Impact of Industrial Practices on the Microbial and Quality Attributes of Fresh Vacuum-Packed Lamb Joints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lamb Sampling
2.2. Fleece Swabbing
2.3. Carcass Chilling
2.4. Aerial Sampling
2.5. Boning-Out Lamb Leg Joints
2.6. Microbiological Analyses
2.7. Microbiological Analyses of Meat Samples
2.8. Visual Colour and Odour Assessment of Raw Samples
2.9. Lipid Oxidation
2.10. Eating Quality
2.11. Statistical Analysis
3. Results and Discussion
3.1. Fleece Management
3.2. Carcass Chilling Time
3.3. Boning-Out of Carcasses
3.4. Vacuum-Packed Storage of Primals
3.5. Raw Meat Quality Assessment
3.6. Eating Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, C.O. Extending the storage life of raw chilled meats. Meat Sci. 1996, 43 (Suppl. S1), 99–109. [Google Scholar] [CrossRef]
- Kennedy, C.; Buckley, D.J.; Kerry, J.P. Display life of sheep meats retail packaged under atmospheres of various volumes and compositions. Meat Sci. 2004, 68, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Mills, J. Sources and Control of Microbial Contamination on Red Meat. In Handbook of Meat and Meat Processing, 2nd ed.; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Hauge, S.J.; Nafstad, O.; Skjerve, E.; Rotterud, O.J.; Nesbakken, T. Effects of shearing and fleece cleanliness on microbiological contamination of lamb carcasses. Int. J. Food Microbiol. 2011, 150, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Department of Agriculture, Food and the Marine. Clean Livestock—Sheep. 2017. Available online: https://wayback.archive-it.org/org-1444/20201127052412/https://www.agriculture.gov.ie/media/migration/foodsafetypublichealthampconsumerissues/controlsonmeat/CleanLivestockPolicySheep230217.pdf (accessed on 6 May 2022).
- Okraszska-Lasica, W.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. Comparison of aerial counts at different sites in beef and sheep abattoirs and the relationship between aerial and beef carcass contamination. Food Microbiol. 2012, 32, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lenahan, M.; O’Brien, S.B.; Kinsella, K.; Sweeney, T.; Sheridan, J.J. Assessment of lamb carcass hygiene before and after chilling at five Irish abattoirs. Food Control 2010, 21, 313–318. [Google Scholar] [CrossRef]
- Muela, E.; Sanudo, C.; Campo, M.M.; Medel, I.; Beltran, J.A. Effects of cooling temperature and hot carcass weight on the quality of lamb. Meat Sci. 2010, 84, 101–107. [Google Scholar] [CrossRef]
- McGeehin, B.; Sheridan, J.J.; Butler, F. Optimising a rapid chilling system for lamb carcasses. J. Food Eng. 2002, 52, 75–81. [Google Scholar] [CrossRef]
- Jones, R.J.; Hussein, H.M.; Zagorec, M.; Brightwell, G.; Tagg, J.R. Isolation of lactic acid bacteria with inhibitory activity against pathogens and spoilage organisms associated with fresh meat. Food Microbiol. 2008, 25, 228–234. [Google Scholar] [CrossRef]
- Gill, C.O. Spoilage, factors affecting-microbiological. In Encyclopedia of Meat Sciences; Jenson, W., Ed.; Elsevier: Oxford, UK, 2004; pp. 1324–1330. [Google Scholar]
- Brightwell, G.; Clemens, R.; Urlich, S.; Boerema, J. Possible involvement of psychrotolerant Enterobacteriaceae in blown pack spoilage of vacuum-packaged raw meats. Int. J. Food Microbiol. 2007, 119, 334–339. [Google Scholar] [CrossRef]
- Mills, J.; Donnison, A.; Brightwell, G. Factors affecting microbial spoilage and shelf-life of chilled vacuum-packed lamb transported to distant markets: A review. Meat Sci. 2014, 98, 71–80. [Google Scholar] [CrossRef]
- Reis, M.M.; Reis, M.G.; Mills, J.; Ross, C.; Brightwell, G. Characterization of volatile metabolites associated with confinement odour during the shelf-life of vacuum packed lamb meat under different storage conditions. Meat Sci. 2016, 113, 80–91. [Google Scholar] [CrossRef] [PubMed]
- AMSA. AMSA Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012. [Google Scholar]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- 2001/471/EC; Commission Decision of 8 June 2001 Laying Down Rules for the Regular Checks on the General Hygiene Carried Out by the Operators in Establishments According to Directive 64/433/EEC on Health Conditions for the Production and Marketing of Fresh Meat and Directive 71/118/EEC on Health Problems Affecting the Production and Placing on the Market of Fresh Poultry Meat (Text with EEA Relevance) (Notified under Document Number C(2001) 1561). European Commission: Brussels, Belgium, 8 June 2001; pp. 48–53.
- Hunt, M.C. Guidelines for Meat Color Evaluation. In Proceedings of the 44th Reciprocal Meat Conference, Manhattan, KS, USA, 9–12 June 1991. [Google Scholar]
- Yantis, J.E. The Role of Sensory Analysis in Quality Control; ASTM International: West Conshohocken, PA, USA, 1992. [Google Scholar]
- Moran, L.; Andres, S.; Bodas, R.; Prieto, N.; Giraldez, F.J. Meat texture and antioxidant status are improved when carnosic acid is included in the diet of fattening lambs. Meat Sci. 2012, 91, 430–434. [Google Scholar] [CrossRef] [PubMed]
- AMSA. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat; American Meat Science Association: Champaign, IL, USA, 2015; pp. 1–106. [Google Scholar]
- Wilkinson, G.N.; Rogers, C.E. Symbolic Description of Factorial Models for Analysis of Variance. J. R. Stat. Society. Ser. C (Appl. Stat.) 1973, 22, 392–399. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 6 May 2022).
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Byrne, B.; Dunne, G.; Lyng, J.; Bolton, D.J. The development of a ‘clean sheep policy’ in compliance with the new Hygiene Regulation (EC) 853/2004 (Hygiene 2). Food Microbiol. 2007, 24, 301–304. [Google Scholar] [CrossRef]
- McGeehin, B.; Sheridan, J.J.; Butler, F. Factors affecting the pH decline in lamb after slaughter. Meat Sci. 2001, 58, 79–84. [Google Scholar] [CrossRef]
- Sheridan, J.J.; Lynch, B.; Harrington, D. The effect of boning and plant cleaning on the contamination of beef cuts in a commercial boning hall. Meat Sci. 1992, 32, 185–194. [Google Scholar] [CrossRef]
- AMPC; MLA. Processor’s Guide to Improving Microbiological Quality and Shelf Life of Meat, 2nd ed.; Meat & Livestock Australia: North Sydney, Australia, 2015. [Google Scholar]
- Kaur, M.; Shang, H.; Tamplin, M.; Ross, T.; Bowman, J.P. Culture-dependent and culture-independent assessment of spoilage community growth on VP lamb meat from packaging to past end of shelf-life. Food Microbiol. 2017, 68, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kiermeier, A.; Tamplin, M.; May, D.; Holds, G.; Williams, M.; Dann, A. Microbial growth, communities and sensory characteristics of vacuum and modified atmosphere packaged lamb shoulders. Food Microbiol. 2013, 36, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Gribble, A.; Mills, J.; Brightwell, G. The spoilage characteristics of Brochothrix thermosphacta and two psychrotolerant Enterobacteriacae in vacuum packed lamb and the comparison between high and low pH cuts. Meat Sci. 2014, 97, 83–92. [Google Scholar] [CrossRef]
- Bell, R.G. Meat packaging: Protection, preservation and presentation. In Meat Science and Applications; Hui, Y.H., Nip, W.K., Rogers, R.W., Young, O.A., Eds.; Marcel-Dekker: New York, NY, USA, 2001; pp. 463–490. [Google Scholar]
- Small, A.H.; Jenson, I.; Kiermeier, A.; Sumner, J. Vacuum-packed beef primals with extremely long shelf life have unusual microbiological counts. J. Food Prot. 2012, 75, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.J.; Doherty, A.M.; Allen, P.; McDowell, D.A.; Blair, I.S.; Harrington, D. The effect of vacuum and modified atmosphere packaging on the shelf-life of lamb primals, stored at different temperatures. Meat Sci. 1997, 45, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Wickramasinghe, N.N.; Ravensdale, J.; Coorey, R.; Chandry, S.P.; Dykes, G.A. The Predominance of Psychrotrophic Pseudomonads on Aerobically Stored Chilled Red Meat. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1622–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippis, F.; La Storia, A.; Villani, F.; Ercolini, D. Strain-Level Diversity Analysis of Pseudomonas fragi after In Situ Pangenome Reconstruction Shows Distinctive Spoilage-Associated Metabolic Traits Clearly Selected by Different Storage Conditions. Appl. Environ. Microbiol. 2019, 85, e02212-18. [Google Scholar] [CrossRef] [Green Version]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.M.; Marnewick, J.J. Volatile compounds associated with microbial growth on normal and high pH beef stored at chill temperatures. J. Appl. Bacteriol. 1989, 66, 281–289. [Google Scholar] [CrossRef]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Singh, T.; Tamplin, M.; Chandry, P.S. Genomic and metabolic characterization of spoilage-associated Pseudomonas species. Int. J. Food Microbiol. 2018, 268, 61–72. [Google Scholar] [CrossRef]
- Gribble, A.; Brightwell, G. Spoilage characteristics of Brochothrix thermosphacta and campestris in chilled vacuum packaged lamb, and their detection and identification by real time PCR. Meat Sci. 2013, 94, 361–368. [Google Scholar] [CrossRef]
- Nychas, G.J.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Smith, N. Chilled meat—Microbiology is only part of the story! In Proceedings of the NZIFST Annual Conference, Rotorua, New Zealand, 24–26 June 2008.
- Hernández Salueña, B.; Sáenz Gamasa, C.; Diñeiro Rubial, J.M.; Alberdi Odriozola, C. CIELAB color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripoll, G.; Joy, M.; Munoz, F. Use of dietary vitamin E and selenium (Se) to increase the shelf life of modified atmosphere packaged light lamb meat. Meat Sci. 2011, 87, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Suleman, R.; Wang, Z.; Aadil, R.M.; Hui, T.; Hopkins, D.L.; Zhang, D. Effect of cooking on the nutritive quality, sensory properties and safety of lamb meat: Current challenges and future prospects. Meat Sci. 2020, 167, 108172. [Google Scholar] [CrossRef]
- Pannier, L.; Corlett, M.; Payne, C.; Pethick, D. Cuts Based MSA Lamb Development—Lamb Turn off and Packaging Effects; Meat and Livestock Australia Limited: Sydney, Australia, 2019; pp. 1–34. [Google Scholar]

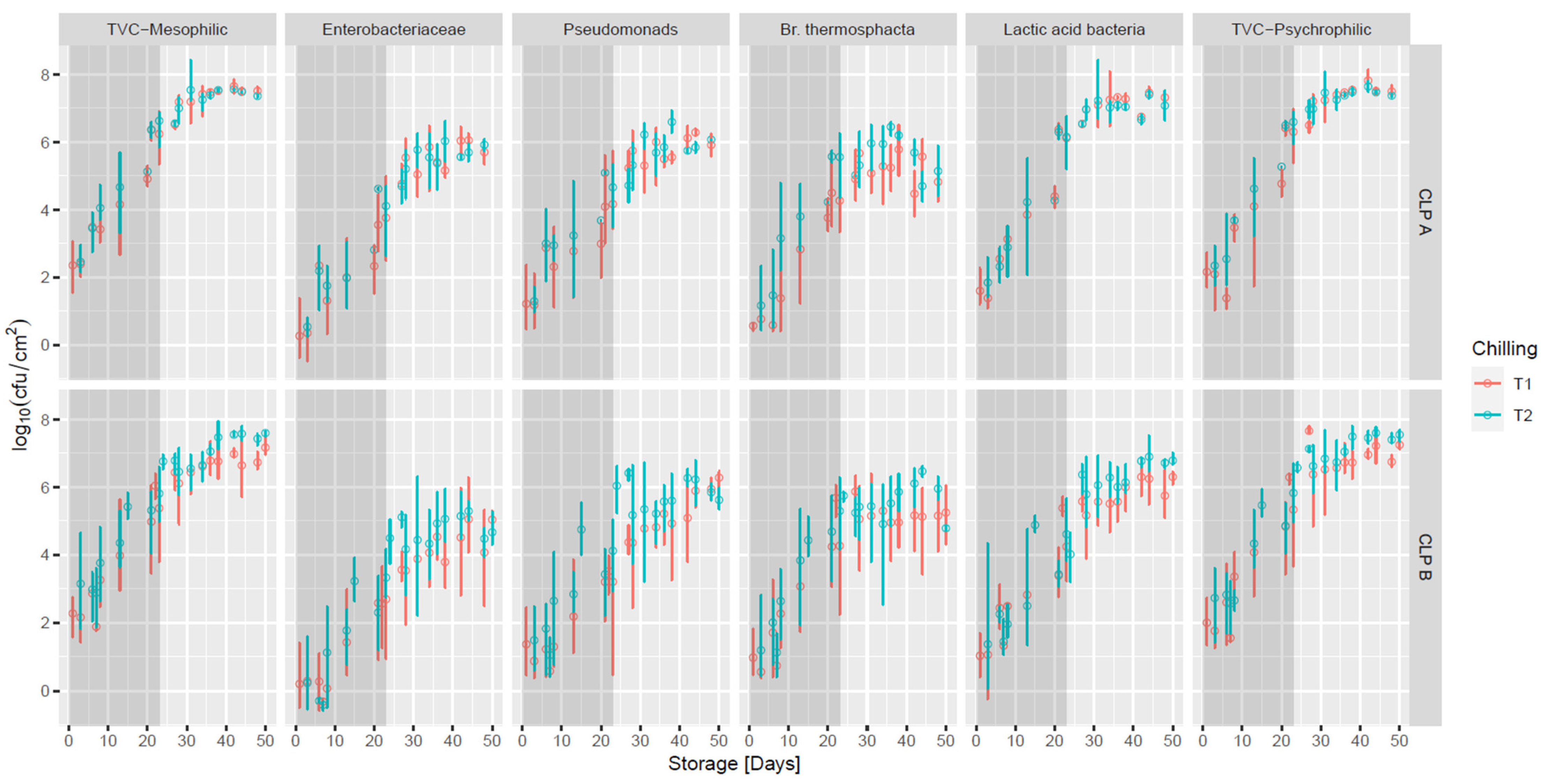
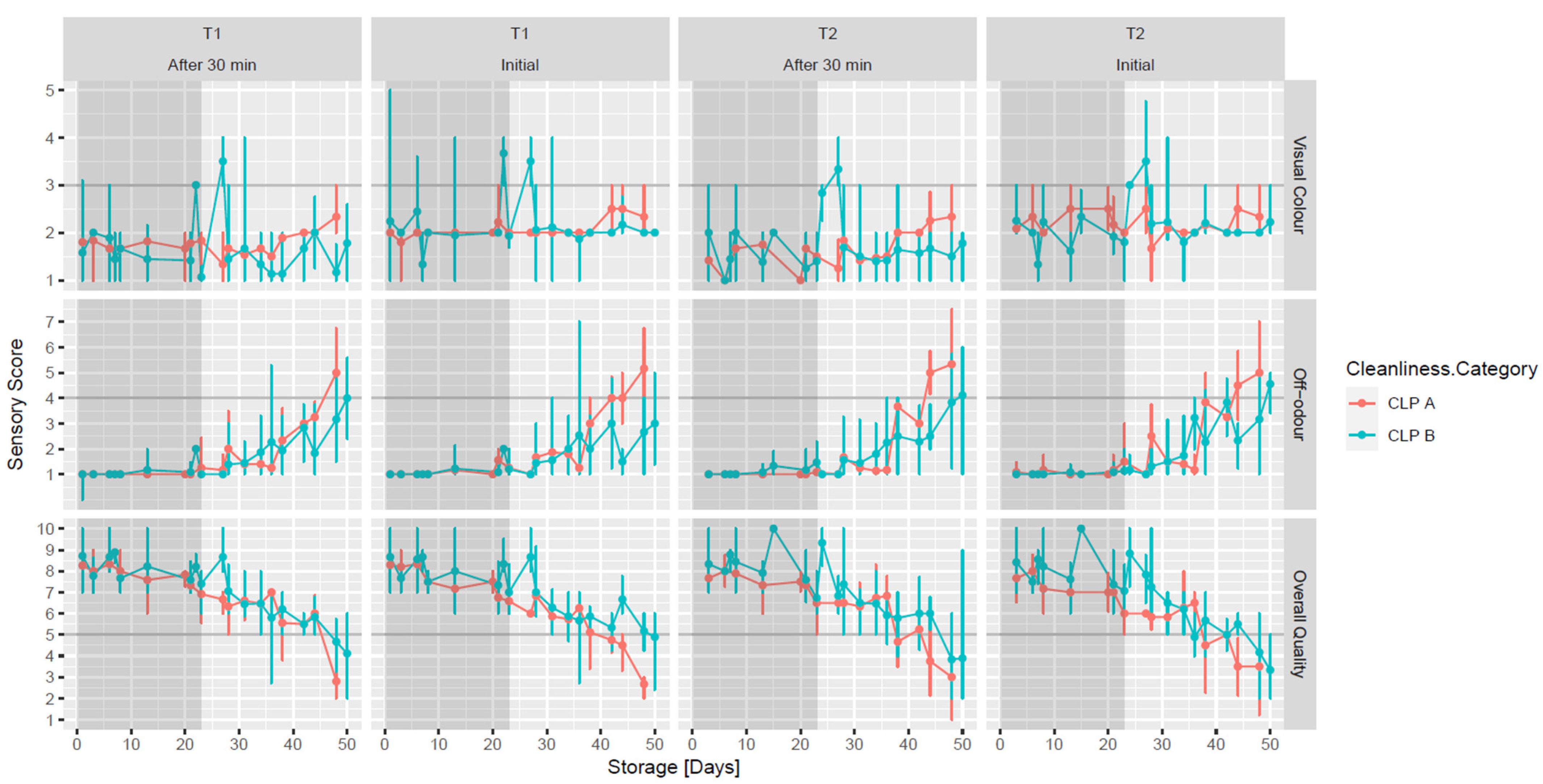
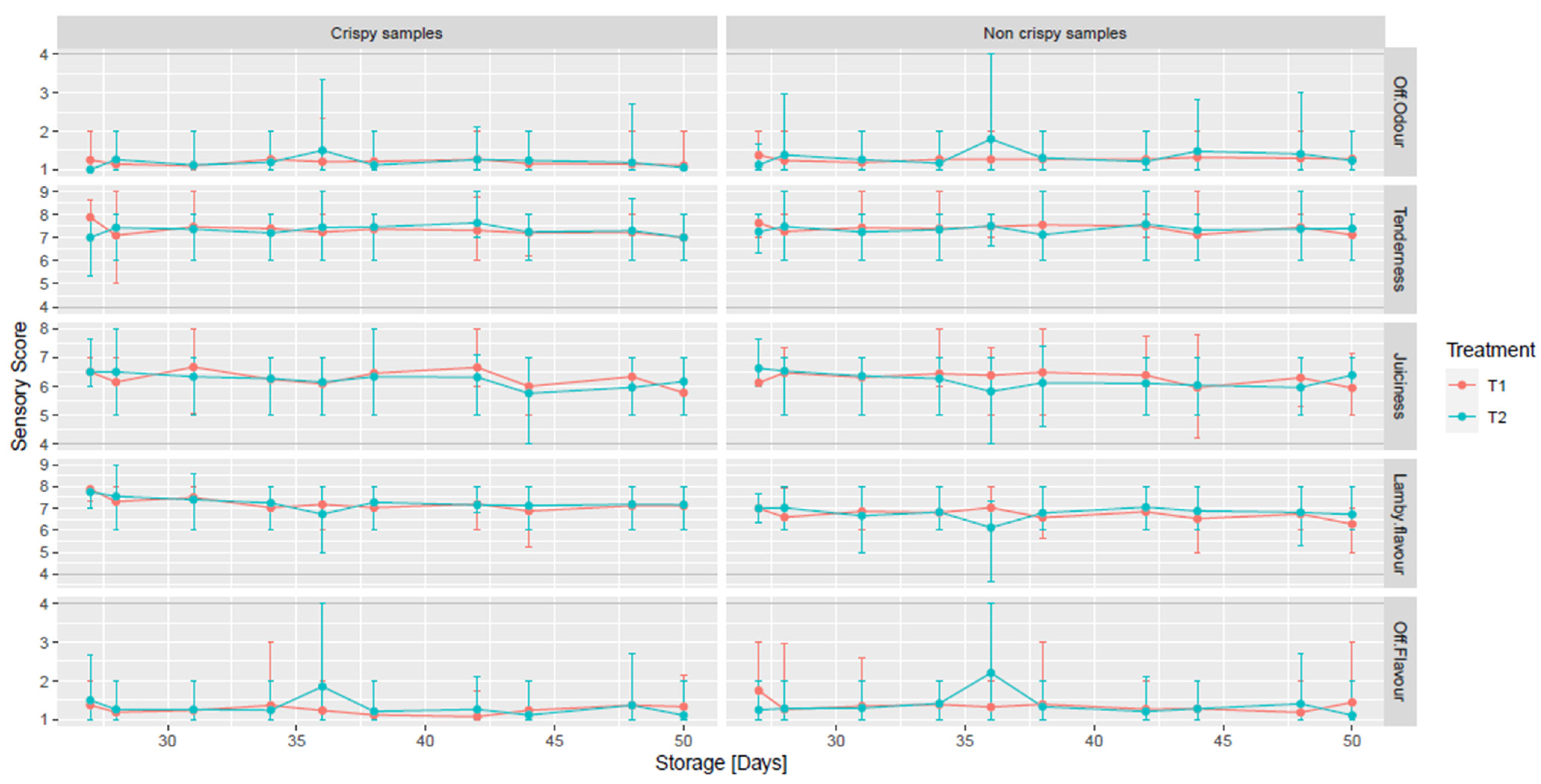
| Microorganisms | CLP Category | Clipping (Abattoir 1) | No Clipping (Abattoir 2) | Clipping V No Clipping (*) |
|---|---|---|---|---|
| 1 TVC-Mesophilic | A | 4.96 ± 0.09 y | 5.78 ± 0.12 a | * |
| B | 4.16 ± 0.07 x | 5.97 ± 0.13 a | * | |
| 1 TVC-Psychrophilic | A | 4.60 ± 0.09 y | 5.54 ± 0.12 a | * |
| B | 3.77 ± 0.07 x | 5.77 ± 0.13 a | * | |
| Lactic acid bacteria | A | 3.50 ± 0.09 y | 3.33 ± 0.13 a | |
| B | 1.97 ± 0.07 x | 3.36 ± 0.12 a | * | |
| Br. thermosphacta | A | 1.55 ± 0.09 y | 0.83 ± 0.12 a | * |
| B | 1.13 ± 0.07 x | 2.66 ± 0.13 b | * | |
| Enterobacteriaceae | A | 1.78 ± 0.09 y | 1.18 ± 0.12 a | |
| B | −0.01 ± 0.07 x | 1.97 ± 0.13 b | * | |
| Pseudomonads | A | 2.29 ± 0.09 y | 2.49 ± 0.12 a | * |
| B | 0.88 ± 0.07 x | 2.80 ± 0.13 a | * |
| Microorganisms | CLP Category | Clipping (Abattoir 1) | No Clipping (Abattoir 2) |
|---|---|---|---|
| 1 TVC-Mesophilic | A | 4.49 ± 0.61 a | TNTC 2 |
| B | 4.60 ± 0.43 a | TNTC | |
| 1 TVC-Psychrophilic | A | 4.27 ± 0.43 a | TNTC |
| B | 4.43 ± 0.30 a | TNTC | |
| Lactic acid bacteria | A | 3.37 ± 0.35 a | TNTC |
| B | 3.80 ± 0.43 a | TNTC | |
| Br. thermosphacta | A | 3.03 ± 0.30 a | TNTC |
| B | 3.67 ± 0.43 a | TNTC | |
| Enterobacteriaceae | A | 2.20 ± 0.43 a | TNTC |
| B | 2.44 ± 0.43 a | TNTC | |
| Pseudomonads | A | 2.85 ± 0.43 a | TNTC |
| B | 2.93 ± 0.30 a | TNTC |
| Microorganisms | CLP Category | Pre-Chill T1 | Pre-Chill T2 | Post-Chill T1 | Post-Chill T2 |
|---|---|---|---|---|---|
| 1 TVC- Mesophilic | A | 2.54 ± 0.30 x | 2.54 ± 0.29 x | 2.46± 0.37 ax | 2.43 ± 0.38 ax |
| B | 2.75 ± 0.25 x | 2.68 ± 0.23 x | 2.37± 0.31 ax | 2.48 ± 0.31 ax | |
| 1 TVC-Psychrophilic | A | 2.07 ± 0.30 x | 2.03 ± 0.29 x | 1.92± 0.37 ax | 2.01 ± 0.38 ax |
| B | 2.23 ± 0.25 x | 2.22 ± 0.23 x | 1.92± 0.31 ax | 2.25 ± 0.31 bx | |
| Lactic acid bacteria | A | 0.95 ± 0.30 x | 0.93 ± 0.29 x | 1.05± 0.37 ax | 1.26 ± 0.38 ax |
| B | 1.26 ± 0.25 x | 0.94 ± 0.23 x | 0.86± 0.31 bx | 0.37 ± 0.31 ax | |
| Br. thermosphacta | A | 0.47 ± 0.30 x | 0.71 ± 0.29 x | 0.26± 0.37 ax | 0.13 ± 0.38 ax |
| B | −0.10 ± 0.25 x | −0.02 ± 0.23 x | −0.11 ± 0.31 ax | 0.51 ± 0.31 bx | |
| Enterobacteriaceae | A | 1.24 ± 0.30 y | 1.23 ± 0.29 y | 0.53 ± 0.37 ax | 0.41 ± 0.38 ax |
| B | 0.00 ± 0.25 x | −0.12 ± 0.23 x | −0.83 ± 0.31 bx | −1.04 ± 0.31 ax | |
| Pseudomonads | A | 1.40 ± 0.30 x | 1.49 ± 0.29 x | 1.14 ± 0.37 ax | 1.14 ± 0.38 ax |
| B | 0.63 ± 0.25 x | 0.44 ± 0.23 x | 0.22 ± 0.31 ax | 0.72 ± 0.31 bx |
| Microorganisms | CLP | Evisceration Area | Chill Room D0 | Chill Room D1 | Chill Room D3 | Boning Hall D1 | Boning Hall D3 |
|---|---|---|---|---|---|---|---|
| 1 TVC Mesophilic | A | 4.48 ± 0.28 x | 3.08 ± 0.23 b | 2.35 ± 0.23 a | 3.03± 0.23 ab | 3.25 ± 0.28 r | 3.48 ± 0.28 r |
| B | 5.02 ± 0.25 x | 3.33 ± 0.19 a | 3.69 ± 0.19 a | 3.15 ± 0.23 a | 3.58 ± 0.24 r | 3.70 ± 0.25 r | |
| 1 TVC Psychrophilic | A | 3.98 ± 0.24 x | 2.87 ± 0.23 a | 2.57 ± 0.23 a | 2.90 ± 0.23 a | 2.64 ± 0.28 r | 3.02 ± 0.28 r |
| B | 4.64 ± 0.20 y | 3.17 ± 0.19 a | 3.03 ± 0.19 a | 2.84 ± 0.23 a | 3.09 ± 0.23 r | 3.00 ± 0.25 r | |
| Lactic acid bacteria | A | 3.58 ± 0.24 x | 2.60 ± 0.23 a | 2.44 ± 0.23 a | 2.50 ± 0.23 a | 2.59 ± 0.28 r | 2.69 ± 0.28 r |
| B | 3.63 ± 0.20 x | 2.56 ± 0.19 a | 2.48 ± 0.19 a | 2.60 ± 0.23 a | 3.49 ± 0.23 s | 2.66 ± 0.25 r | |
| Br. thermosphacta | A | 2.57 ± 0.24 x | 2.20 ± 0.23 a | 2.20 ± 0.23 a | 2.20 ± 0.23 a | 2.20 ± 0.28 r | 2.27 ± 0.28 r |
| B | 2.50 ± 0.20 x | 2.20 ± 0.19 a | 2.20 ± 0.19 a | 2.18 ± 0.23 a | 2.53 ± 0.23 r | 2.43 ± 0.25 r | |
| Enterobacteriaceae | A | 3.33 ± 0.24 x | 2.20 ± 0.23 a | 2.20 ± 0.23 a | 2.20 ± 0.23 a | 2.20 ± 0.28 r | 2.20 ± 0.28 r |
| B | 2.85 ± 0.20 x | 2.20 ± 0.19 a | 2.20 ± 0.19 a | 2.18 ± 0.23 a | 2.87 ± 0.23 s | 2.43 ± 0.25 r | |
| Pseudomonads | A | 3.09 ± 0.24 x | 2.20 ± 0.23 a | 2.50 ± 0.23 a | 2.54 ± 0.23 a | 2.20 ± 0.28 r | 2.57 ± 0.28 r |
| B | 3.18 ± 0.20 x | 2.44 ± 0.19 a | 2.20 ± 0.19 a | 2.18 ± 0.23 a | 2.25 ± 0.23 r | 2.51 ± 0.25 r |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Alba, M.; Burgess, C.M.; Pollard, K.; Perussello, C.; Frías-Celayeta, J.M.; Walsh, D.; Carroll, J.; Crofton, E.; Griffin, C.; Botinestean, C.; et al. Impact of Industrial Practices on the Microbial and Quality Attributes of Fresh Vacuum-Packed Lamb Joints. Foods 2022, 11, 1850. https://doi.org/10.3390/foods11131850
de Alba M, Burgess CM, Pollard K, Perussello C, Frías-Celayeta JM, Walsh D, Carroll J, Crofton E, Griffin C, Botinestean C, et al. Impact of Industrial Practices on the Microbial and Quality Attributes of Fresh Vacuum-Packed Lamb Joints. Foods. 2022; 11(13):1850. https://doi.org/10.3390/foods11131850
Chicago/Turabian Stylede Alba, María, Catherine M. Burgess, Katie Pollard, Camila Perussello, Jesús M. Frías-Celayeta, Des Walsh, Joan Carroll, Emily Crofton, Carol Griffin, Cristina Botinestean, and et al. 2022. "Impact of Industrial Practices on the Microbial and Quality Attributes of Fresh Vacuum-Packed Lamb Joints" Foods 11, no. 13: 1850. https://doi.org/10.3390/foods11131850
APA Stylede Alba, M., Burgess, C. M., Pollard, K., Perussello, C., Frías-Celayeta, J. M., Walsh, D., Carroll, J., Crofton, E., Griffin, C., Botinestean, C., & Duffy, G. (2022). Impact of Industrial Practices on the Microbial and Quality Attributes of Fresh Vacuum-Packed Lamb Joints. Foods, 11(13), 1850. https://doi.org/10.3390/foods11131850







