| 16 | β-Elemene | ND | 0.49 ± 0.04 ab | 0.38 ± 0.03 bc | 0.48 ± 0.04 ab | 0.50 ± 0.06 a | 0.44 ± 0.10 ab | 0.27 ± 0.04 c |
| 17 | β-Caryophyllene | ND | 0.55 ± 0.14 ab | 0.64 ± 0.09 ab | 0.47 ± 0.05 b | 0.56 ± 0.17 ab | 0.54 ± 0.10 ab | 0.76 ± 0.08 a |
| 18 | β-Maaliene | ND | 0.97 ± 0.00 b | ND | ND | 1.67 ± 0.01 a | 0.63 ± 0.27 b | ND |
| 19 | γ-Gurjunene | ND | 0.81 ± 0.13 b | 0.84 ± 0.01 b | 1.48 ± 0.46 a | 1.53 ± 0.00 a | 1.19 ± 0.26 ab | 0.07 ± 0.01 c |
| 20 | γ-Selinene | 5.08 ± 0.01 cd | 0.47 ± 0.00 e | 7.77 ± 0.77 a | 4.59 ± 0.64 cd | 6.83 ± 0.83 ab | 6.03 ± 1.67 bc | 4.05 ± 0.06 d |
| 21 | (+)-Longifolene | ND | 0.51 ± 0.00 b | 1.41 ± 0.51 a | 0.04 ± 0.00 c | 0.31 ± 0.00 bc | ND | 0.15 ± 0.01 c |
| 22 | α-Panasinsanene | ND | 4.76 ± 0.73 ab | 6.24 ± 1.21 ab | 4.66 ± 0.71 ab | ND | 4.52 ± 1.56 b | 6.59 ± 0.56 a |
| 23 | (+)-Aromadendrene | ND | 1.36 ± 0.19 a | 0.10 ± 0.00 b | ND | ND | ND | 0.04 ± 0.00 c |
| 24 | Alloaromadendrene | ND | 1.29 ± 0.00 ab | 1.56 ± 0.28 ab | 1.03 ± 0.15 ab | 0.86 ± 0.84 b | 0.83 ± 0.10 b | 1.76 ± 0.24 a |
| 25 | β-Patchoulene | ND | ND | ND | 5.90 ± 0.21 b | ND | ND | 7.06 ± 0.56 a |
| 26 | β-Humulene | ND | 0.97 ± 0.10 | ND | ND | ND | ND | ND |
| 27 | α-Guaiene | ND | ND | ND | 0.04 ± 0.00 b | ND | 0.07 ± 0.00 a | ND |
| Acids | ||||||||
| 28 | Acetic acid | ND | 4.47 ± 0.60 a | ND | 4.40 ± 0.15 a | ND | 2.63 ± 0.03 b | ND |
| 29 | DL-Alanine | ND | ND | 0.41 ± 0.01 a | ND | ND | 0.23 ± 0.02 b | 0.11 ± 0.01 c |
| Alcohols | ||||||||
| 30 | Linalool | 0.90 ± 0.01 e | 7.27 ± 0.80 a | 3.06 ± 0.59 c | 4.24 ± 0.66 b | 2.41 ± 0.19 cd | 1.32 ± 0.31 e | 1.80 ± 0.08 de |
| 31 | (−)-4-Terpineol | 1.55 ± 0.05 e | 10.19 ± 0.92 a | 4.78 ± 0.15 b | 5.38 ± 0.41 b | 3.41 ± 0.39 c | 2.29 ± 0.15 de | 2.99 ± 0.30 cd |
| 32 | α-Terpineol | 0.15 ± 0.08 b | 2.14 ± 1.16 a | 0.62 ± 0.46 b | 0.73 ± 0.13 b | 0.43 ± 0.36 b | 0.15 ± 0.04 b | 0.50 ± 0.12 b |
| 33 | Citronellol | ND | 0.51 ± 0.00 a | 0.14 ± 0.06 b | 0.05 ± 0.01 c | 0.11 ± 0.02 bc | 0.05 ± 0.00 c | 0.16 ± 0.06 b |
| 34 | Ethanol | ND | 2.90 ± 0.21 a | 3.19 ± 0.74 a | 1.23 ± 0.05 b | 0.59 ± 0.02 c | 0.59 ± 0.04 c | 0.56 ± 0.03 c |
| 35 | 1-Octanol | ND | 0.47 ± 0.00 a | 0.16 ± 0.17 b | 0.09 ± 0.05 b | ND | 0.14 ± 0.00 b | 0.10 ± 0.03 b |
| 36 | 1,4-Anhydroerythritol | ND | ND | ND | 0.78 ± 0.83 a | ND | 0.81 ± 0.33 a | 0.94 ± 0.22 a |
| Ketones | ||||||||
| 37 | Carvone | 0.64 ± 0.05 b | 0.21 ± 0.14 bc | 2.21 ± 0.15 a | 2.34 ± 0.29 a | 0.52 ± 0.21 bc | 0.06 ± 0.02 c | 2.25 ± 0.30 a |
| 38 | Nootkatone | ND | 0.42 ± 0.04 a | ND | 0.11 ± 0.03 c | ND | 0.37 ± 0.00 a | 0.32 ± 0.01 b |
| 39 | (+)-Nootkatone | 0.18 ± 0.02 c | 0.01 ± 0.00 e | 0.58 ± 0.10 a | 0.11 ± 0.04 d | 0.31 ± 0.02 b | 0.03 ± 0.03 de | 0.36 ± 0.00 b |
| Aldehydes | ||||||||
| 40 | Hexanal | ND | 0.33 ± 0.23 | ND | ND | ND | ND | ND |
| 41 | Pentanal | ND | ND | ND | ND | ND | 0.13 ± 0.02 | ND |
| 42 | (Z)-citral | ND | ND | 0.04 ± 0.00 c | ND | ND | 0.05 ± 0.00 b | 0.14 ± 0.02 a |
| Piperazines | ||||||||
| 43 | 2-Methylpiperazine | ND | 0.13 ± 0.04 bc | 0.20 ± 0.07 b | 0.57 ± 0.05 a | ND | 0.02 ± 0.01 d | 0.06 ± 0.01 cd |
| No. | Compound | OT a | OAV b | Odor Quality c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OJ | Lp | Lf | La | Lr | Lc | Bl | ||||
| 1 | Ethyl acetate | 0.1 | ND | ND | ND | ND | 5.1 | ND | ND | Pineapple |
| 2 | Ethyl butyrate | 0.001 | 1190 | 2770 | 770 | 1170 | 190 | 520 | 350 | Apple |
| 3 | Ethyl3-hydroxyhexanoate | 0.01 | ND | 86 | 43 | 13 | 94 | 12 | 299 | Fruity |
| 4 | α-Pinene | 0.033 | 39 | 70 | 12 | 11 | 18 | 20 | 8 | Pine, turpentine |
| 5 | β-Myrcene | 0.05 | 174 | 100 | 94 | 101 | 119 | 165 | 83 | Spice, must, balsamic |
| 6 | D-Limonene | 0.034 | 10,724 | 5537 | 8283 | 9080 | 9909 | 10,489 | 9026 | Citrus, mint |
| 7 | 2-Carene | 0.006 | ND | 72 | 15 | 72 | 35 | 28 | 78 | Turpentine |
| 8 | 3-Carene | 0.005 | ND | 190 | 118 | 124 | 138 | 152 | ND | Lemon, resin, citrus |
| 9 | Terpinolene | 0.2 | ND | 1 | 3 | <1 | <1 | 1 | 2 | Pine, plastic |
| 10 | α-Terpinene | 0.056 | ND | 7 | ND | <1 | 2 | 2 | 1 | Lemon, mint, oil |
| 11 | p-Cymene | 0.0133 | ND | 71 | ND | 55 | ND | ND | ND | Citrus, gasoline |
| 12 | Valencene | 10.5 | 8 | 5 | 10 | 6 | 9 | 6 | 10 | Green, oil, orange-like |
| 13 | β-Caryophyllene | 0.16 | ND | 3 | 4 | 3 | 4 | 3 | 5 | Sweet, woody, lilac |
| 14 | β-Humulene | 0.16 | ND | 6 | ND | ND | ND | ND | ND | Woody, balsamic |
| 15 | γ-Selinene | 0.036 | 141 | 13 | 216 | 128 | 190 | 168 | 113 | Woody, herb |
| 16 | Acetic acid | 0.013 | ND | 344 | ND | 338 | ND | 202 | ND | Sour, pungent |
| 17 | Linalool | 0.0015 | 600 | 4847 | 2040 | 2827 | 1607 | 880 | 1200 | Flower, lavender |
| 18 | (−)-4-Terpineol | 0.4 | 4 | 25 | 12 | 13 | 9 | 6 | 7 | Rose |
| 19 | α-Terpineol | 0.3 | <1 | 7 | 2 | 2 | 1 | <1 | 2 | Pine, terpene, lilac |
| 20 | Citronellol | 0.01 | ND | 51 | 14 | 5 | 11 | 5 | 16 | Rose |
| 21 | Ethanol | 0.01 | ND | 290 | 319 | 123 | 59 | 59 | 56 | Sweet, apple, pungent |
| 22 | 1-Octanol | 0.11 | ND | 4 | 1 | <1 | ND | 1 | <1 | Moss, nut, mushroom |
| 23 | Carvone | 0.086 | 7 | 2 | 26 | 27 | 6 | <1 | 26 | Mint, basil, fennel |
| 24 | Nootkatone | 0.18 | ND | 2 | ND | <1 | ND | 2 | 2 | Grapefruit |
| 25 | (+)-Nootka tone | 0.001 | 180 | 10 | 580 | 110 | 310 | 30 | 360 | Orange-like |
| 26 | Hexanal | 0.005 | ND | 66 | ND | ND | ND | ND | ND | Grass, tallow, fat |
| 27 | Pentanal | 0.012 | ND | ND | ND | ND | ND | 11 | ND | Almond, malt |
| 28 | (Z)-citral | 0.05 | ND | ND | <1 | ND | ND | 1 | 3 | Lemon |
| Model | Factors | Regression Coefficients | t-Value | p-Value | R2 | F-Value | Sig. |
|---|---|---|---|---|---|---|---|
| 1 | ethanol | 5.136 (constant) | 12.560 | 0.000 | 0.667 | 10.006 | 0.025 |
| 0.007 | 3.163 | 0.025 | |||||
| 2 | 4.099 (constant) | 10.570 | 0.000 | 0.913 | 20.875 | 0.008 | |
| ethanol | 0.005 | 3.679 | 0.021 | ||||
| β-caryophyllene | 0.413 | 3.353 | 0.028 |
| Model | Taste Quality (Y) | Factors (X) | Regression Coefficients | t-Value | p-Value | R2 | F-Value | Sig. |
|---|---|---|---|---|---|---|---|---|
| 1 | sweetness | organic acid | 7.268 (constant) | 10.028 | 0.000 | 0.698 | 11.560 | 0.019 |
| −0.284 | −3.400 | 0.019 | ||||||
| 2 | sourness | SSC/TA ratio | 11.183 (constant) | 33.319 | 0.000 | 0.973 | 180.776 | 0.000 |
| −0.432 | −13.445 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices. Foods 2022, 11, 1920. https://doi.org/10.3390/foods11131920
Quan Q, Liu W, Guo J, Ye M, Zhang J. Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices. Foods. 2022; 11(13):1920. https://doi.org/10.3390/foods11131920
Chicago/Turabian StyleQuan, Qi, Wei Liu, Jiajing Guo, Meiling Ye, and Juhua Zhang. 2022. "Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices" Foods 11, no. 13: 1920. https://doi.org/10.3390/foods11131920
APA StyleQuan, Q., Liu, W., Guo, J., Ye, M., & Zhang, J. (2022). Effect of Six Lactic Acid Bacteria Strains on Physicochemical Characteristics, Antioxidant Activities and Sensory Properties of Fermented Orange Juices. Foods, 11(13), 1920. https://doi.org/10.3390/foods11131920





