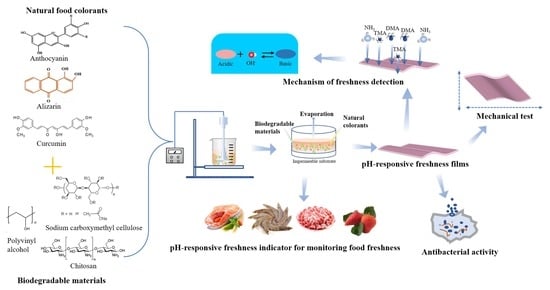
Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness
Abstract
:1. Introduction
2. Methods for Detecting Food Freshness
3. Overview of pH-Responsive Freshness Indicators
3.1. Synthetic Colorants
3.2. Natural Colorants
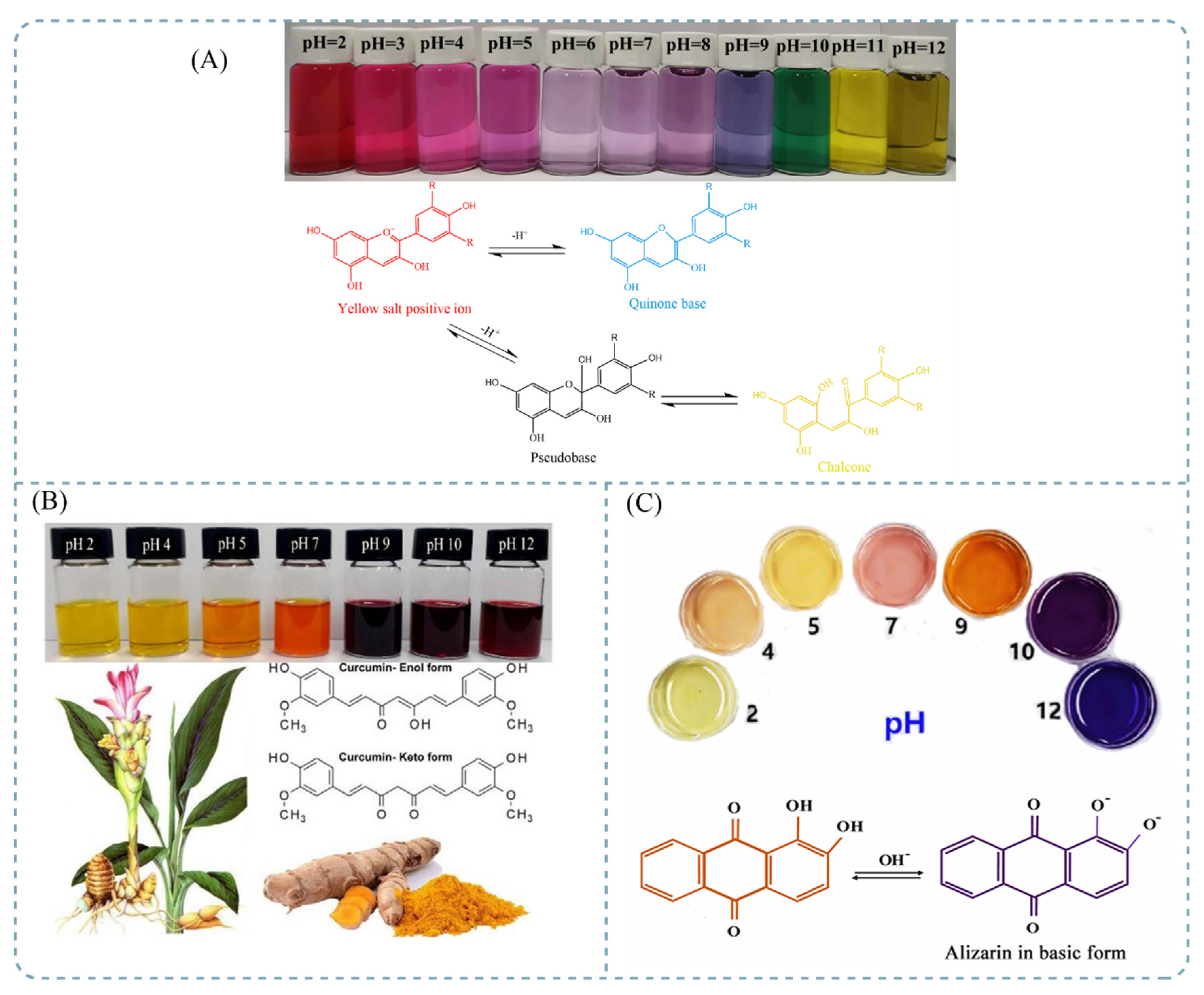
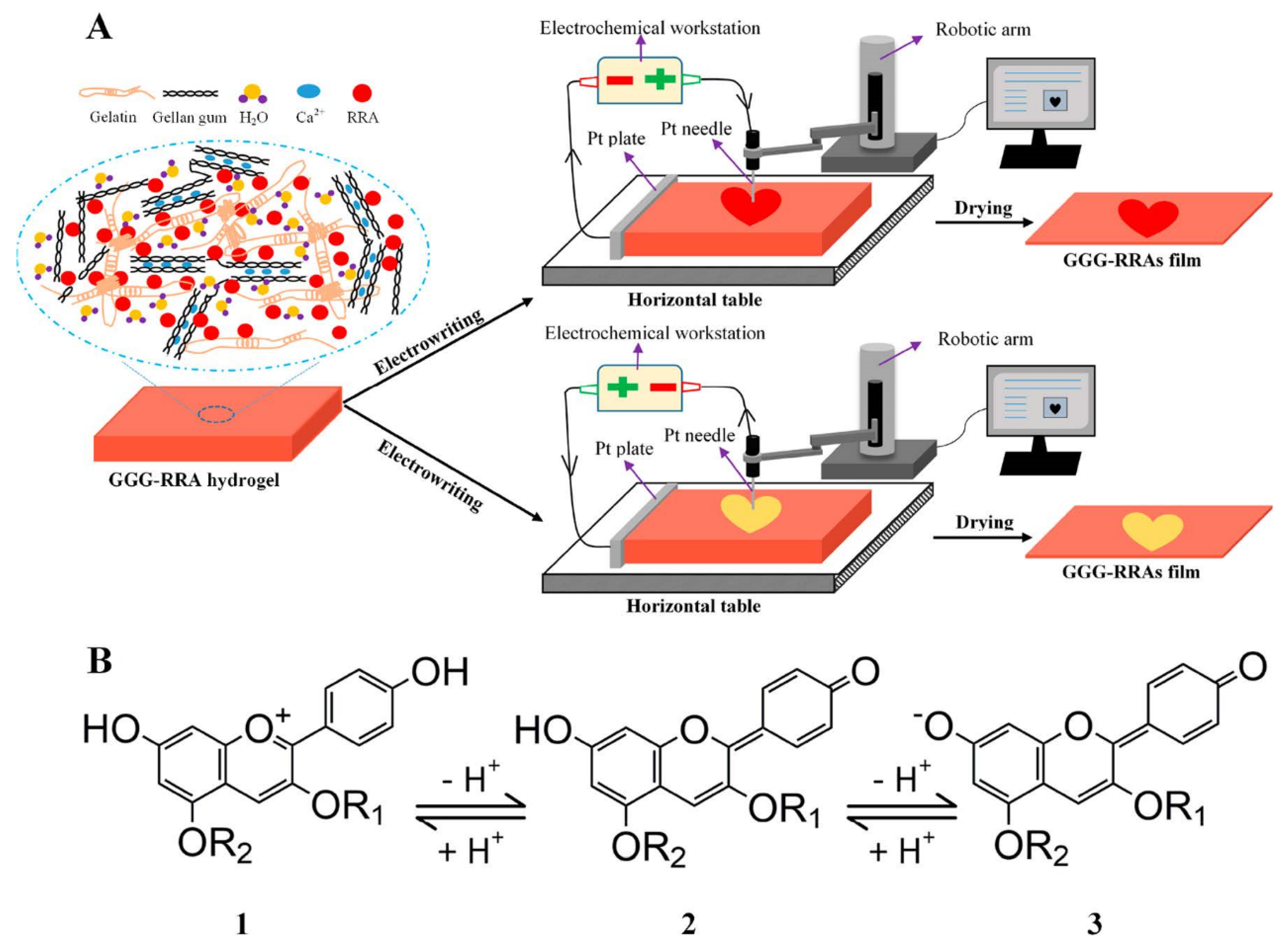
3.2.1. Anthocyanin
3.2.2. Curcumin
3.2.3. Alizarin
3.2.4. Betalain
3.2.5. Shikonin
3.3. Polymer Support
3.3.1. Synthetic Polymers
3.3.2. Biopolymers
4. Preparation of pH-Responsive Freshness Indicators
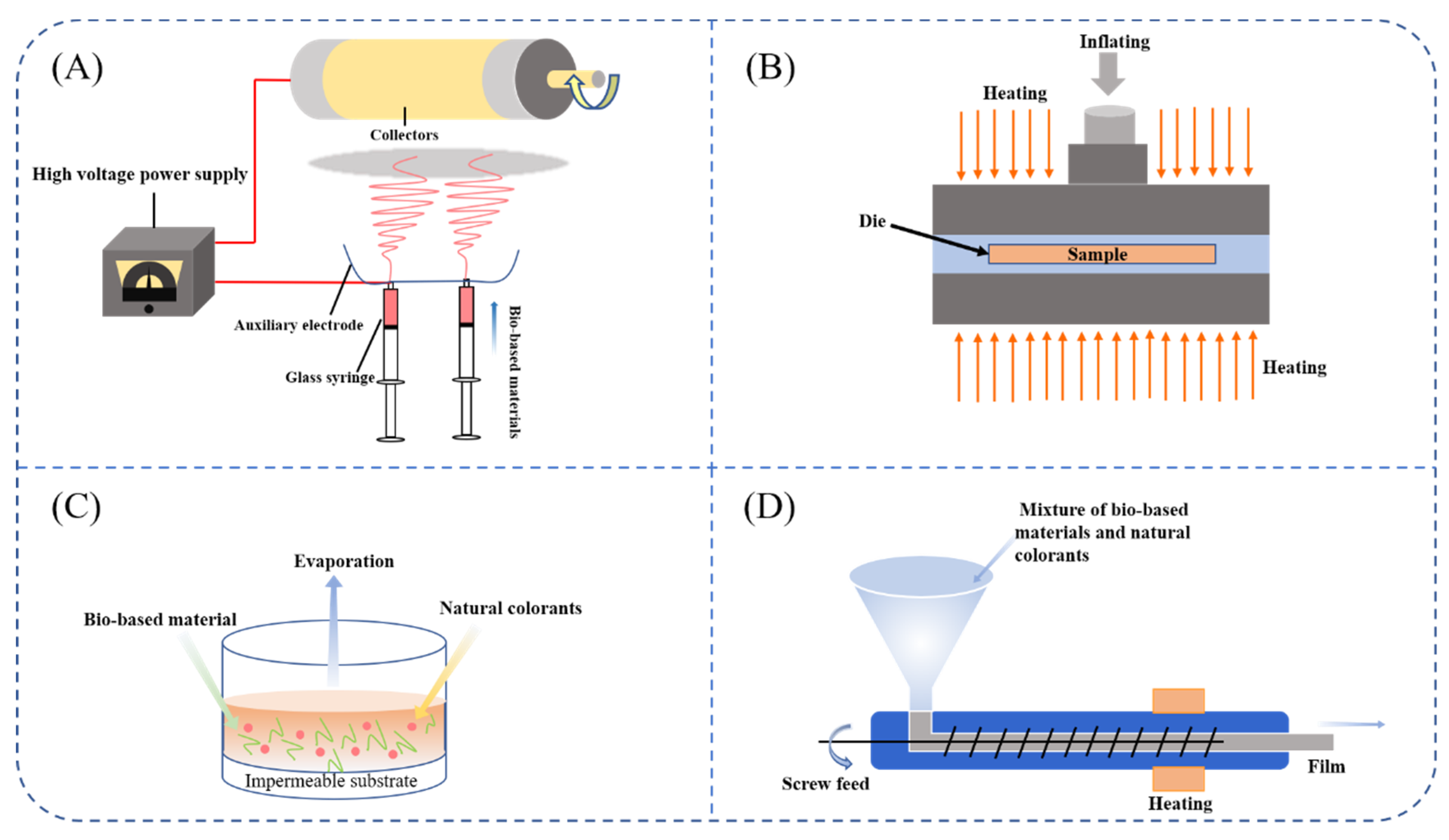
4.1. Freshness Indicator Preparation by Solvent Casting
4.2. Freshness Indicator Preparation by Extrusion
4.3. Freshness Indicator Preparation by Electrospinning
4.4. Freshness Indicator Preparation by Compression Molding
4.5. Freshness Indicator Preparation by Other Methods
5. Application of Natural-Colorants-Based pH-Responsive Freshness Indicators
5.1. Freshness Monitoring of Meat and Seafood Products
5.2. Freshness Monitoring of Milk and Dairy Products
5.3. Freshness Monitoring of Fruits and Vegetables
6. Conclusions and Future Perspective
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, D.; Yang, L.; Shang, M.; Zhong, Y. Research progress of packaging indicating materials for real-time monitoring of food quality. Mater. Express 2019, 9, 377–396. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.-T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food. Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [Green Version]
- Steenis, N.D.; van Herpen, E.; van der Lans, I.A.; Ligthart, T.N.; van Trijp, H.C.M. Consumer response to packaging design: The role of packaging materials and graphics in sustainability perceptions and product evaluations. J. Clean. Prod. 2017, 162, 286–298. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Gouveia Costa, H.L.; Lopes, D.R.; Prentice, C. Intelligent packaging with pH indicator potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Fernandez-Pan, I.; Carrion-Granda, X.; Mate, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A—Chem. 2014, 31, 374–395. [Google Scholar] [CrossRef]
- Zhao, C.J.; Han, J.W.; Yang, X.T.; Qian, J.P.; Fan, B.L. A review of computational fluid dynamics for forced-air cooling process. Appl. Energy 2016, 168, 314–331. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Vicente Ros-Lis, J.; Vivancos, J.L.; Martinez-Manez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Kuswandi, B.; Wicaksono, Y.; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Heising, J.K.; Dekker, M.; Bartels, P.V.; Van Boekel, M.A.J.S. Monitoring the quality of perishable foods: Opportunities for intelligent packaging. Crit. Rev. Food Sci. Nutr. 2014, 54, 645–654. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M. Fabrication and characterization of alizarin colorimetric indicator based on cellulose-chitosan to monitor the freshness of minced beef. Sens. Actuator B—Chem. 2019, 285, 519–528. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [Green Version]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Sobhi, M.; Piryaei, M. Preparation of a novel green optical pH sensor based on immobilization of red grape extract on bioorganic agarose membrane. Sens. Actuator B—Chem. 2016, 224, 391–395. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Toxicology of food dyes. Int. J. Occup. Environ. Health 2012, 18, 220–246. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, P.; Guo, M.; Jiang, S.; Li, X.; Jiang, S. Films based on kappa-carrageenan incorporated with curcumin for freshness monitoring. Food Hydrocoll. 2018, 83, 134–142. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total volatile basic nitrogen and trimethylamine in muscle foods: Potential formation pathways and effects on human health. Compr. Rev. Food. Sci. Food Saf. 2021, 20, 3620–3666. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef]
- Ezati, P.; Tajik, H.; Moradi, M.; Molaei, R. Intelligent pH-sensitive indicator based on starch-cellulose and alizarin dye to track freshness of rainbow trout fillet. Int. J. Biol. Macromol. 2019, 132, 157–165. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants trends and perspective. TrAC—Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Kim, J.; Moreira, R.; Castell-Perez, E. Simulation of pathogen inactivation in whole and fresh-cut cantaloupe (Cucumis melo) using electron beam treatment. J. Food Eng. 2010, 97, 425–433. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Zhang, X.; Liu, Y.; Qin, Y.; Liu, J. Effects of anthocyanin-rich purple and black eggplant extracts on the physical, antioxidant and pH-sensitive properties of chitosan film. Food Hydrocoll. 2019, 94, 93–104. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Nicolai, B.M.; Hertog, M.L.A.T.M. Optimization of hs spme fast gc-ms for high-throughput analysis of strawberry aroma. Food Anal. Meth. 2013, 6, 512–520. [Google Scholar] [CrossRef]
- Hui, G.; Jin, J.; Deng, S.; Ye, X.; Zhao, M.; Wang, M.; Ye, D. Winter jujube (zizyphus jujuba mill.) quality forecasting method based on electronic nose. Food Chem. 2015, 170, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, G.; Luo, L.; Chen, G. Study on seafood volatile profile characteristics during storage and its potential use for freshness evaluation by headspace solid phase microextraction coupled with gas chromatography-mass spectrometry. Anal. Chim. Acta 2010, 659, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lu, X.; Wang, Q.; Ma, M. Online automatic grading of salted eggs based on machine vision. Int. J. Agric. Biol. Eng. 2015, 8, 35–41. [Google Scholar] [CrossRef]
- Han, F.; Huang, X.; Teye, E.; Gu, F.; Gu, H. Nondestructive detection of fish freshness during its preservation by combining electronic nose and electronic tongue techniques in conjunction with chemometric analysis. Anal. Methods 2014, 6, 529–536. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive food packaging: Recent progress and technological prospects. Compr. Rev. Food Sci. F. 2016, 15, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.W.; Jafari, S.M. pH-sensitive (halochromic) smart packaging films based on natural food colorants for the monitoring of food quality and safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Suslick, K.S.; Rakow, N.A.; Sen, A. Colorimetric sensor arrays for molecular recognition. Tetrahedron 2004, 60, 11133–11138. [Google Scholar] [CrossRef]
- Munir, S.; Hu, Y.; Liu, Y.; Xiong, S. Enhanced properties of silver carp surimi-based edible films incorporated with pomegranate peel and grape seed extracts under acidic condition. Food Packag. Shelf 2019, 19, 114–120. [Google Scholar] [CrossRef]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Zaidi, N.S.R.; Mah, S.K. Poly (vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf 2020, 24, 100463. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomez, A.; Cerdan-Cartagena, F.; Suardiaz-Muro, J.; Boluda-Aguilar, M.; Esther Hernandez-Hernandez, M.; Angeles Lopez-Serrano, M.; Lopez-Coronado, J. Radiofrequency identification and surface acoustic wave technologies for developing the food intelligent packaging concept. Food Eng. Rev. 2015, 7, 11–32. [Google Scholar] [CrossRef]
- Fleischmann, C.; Cheng, J.; Tabatabai, M.; Ritter, H. Extended applicability of classical phenolphthalein: Color changing polymeric materials derived from pH-sensitive acrylated phenolphthalein derivatives. Macromolecules 2012, 45, 5343–5346. [Google Scholar] [CrossRef]
- Shukla, V.; Kandeepan, G.; Vishnuraj, M.R. Development of on-package indicator sensor for real-time monitoring of buffalo meat quality during refrigeration storage. Food Anal. Meth. 2015, 8, 1591–1597. [Google Scholar] [CrossRef]
- Gavrilenko, N.A.; Saranchina, N.V.; Sukhanov, A.V.; Fedan, D.A. Reversible pH-sensitive element based on bromocresol purple immobilized into the polymethacrylate matrix. Mendeleev Commun. 2018, 28, 450–452. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, L.-T. Colorimetric array indicator for nh3 and co2 detection. Sens. Actuator B—Chem. 2018, 255, 3216–3226. [Google Scholar] [CrossRef]
- Pacquit, A.; Lau, K.T.; McLaughlin, H.; Frisby, J.; Quilty, B.; Diamond, D. Development of a volatile amine sensor for the monitoring of fish spoilage. Talanta 2006, 69, 515–520. [Google Scholar] [CrossRef]
- Shao, P.; Liu, L.; Yu, J.; Zheng, L.; Sun, P. Novel aldehyde sensitive bio-based colorimetric film for kiwi fruit freshness monitoring. LWT 2022, 159, 113177. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Oktaviana, R.; Abdullah, A.; Heng, L.Y. A novel on-package sticker sensor based on methyl red for real-time monitoring of broiler chicken cut freshness. Packag. Technol. Sci. 2014, 27, 69–81. [Google Scholar] [CrossRef]
- Kuswandi, B.; Damayanti, F.; Jayus; Abdullah, A.; Heng, L.Y. Simple and low-cost on-package sticker sensor based on litmus paper for real-time monitoring of beef freshness. J. Math. Fundam. Sci. 2015, 47, 236–251. [Google Scholar] [CrossRef]
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a novel colorimetric indicator label for monitoring freshness of intermediate-moisture dessert spoilage. Talanta 2010, 81, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on ph indicators for real-time monitoring of beef. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Li, L.; Wang, Q.; Jiang, S.; Chen, M.; Li, X.; Jiang, S. Ph-responsive antibacterial film based polyvinyl alcohol/poly (acrylic acid) incorporated with aminoethyl-phloretin and application to pork preservation. Food Res. Int. 2021, 147, 110532. [Google Scholar] [CrossRef]
- Huang, X.w.; Zou, X.b.; Shi, J.y.; Li, Z.h.; Zhao, J.w. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Feketea, G.; Tsabouri, S. Common food colorants and allergic reactions in children: Myth or reality? Food Chem. 2017, 230, 578–588. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jimenez, A.R.; Paredes-Lopez, O. Natural pigments: Carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- Vatai, T.; Skerget, M.; Knez, Z. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar] [CrossRef]
- Jafari, S.M.; Khazaei, K.M.; Assadpour, E. Production of a natural color through microwave-assisted extraction of saffron tepal’s anthocyanins. Food Sci. Nutr. 2019, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Roeck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Wells, N.; Yusufu, D.; Mills, A. Colourimetric plastic film indicator for the detection of the volatile basic nitrogen compounds associated with fish spoilage. Talanta 2019, 194, 830–836. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. A comparative study on color stability of anthocyanin hybrid pigments derived from 1d and 2d clay minerals. Materials 2019, 12, 3287. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lu, S.; Chen, X. A visual pH sensing film using natural dyes from bauhinia blakeana dunn. Sens. Actuator B-Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins-nature’s bold, beautiful, and health-promoting colors. Foods 2019, 8, 550. [Google Scholar] [CrossRef] [Green Version]
- Castaneda-Ovando, A.; de Lourdes Pacheco-Hernandez, M.; Elena Paez-Hernandez, M.; Rodriguez, J.A.; Andres Galan-Vidal, C. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Almasi, H.; Forough, M.; Ezati, P. A novel pH-sensing indicator based on bacterial cellulose nanofibers and black carrot anthocyanins for monitoring fish freshness. Carbohydr. Polym. 2019, 222, 11530. [Google Scholar] [CrossRef]
- Romero, A.; Sharp, J.L.; Dawson, P.L.; Darby, D.; Cooksey, K. Evaluation of two intelligent packaging prototypes with a ph indicator to determine spoilage of cow milk. Food Packag. Shelf Life 2021, 30, 100720. [Google Scholar] [CrossRef]
- Halasz, K.; Csoka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric ph indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Z.; Jing, P. Black rice anthocyanins embedded in self-assembled chitosan/chondroitin sulfate nanoparticles enhance apoptosis in hct-116 cells. Food Chem. 2019, 301, 125280. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Xu, Z.; Wicker, L. Binding kinetics of blueberry pectin-anthocyanins and stabilization by non-covalent interactions. Food Hydrocoll. 2020, 99, 105354. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. pH-responsive pectin-based multifunctional films incorporated with curcumin and sulfur nanoparticles. Carbohydr. Polym. 2020, 230, 115638. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of carboxymethyl cellulose/agar-based functional films hybridized with alizarin and grapefruit seed extract. ACS Appl. Bio Mater. 2021, 4, 4470–4478. [Google Scholar] [CrossRef]
- Cvek, M.; Paul, U.C.; Zia, J.; Mancini, G.; Sedlarik, V.; Athanassiou, A. Biodegradable films of PLA/PPC and curcumin as packaging materials and smart indicators of food spoilage. ACS Appl. Mater. Interfaces 2022, 14, 14654–14667. [Google Scholar] [CrossRef]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—from molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, Y.; Han, J.; Cao, B.; Yin, H.; Shi, Y. Enhanced photoelectrical properties of alizarin-based natural dye via structure modulation. Sol. Energy 2019, 185, 315–323. [Google Scholar] [CrossRef]
- Qi, X.-N.; Che, Y.-X.; Qu, W.-J.; Zhang, Y.-M.; Yao, H.; Lin, Q.; Wei, T.-B. Design and fabricating biogenic amine-responsive platform based on self-assembly property of phenazine derivative for visual monitoring of meat spoilage. Sens. Actuators B Chem. 2021, 333, 129430. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. Ph-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. Advances and future directions in betalain metabolic engineering. New Phytol. 2019, 224, 1472–1478. [Google Scholar] [CrossRef] [Green Version]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT-Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Khan, M.I. Stabilization of betalains: A review. Food Chem. 2016, 197, 1280–1285. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. J. Agric. Food Chem. 2006, 54, 390–398. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef]
- Dong, H.; Ling, Z.; Zhang, X.; Zhang, X.; Ramaswamy, S.; Xu, F. Smart colorimetric sensing films with high mechanical strength and hydrophobic properties for visual monitoring of shrimp and pork freshness. Sens. Actuators B Chem. 2020, 309, 127752. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, Y.; Zou, Y.; Dong, Q.; Ding, F.; Liu, X.; Li, H. A novel colorimetric indicator based on agar incorporated with arnebia euchroma root extracts for monitoring fish freshness. Food Hydrocoll. 2019, 90, 198–205. [Google Scholar] [CrossRef]
- Yoshida, C.M.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT-Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Chen, H.; Bhandari, B. Freshness monitoring technology of fish products in intelligent packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 1279–1292. [Google Scholar] [CrossRef]
- Kuswandi, B.; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Li, H.; Liu, X.; Guo, T.; Zhang, G.; Xiong, Y. A renewable intelligent colorimetric indicator based on polyaniline for detecting freshness of tilapia. Packag. Technol. Sci. 2018, 31, 133–140. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, X.; Zhang, J.; Yang, Z.; Sun, Y.; Li, Z.; Huang, X.; Holmes, M.; Gong, Y.; Povey, M. Extruded low density polyethylene-curcumin film: A hydrophobic ammonia sensor for intelligent food packaging. Food Packag. Shelf Life 2020, 26, 100595. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Pectin/pullulan blend films for food packaging: Effect of blending ratio. Food Chem. 2021, 347, 129022. [Google Scholar] [CrossRef]
- Kerry, J.; Butler, P. Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, J.; Xu, Z.; Wicker, L. Blueberry pectin and increased anthocyanins stability under in vitro digestion. Food Chem. 2020, 302, 125343. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.F.; Andrade, C.T. Optimized pH-responsive film based on a eutectic mixture-plasticized chitosan. Carbohydr. Polym. 2017, 165, 238–246. [Google Scholar] [CrossRef]
- Mills, A.; Wild, L.; Chang, Q. Plastic colorimetric film sensors for gaseous ammonia. Mikrochim. Acta 1995, 121, 225–236. [Google Scholar] [CrossRef]
- Yildiz, E.; Sumnu, G.; Kahyaoglu, L.N. Monitoring freshness of chicken breast by using natural halochromic curcumin loaded chitosan/peo nanofibers as an intelligent package. Int. J. Biol. Macromol. 2021, 170, 437–446. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of active fish gelatin films with anthocyanins by compression molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Gaviria, Y.A.R.; Palencia, N.S.N.; Capello, C.; Trevisol, T.C.; Monteiro, A.R.; Valencia, G.A. Nanostructured pH-indicator films based on cassava starch, laponite, and jambolan (syzygium cumini) fruit manufactured by thermo-compression. Starch-Stärke 2021, 73, 2000208. [Google Scholar] [CrossRef]
- He, C.; Zhang, M.; Guo, C. 4d printing of mashed potato/purple sweet potato puree with spontaneous color change. Innov. Food Sci. Emerg. Technol. 2020, 59, 102250. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun functional materials toward food packaging applications: A review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Tan, J.; Yu, S.Y.; Yousefzadeh, M.; Lyu, T.t.; Jiao, Z.W.; Li, H.y.; Ramakrishna, S. High-efficiency preparation of polypropylene nanofiber by melt differential centrifugal electrospinning. J. Appl. Polym. Sci. 2020, 137, 48299. [Google Scholar] [CrossRef]
- Wang, G.; Sun, X.; Bai, J.; Han, L. Preparation of Fe–C nanofiber composites by metal organic complex and potential application in supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 4665–4675. [Google Scholar] [CrossRef]
- Wortmann, M.; Frese, N.; Sabantina, L.; Petkau, R.; Kinzel, F.; Gölzhäuser, A.; Moritzer, E.; Hüsgen, B.; Ehrmann, A. New polymers for needleless electrospinning from low-toxic solvents. Nanomaterials 2019, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.W.; Park, Y.; Kim, J.; Park, C.H. Multi-jet electrospinning of polystyrene/polyamide 6 blend: Thermal and mechanical properties. Fash. Text. 2017, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication and testing of PVA/chitosan bilayer films for strawberry packaging. Coatings 2017, 7, 109. [Google Scholar] [CrossRef]
- Langroodi, A.M.; Tajik, H.; Mehdizadeh, T.; Moradi, M.; Kia, E.M.; Mahmoudian, A. Effects of sumac extract dipping and chitosan coating enriched with zataria multiflora boiss oil on the shelf-life of meat in modified atmosphere packaging. LWT 2018, 98, 372–380. [Google Scholar] [CrossRef]
- Phuhongsung, P.; Zhang, M.; Bhandari, B. 4d printing of products based on soy protein isolate via microwave heating for flavor development. Food Res. Int. 2020, 137, 109605. [Google Scholar] [CrossRef]
- Sun, G.; Chi, W.; Zhang, C.; Xu, S.; Li, J.; Wang, L. Developing a green film with pH-sensitivity and antioxidant activity based on ĸ-carrageenan and hydroxypropyl methylcellulose incorporating prunus maackii juice. Food Hydrocoll. 2019, 94, 345–353. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Goodarzi, M.M.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Development of an easy-to-use colorimetric pH label with starch and carrot anthocyanins for milk shelf life assessment. Int. J. Biol. Macromol. 2020, 153, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-z.; Zhang, M.; Bhandari, B.; Yang, C.-h. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2020, 100, 105438. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Chen, M.; Ge, Y.; Ma, J.; Hu, Y.; Pang, J.; Yan, Z. Effect of oxidized chitin nanocrystals and curcumin into chitosan films for seafood freshness monitoring. Food Hydrocoll. 2019, 95, 308–317. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (hylocereus polyrhizus) peel into starch/polyvinyl alcohol films. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Kang, S.; Wang, H.; Xia, L.; Chen, M.; Li, L.; Cheng, J.; Li, X.; Jiang, S. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020, 229, 115402. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of echium amoenum anthocyanins into bacterial cellulose film: A novel colorimetric pH indicator for freshness/spoilage monitoring of shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of pH-sensitive and antioxidant packaging films based on kappa-carrageenan and mulberry polyphenolic extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual pH-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2020, 40, e12725. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Zandraa, O.; Pummerová, M.; Sáha, P. Essential oil based pvp-cmc-bc-gg functional hydrogel sachet for ‘cheese’: Its shelf life confirmed with anthocyanin (isolated from red cabbage) bio stickers. Foods 2020, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: Freshness indicators based on anthocyanin extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.S.; Luo, Q.Y.; Li, M.l.; Liu, X.Y.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2020, 143, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Shi, J.; Zou, X.; Wang, S.; Jiang, C.; Zhang, J.; Huang, X.; Zhang, W.; Holmes, M. Novel colorimetric films based on starch/polyvinyl alcohol incorporated with roselle anthocyanins for fish freshness monitoring. Food Hydrocoll. 2017, 69, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, H.; Guo, M.; Li, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Extract from lycium ruthenicum murr. Incorporating κ-carrageenan colorimetric film with a wide pH–sensing range for food freshness monitoring. Food Hydrocoll. 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, Z.; Xie, F.; Tang, S.; Fang, J.; Wang, X. 3d printed nanocellulose-based label for fruit freshness keeping and visual monitoring. Carbohydr. Polym. 2021, 273, 118545. [Google Scholar] [CrossRef]
- Kurnianto, M.; Poerwanto, B.; Wahyono, A.; Apriliyanti, M.; Lestari, I. Monitoring of Banana Deteriorations using Intelligent-packaging Containing Brazilien Extract (Caesalpina sappan L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012043. [Google Scholar] [CrossRef]
- Stoll, L.; Costa, T.M.H.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Microencapsulation of anthocyanins with different wall materials and its application in active biodegradable films. Food Bioprocess Technol. 2016, 9, 172–181. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Ščetar, M.; Bosiljkov, T.; Galić, K. Comparison of two pH responsive color changing bio-based films containing wasted fruit pomace as a source of colorants. J. Food Sci. 2019, 84, 2490–2498. [Google Scholar] [CrossRef]
- Gómez, I.; Janardhanan, R.; Ibañez, F.C.; Beriain, M.J. The effects of processing and preservation technologies on meat quality: Sensory and nutritional aspects. Foods 2020, 9, 1416. [Google Scholar] [CrossRef]
- Othman, M.; Yusup, A.A.; Zakaria, N.; Khalid, K. In Bio-polymer chitosan and corn starch with extract of hibiscus rosa-sinensis (hibiscus) as ph indicator for visually-smart Food Packaging. AIP Conf. Proc. 2018, 1985, 050004. [Google Scholar] [CrossRef]
- Dudnyk, I.; Janeček, E.-R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Zou, X.; Shi, J.; Zhai, X.; Liu, L.; Li, Z.; Holmes, M.; Gong, Y.; Povey, M. A visual indicator based on curcumin with high stability for monitoring the freshness of freshwater shrimp, Macrobrachium rosenbergii. J. Food Eng. 2021, 292, 110290. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Shi, Q.; Zhang, Y.; Liu, J.; Wu, X.; Fang, Z. Development and characterization of active and pH-sensitive films based on psyllium seed gum incorporated with free and microencapsulated mulberry pomace extracts. Food Chem. 2021, 352, 129333. [Google Scholar] [CrossRef]
- Karaman, A.; Özer, B.; Pascall, M.A.; Alvarez, V. Recent advances in dairy packaging. Food Rev. Int. 2015, 31, 295–318. [Google Scholar] [CrossRef]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from brassica oleraceae (red cabbage) as time–temperature indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M. Natural biomaterial-based edible and pH-sensitive films combined with electrochemical writing for intelligent food packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; Sganzerla, W.G.; Maciel, M.V.d.O.B.; de Melo, A.P.Z.; da Rosa Almeida, A.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Development of poly (ethylene oxide) bioactive nanocomposite films functionalized with zein nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124268. [Google Scholar] [CrossRef]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Chen, H.-z.; Zhang, M.; Bhandari, B.; Guo, Z. Applicability of a colorimetric indicator label for monitoring freshness of fresh-cut green bell pepper. Postharvest Biol. Technol. 2018, 140, 85–92. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ribeiro, C.P.P.; Uliana, N.R.; Rodrigues, M.B.C.; da Rosa, C.G.; Ferrareze, J.P.; de Lima Veeck, A.P.; Nunes, M.R. Bioactive and pH-sensitive films based on carboxymethyl cellulose and blackberry (Morus nigra L.) anthocyanin-rich extract: A perspective coating material to improve the shelf life of cherry tomato (Solanum lycopersicum L. Var. Cerasiforme). Biocatal. Agric. Biotechnol. 2021, 33, 101989. [Google Scholar] [CrossRef]
- Torres-León, C.; Vicente, A.A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible films and coatings based on mango (var. Ataulfo) by-products to improve gas transfer rate of peach. LWT 2018, 97, 624–631. [Google Scholar] [CrossRef] [Green Version]

| Materials | Colorants | Methods | Food Sample | Reference |
|---|---|---|---|---|
| Pectin/Sulfur nanoparticles | Curcumin | Solvent Casting | Shrimp | [77] |
| Chitosan/Microcrystalline cellulose | Curcumin | Solvent Casting | No date | [104] |
| Poly (vinyl butyral) or ethylcellulose/Tributyl phosphate | Bromophenol blue, Bromocresol green or Chlorophenol red | Extrusion | No date | [105] |
| Low-density Polyethylene/SiO2 nanoparticles | Bromophenol blue | Extrusion | Fish | [64] |
| Low-density polyethylene (LDPE) | Curcumin | Extrusion | Silver carp/Beef | [96] |
| Chitosan/Polyethylene oxide | Curcumin | Electrospinning | Chicken breast | [106] |
| Fish gelatin | Anthocyanin | Compression Molding | No date | [107] |
| Cassava starch/Laponite | Anthocyanin | Compression Molding | Round steak | [108] |
| Potato flakes/Sodium alginate powder/Citric acid | Anthocyanin | 4D printing | No date | [109] |
| Tested Food | Natural Colorants | Source of Natural Colorants | Polymer Materials | pH Values/Color Variations | Colorant Concentrations | Reference |
|---|---|---|---|---|---|---|
| Pork and shrimp | Curcumin | Curcuma longa | k-carrageenan | 3 and 13 Pink and blue-green colors in different solutions | Ethanol solutions (10 mL, ethanol/water = 4/1, w/w) containing 0, 1, 3, 5, and 7% (w/w) based on k-carrageenan | [23] |
| Pork | Anthocyanin | Prunus maackii | κ-carrageenan/hydroxypropyl methylcellulose | 3–11 Dark red to gray-blue colors | 0, 2, 4, 8, and 16% (w/w) based on total mixed hydrogels | [120] |
| Chicken | Anthocyanin | Blueberry residue | Cassava starch | 2–11 Red/pink /purple (pH ≤ 5) and yellowish (pH ≥ 6) | 4 g blueberry residue powder/100 g cassava starch | [121] |
| Chicken/fish | Betalains | Amaranthus leaf | Polyvinyl alcohol/gelatin | 2–11 Pink (pH ≤ 4) to bluish pink (pH ≤ 6) to blue (pH ≤ 9) and to gray (pH ≤ 11) | 5% Amaranthus leaf extract (v/v) based on total mixed hydrogels | [122] |
| Minced beef | Alizarin | Roots of Madder family plants | Cellulose/chitosan | 2–11 Yellow to dark purple | 1% (w/v) based on total mixed hydrogels | [16] |
| Seafood/meat | Alizarin | Roots of Madder family | Carboxymethyl Cellulose/Agar | 2–12 Yellow at acidic pHs, pale pink at neutral pH, and red to purple to blue at basic pHs | 1.0% (w/w) based on mixed hydrogels | [83] |
| Fish | Anthocyanin | Black carrot | Bacterial nanocellulose | 2–11 Red to khaki | 6 mg/mL | [70] |
| Fish (Bighead carp) | Curcumin (CR)/anthocyanin (ATH) | Curcuma longa/Purple sweet potatoes | Starch/polyvinyl alcohol | 5–11 Yellow to reddish brown (CR) Pinkish purple to blue to green (ATH) | 4%(v/v) mixture of curcumin and anthocyanin solution at a ratio of 8:2 (v/v) | [123] |
| Fish (Hair tail) and shrimp | Curcumin | Curcuma longa | Chitosan (CS)/oxidized chitin nanocrystal (O-ChNCs) | 3–10 Yellow to orange red | 10% (w/w, CS and O-ChNCs basis) | [124] |
| Fish (Rainbow trout fillet) | Alizarin | Roots of Madder family plants | Starch-cellulose | 2–11 Yellow to purple | 1% (w/v, mixed starch/glycerol basis) | [26] |
| Shrimp | Betalains | Hylocereus polyrhizus | Starch/polyvinyl alcohol | 3–12 Red (pH ≤ 7) to orange (pH = 8–9) and to yellow (pH = 10–12) | 0.25, 0.50 and 1.00% (w/w, starch basis) | [125] |
| Shrimp | Anthocyanin | Red rose | Polyvinyl alcohol/okra mucilage polysaccharide | 2–12 Red (pH = 2) to pink (pH= 3–5) to blue (pH= 7–10) and to yellow green (pH = 12) | 1, 2, 3, and 4% (w/w, based on PVA) | [126] |
| Shrimp | Anthocyanin | Echium amoenum flowers | Bacterial cellulose | 2–12 Red to yellow | 1:1 dilution of extract solution | [127] |
| Milk | Anthocyanin | Mulberry | κ-carrageenan | 2–13 Red to purple and to gray | 1.5, 2.5, 3.5, and 4.5% (w/w, κ-carrageenan basis) | [128] |
| Milk | Anthocyanin | Grape skins | Tara gum/cellulose | 1–10 Bright red to dark green | 5 g/100 g, 10 g/ 100 g, and 15 g/100 g (tara gum basis) | [129] |
| Milk | Anthocyanin | Red cabbage | Polyvinyl alcohol/ starch | No date | No date | [130] |
| Cheese | Anthocyanin | Red Cabbage | Polyvinylpyrrolidone/CMC/Bacterial cellulose/Guar gum | 1–14 Reddish (acidic) to blue (neutral) to green and to yellow (alkaline) | No date | [131] |
| Indicator | Food | pH Values | Color Variation | Reference |
|---|---|---|---|---|
| Based on starch-cellulose and alizarin dye | Fish (rainbow trout fillet) 4 °C | 2–11 |  | [26] |
| Based on starch/polyvinyl alcohol and roselle anthocyanins | Fish (4 °C) | 2–12 | 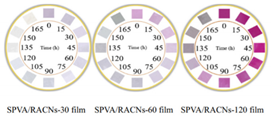 | [135] |
| Based on polyvinyl alcohol/sodium carboxymethyl cellulose and red cabbage anthocyanin | Pork (25 °C) | 2–12 |  | [5] |
| Based on polyvinyl alcohol/okra mucilage polysaccharide and rose anthocyanins | Shrimp | 2–12 |  | [126] |
| Based on κ-carrageenan and anthocyanins | Milk | 2–10 |  | [136] |
| Based on cellulose nanofibers and blueberry anthocyanin | Lychees | 2–12 |  | [137] |
| Based on polyvinyl alcohol/glucomannan and anthocyanins | Banana | 3–8 |  | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Liu, L.; Cui, Z.; Zhong, Y. Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness. Foods 2022, 11, 1884. https://doi.org/10.3390/foods11131884
Liu D, Zhang C, Pu Y, Chen S, Liu L, Cui Z, Zhong Y. Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness. Foods. 2022; 11(13):1884. https://doi.org/10.3390/foods11131884
Chicago/Turabian StyleLiu, Danfei, Changfan Zhang, Yumei Pu, Siyuan Chen, Lei Liu, Zijie Cui, and Yunfei Zhong. 2022. "Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness" Foods 11, no. 13: 1884. https://doi.org/10.3390/foods11131884
APA StyleLiu, D., Zhang, C., Pu, Y., Chen, S., Liu, L., Cui, Z., & Zhong, Y. (2022). Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness. Foods, 11(13), 1884. https://doi.org/10.3390/foods11131884