Combination of Chitosan, Tea Polyphenols, and Nisin on the Bacterial Inhibition and Quality Maintenance of Plant-Based Meat
Abstract
1. Introduction
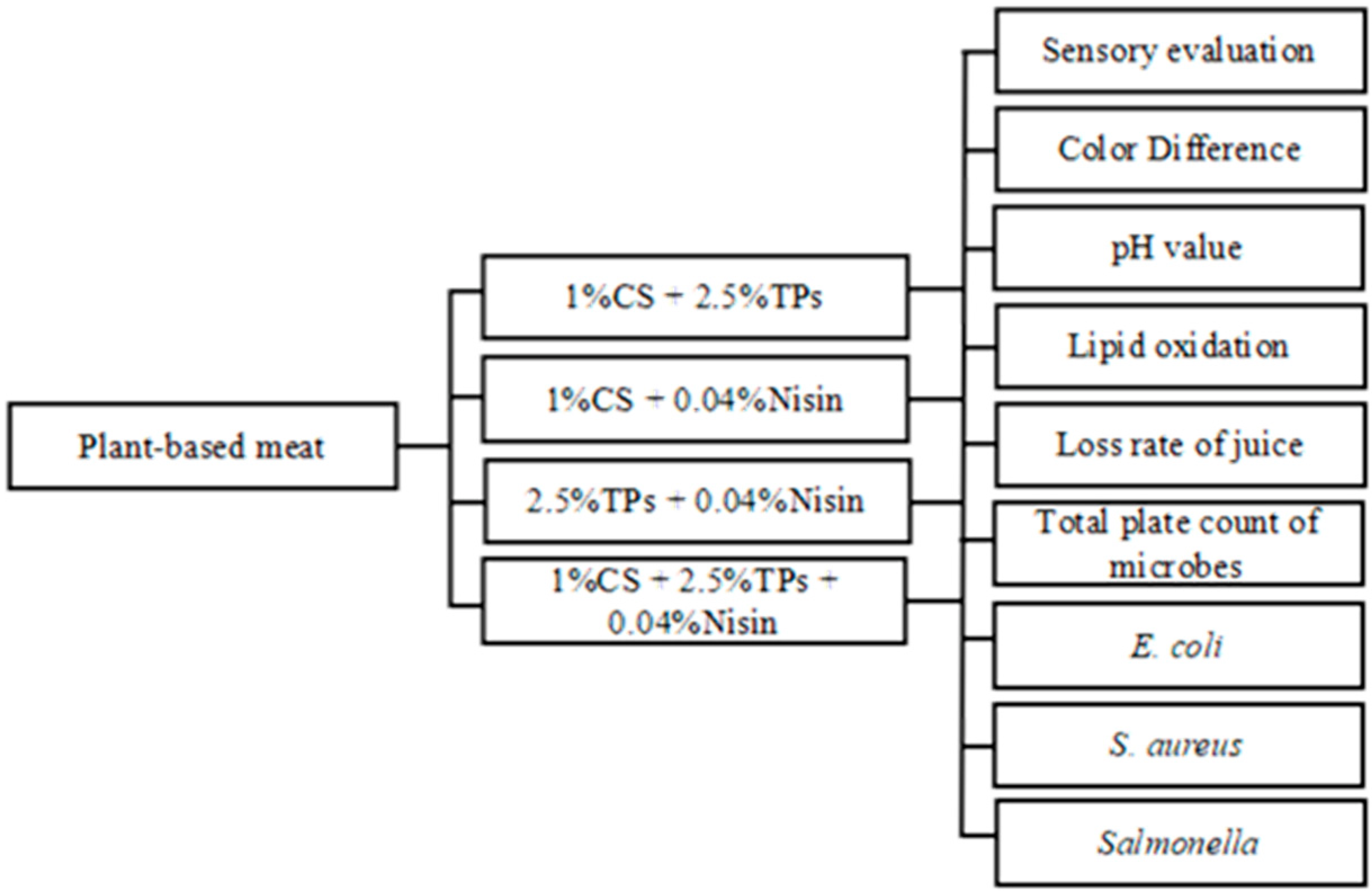
2. Materials and Methods
2.1. Materials
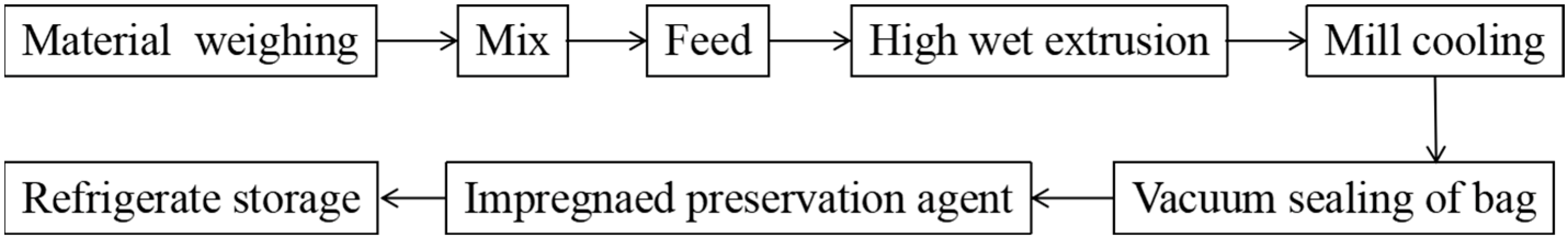
2.2. Plant-Based Meat Production and Treatment with Preservatives
2.3. Sensory Evaluation
2.4. Determination of Color Difference
2.5. Determination of pH Value
2.6. Determination of Lipid Oxidation
2.7. Loss Rate of Juice
2.8. Microbial Analysis
- (1)
- A total of 1 mL of the suspension was injected onto an agar plate medium. The inoculated plates were placed in an incubator at 37 °C. After one day of incubation, the total viable counts were measured.
- (2)
- A total of 1 mL of the suspension was injected onto VRBA. The inoculated plates were placed in an incubator at 37 °C. From the VRBA medium, a plate with 15–150 suspected E. coli colonies was selected after a day of incubation. Ten colonies were picked into ten tubes with BGLB medium from the plate to observe gas production and record the number of gas-producing tubes. The proportion of gas-producing tubes was multiplied by the number of counted plate colonies, and the dilution was the number of coliform colonies per gram sample.
- (3)
- To determine S. aureus, the bacterial suspension was prepared from the plant-based meat and 1 mL of the suspension was injected onto chromogenic S. aureus agar and cultured for 24 h before the number of colonies in red was recorded.
- (4)
- Similarly, for Salmonella, the plant-based meat broth suspension was pre-enriched and enriched, seeded from the RVS broth onto chromogenic Salmonella media, and cultured for 24 h before it was observed whether red colonies were produced.
2.9. Statistical Analysis
3. Results and Discussion
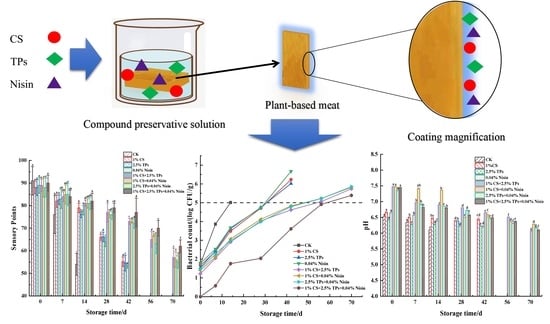
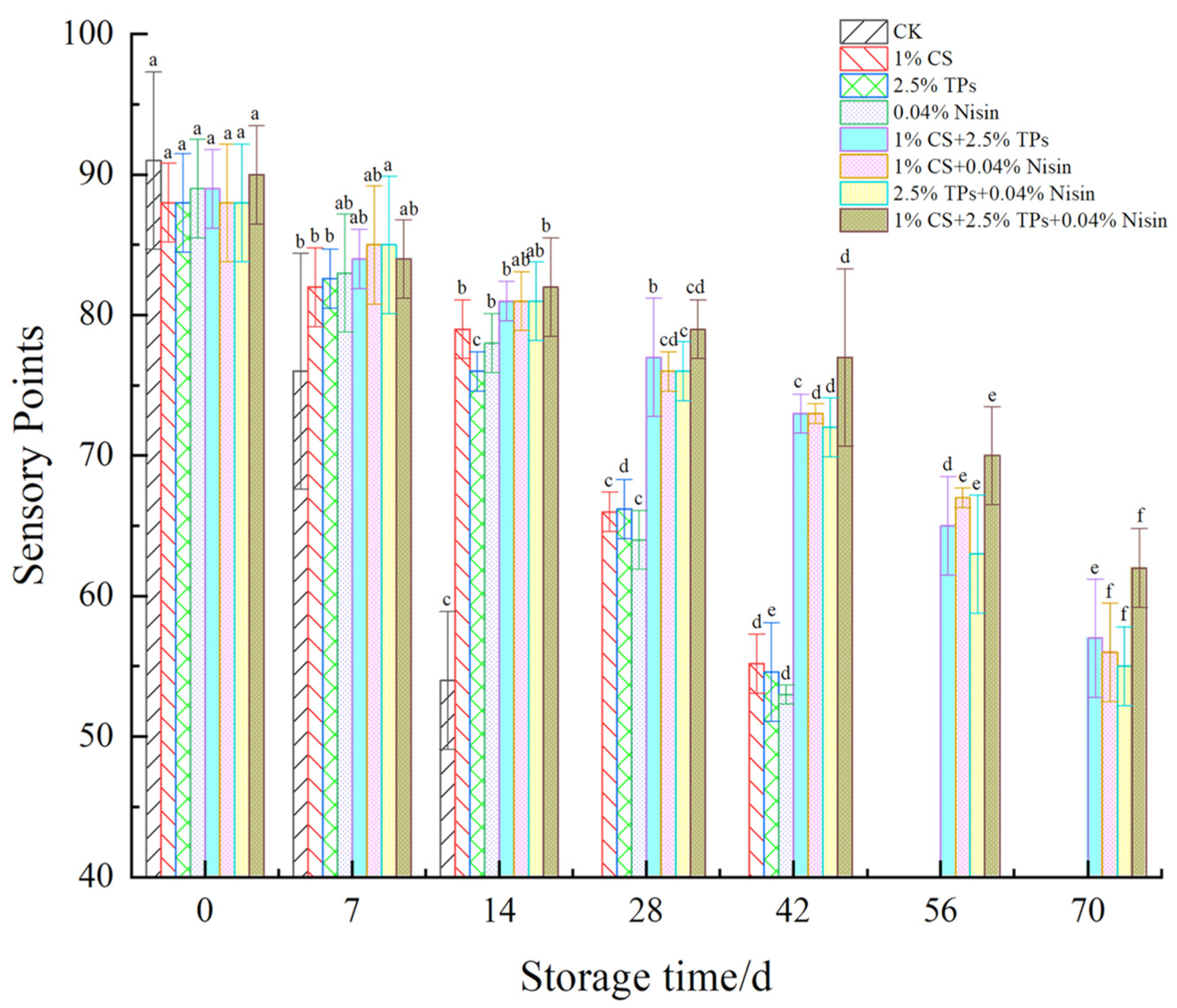
3.1. Sensory Evaluation
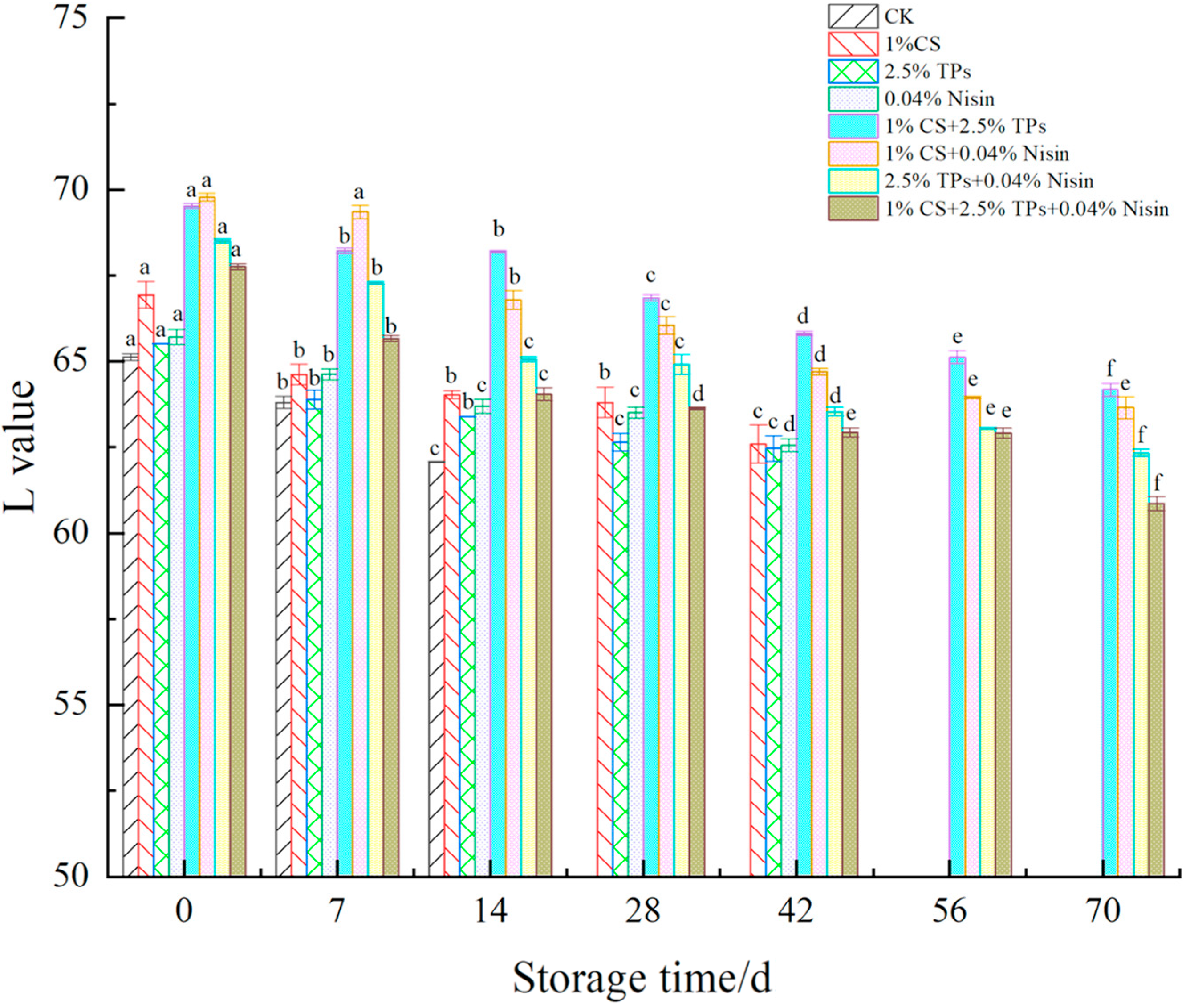
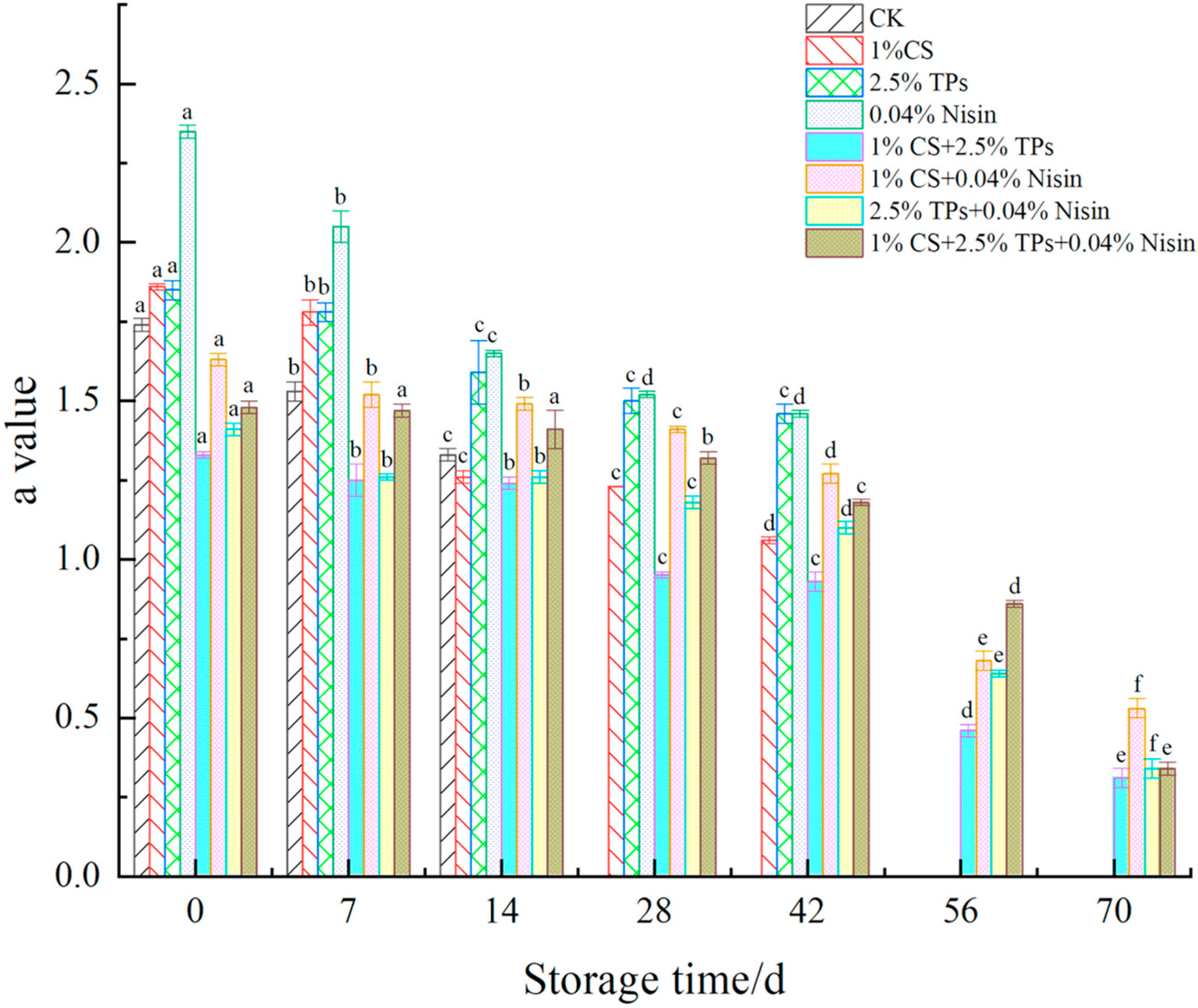
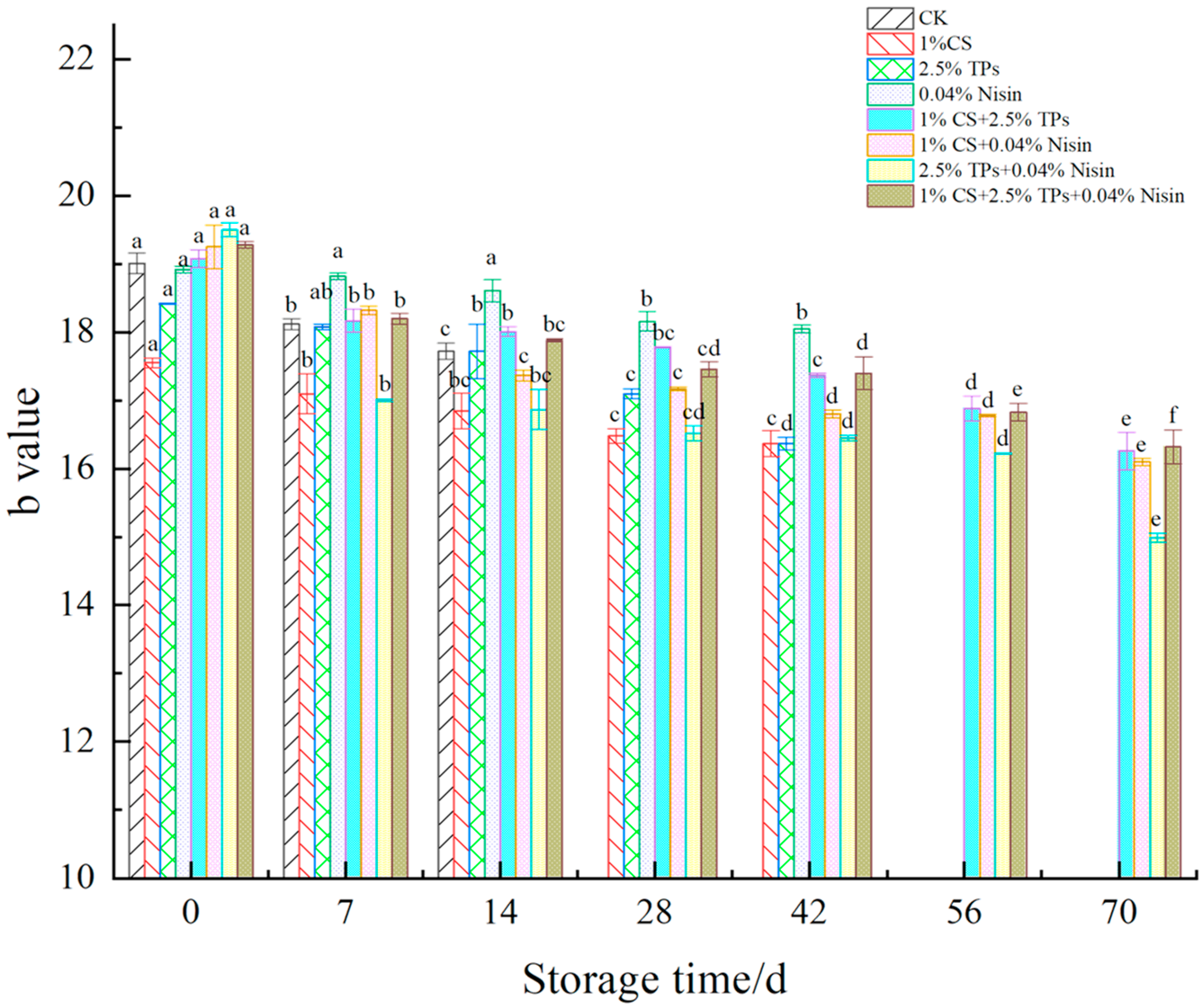
3.2. Color Difference
3.3. pH Value
3.4. Lipid Oxidation
3.5. Loss Rate of Juice
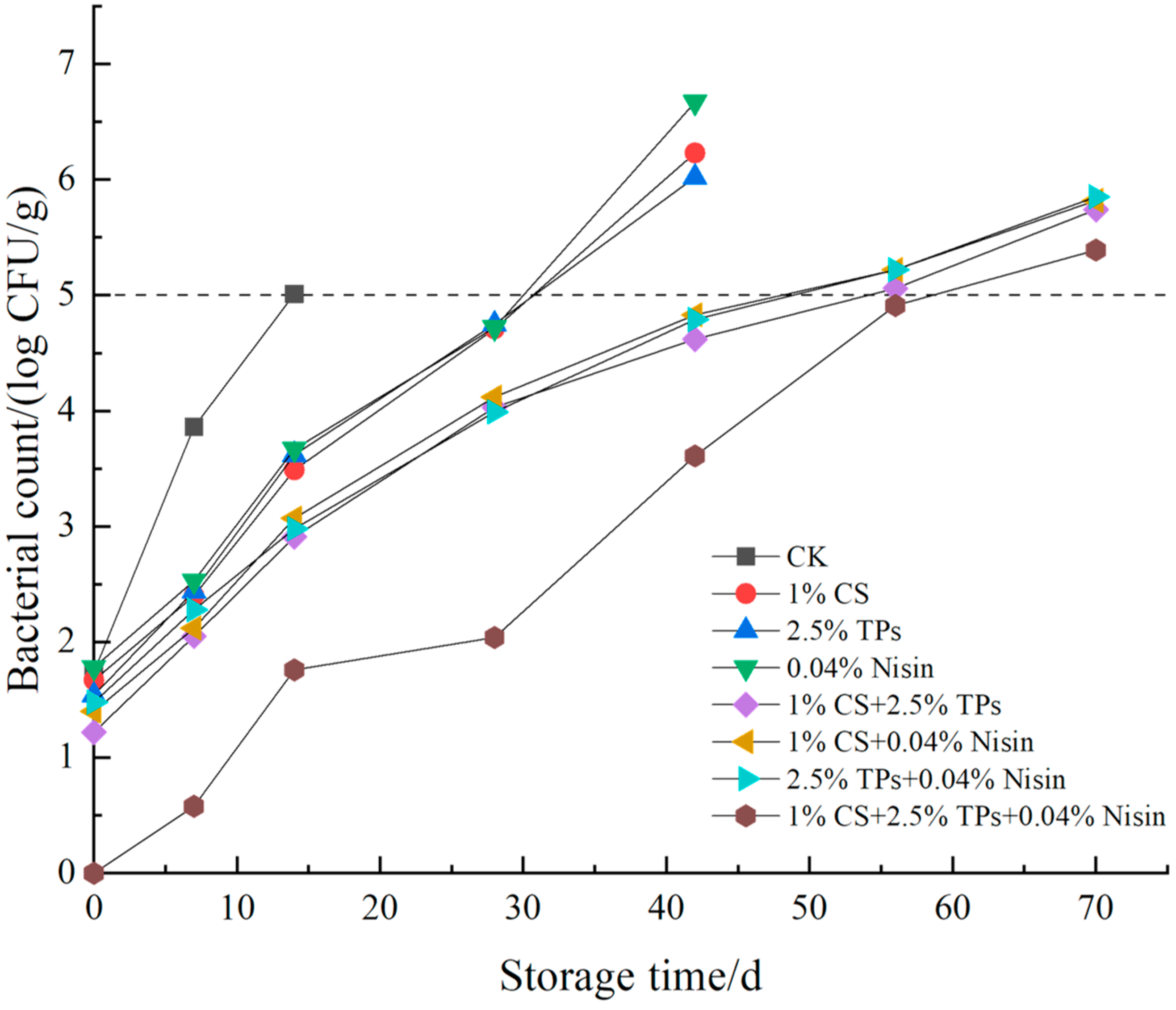
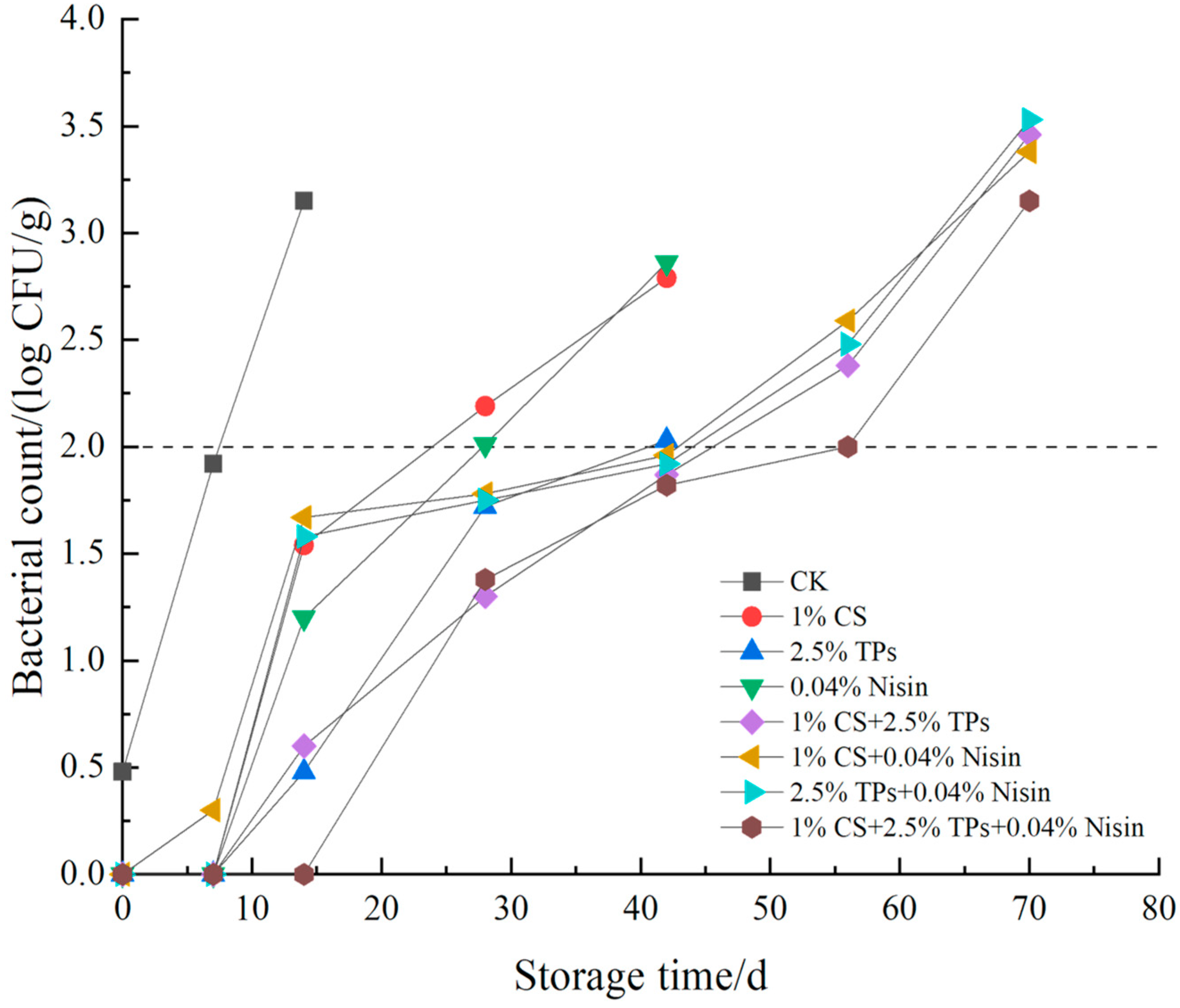
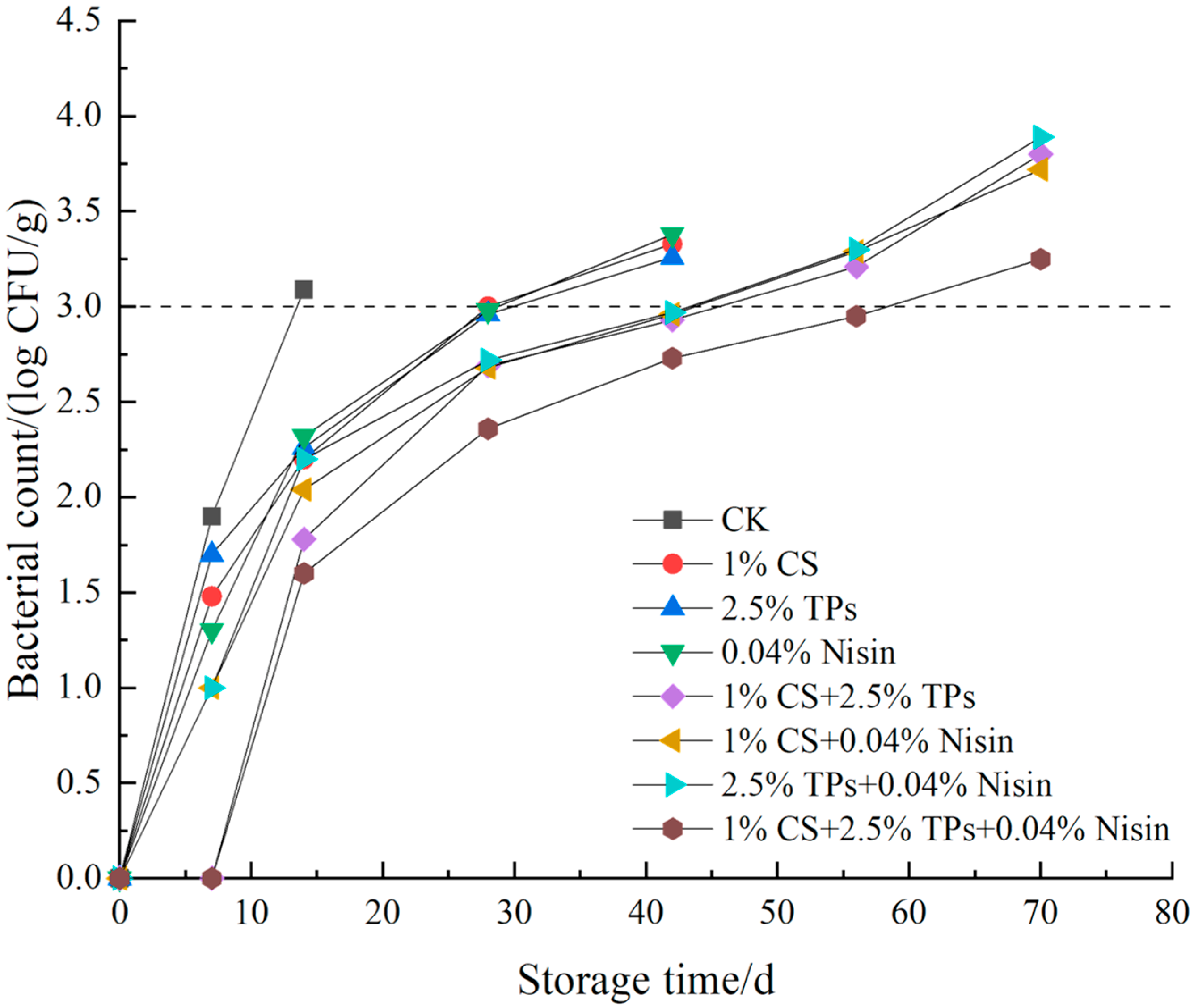
3.6. Microbial Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Evans, N.M.; Liu, H.Z.; Shao, S.Q. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Malav, O.P.; Talukder, S.; Gokulakrishnan, P.; Chand, S. Meat analog: A review. Crit. Rev. Food Sci. 2015, 55, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Ismil, I.; Hwang, Y.H.; Joo, S.T. Meat Analog as future food: A Review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef]
- Jie, H.C.; Simon, M.L.; Allan, K.H.; Michael, E.P. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. Neth. 2019, 19, 100102. [Google Scholar]
- Joshi, V.; Kumar, S. Meat Analogues: Plant based alternatives to meat products—A review. Int. J. Food Ferment. Technol. 2015, 5, 107. [Google Scholar] [CrossRef]
- Manski, J.M.; van Riemsdijk, L.E.; van der Goot, A.J.; Boom, R.M. Importance of intrinsic properties of dense caseinate dispersions for structure formation. Biomacromolecules 2007, 8, 3540. [Google Scholar] [CrossRef]
- Dick, A.; Bhandari, B.; Prakash, S. 3D Printing of Meat. Meat Sci. 2019, 153, 35–44. [Google Scholar] [CrossRef]
- Corvan, D.W.; Peter, F.; Atze, J.V. Meat alternatives: An integrative comparison. Trends Food Sci. Technol. 2019, 88, 505–512. [Google Scholar]
- Arora, B.; Kamal, S.; Sharma, V.P. Effect of binding agents on quality characteristics of mushroom based sausage analogue. J. Food Process. Pres. 2017, 41, e13134. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.; Hsieh, F. Extrusion process parameters, sensory characteristics, and structural properties of a high moisture soy protein meat analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Gadea, D.J.; Atze, J.G.; Stankiewicz, A.I.; Stefanidis, G.D. On the use of the couette cell technology for large scale production of textured soy-based meat replacers. J. Food Eng. 2016, 169, 205–213. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Gobel, J.; Atze, J.G.; Jan, V.D.G.A.; Stefanidis, G.D. Production of structured soy-based meat analogues using simple shear and heat in a couette cell. J. Food Eng. 2015, 160, 34–41. [Google Scholar] [CrossRef]
- Elzerman, J.E.; Keulemans, L.; Sap, R.; Luninget, P.A. Situational appropriateness of meat products, meat substitutes and meat alternatives as perceived by Dutch consumers. Food Qual. Prefer. 2020, 88, 104108. [Google Scholar] [CrossRef]
- Michel, F.; Hartmann, C.; Siegrist, M. Consumers’ associations, perceptions and acceptance of meat and plant-based meat alternatives. Food Qual. Prefer. 2020, 87, 104063. [Google Scholar] [CrossRef]
- Loo, E.; Caputo, V.; Lusk, J.L. Consumer preferences for farm-raised meat, lab-grown meat, and plant-based meat alternatives: Does information or brand matter. Food Policy 2020, 95, 101931. [Google Scholar]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Choudhury, D.; Singh, S.; Si, J.; Han, S.; Tan, L.P. Commercialization of plant-based meat alternatives. Trends Plant Sci. 2020, 25, 1055–1058. [Google Scholar] [CrossRef]
- Geeraerts, W.; Vuyst, L.D.; Leroy, F. Ready-to-eat meat alternatives, a study of their associated bacterial communities. Food Biosci. 2020, 37, 100681. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of alternative proteins: Technological, environmental and regulatory aspects of cultured meat, plant-based meat, insect protein and single-cell protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.J.; Dunay, A.; Battay, M.; Illés, C.B.; Bittsánszky, A.; Süth, M. Microbial Spoilage of Plant-Based Meat Analogues. Appl. Sci. 2021, 11, 8309. [Google Scholar] [CrossRef]
- Sun, C.X.; Ge, J.; He, J.; Gan, R.Y.; Fang, Y.P. Processing, Quality, Safety, and Acceptance of Meat Analogue Products. Engineering 2021, 5, 674–678. [Google Scholar] [CrossRef]
- Charis, M.G. Sustainable Meat Production and Processing, 1st ed.; Andre Gerhard Wolff: London, UK, 2019; pp. 112–113. [Google Scholar]
- Hu, Z.; Gänzle, M.G. Challenges and opportunities related to the use of chitosan as a food preservative. J. Appl. Microbiol. 2019, 126, 1318–1331. [Google Scholar] [CrossRef]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial coatings based on chitosan for pharmaceutical and biomedical applications. Curr. Pharm. Design 2018, 24, 866–885. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and antimicrobial properties of chitosan-nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef]
- Gedarawatte, S.; Ravensdale, J.T.; Johns, M.L.; Aziziet, A.; Coorey, R. Effectiveness of gelatine and chitosan spray coating for extending shelf life of vacuum-packaged beef. Int. J. Food Sci. Technol. 2021, 56, 4026–4037. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Ding, W. Eugenol nanocapsules embedded with gelatin-chitosan for chilled pork preservation. Int. J. Biol. Macromol. 2020, 158, 837–844. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation of tofu using chitosan as a coagulant for improved shelf-life. Int. J. Food Sci. Technol. 2004, 39, 133–141. [Google Scholar] [CrossRef]
- Bora, A.; Ma, S.; Li, X.; Lu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2017, 105, 241–249. [Google Scholar] [CrossRef]
- Yang, A.; Cheng, F.; Tong, P.; Chen, H. Effect of tea polyphenol and nisin on the quality of tortoise (Trachemys scripta elegans) meat during chilled storage. J. Food Process. Pres. 2017, 41, e13308. [Google Scholar] [CrossRef]
- Ju, J.; Liao, L.; Qiao, Y.; Xiong, G.; Li, D.; Wang, C.; Hu, J.; Wang, L.; Wu, W.; Ding, A. The effects of vacuum package combined with tea polyphenols (V+TP) treatment on quality enhancement of weever (Micropterus salmoides) stored at 0 °C and 4 °C. LWT 2018, 91, 484–490. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ahmed, S.; Qin, W.; Liu, Y. Effects of various antimicrobial polyvinyl alcohol/tea polyphenol composite films on the shelf life of packaged strawberries. LWT 2019, 113, 108297. [Google Scholar] [CrossRef]
- Lu, T.M.; Lee, C.C.; Mau, J.L.; Lin, S.D. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010, 119, 1090–1095. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox. Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Sousa, R.; Otoni, C.G.; Moraes, A.; Souza, V.; Medeiros, E. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Cutter, C.N. Development and evaluation of pullulan-based composite antimicrobial films (CAF) incorporated with nisin, thymol and lauric arginate to reduce foodborne pathogens associated with muscle foods. Int. J. Food Microbiol. 2020, 320, 108519. [Google Scholar] [CrossRef]
- Penna, T.; Jozala, A.F.; Letícia, C.D.; Pessoa, A.; Cholewa, O. Production of nisin by Lactococcus lactis in media with skimmed milk. Appl. Biochem. Biotech. 2005, 122, 619–637. [Google Scholar] [CrossRef]
- Tang, H.; Darwish, W.S.; Jinkgui, M. Microbial quality and formation of biogenic amines in the meat and edible offal of Camelus dromedaries with a protection trial using gingerol and nisin. Food Sci. Nutr. 2020, 8, 2094–2101. [Google Scholar] [CrossRef]
- Verdi, M.C.; Melian, C.; Castellano, P.; Vignolo, G.; Massani, M.B. Synergistic antimicrobial effect of lactocin al705 and nisin combined with organic acid salts against Listeria innocua 7 in broth and a hard cheese. Int. J. Food Sci. Technol. 2020, 55, 267–275. [Google Scholar] [CrossRef]
- Sun, J.; Hui, X.Z.; Guo, J.; Dong, G.X. The bacteriostasis study of nisin for the raspberry health draft beer. Phys. Procedia 2012, 25, 973–977. [Google Scholar] [CrossRef][Green Version]
- de Souza de Azevedo, P.O.; Azevedo, D.; Converti, A.; Gierus, M.; de Souza Oliveira, R.P. Application of nisin as biopreservative of pork meat by dipping and spraying methods. Braz. J. Microbiol. 2019, 50, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encap-sulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- He, L.; Zou, L.; Yang, Q.; Xia, J.; Zhou, K.; Zhu, Y. Antimicrobial activities of nisin, tea polyphenols, and chitosan and their combinations in chilled mutton. J. Food Sci. 2016, 81, M1466–M1471. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naeem, H.; Zayed, N.; Mansour, H.A. Effect of chitosan and lauric arginate edible coating on bacteriological quality, deterioration criteria, and sensory attributes of frozen stored chicken meat. LWT 2021, 150, 111928. [Google Scholar] [CrossRef]
- Meng, D.M.; Sun, X.Q.; Sun, S.N.; Li, W.J.; Lv, Y.J.; Fan, Z.C. The potential of antimicrobial peptide Hispidalin application in pork preservation during cold storage. J. Food Process. Preserv. 2020, 44, e14443. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Fan, X.; Jin, T.; Niemira, B.A. Influence of antimicrobial agents on the thermal sensitivity of foodborne pathogens: A review. J. Food Prot. 2019, 82, 628–644. [Google Scholar] [CrossRef]
- Hamad, A.; Ramadhan, M.B.; Dewi, D.Y.S.; Djalil, A.D.; Hartanti, D. Preservation potential of lemon basil essential oil on tofu: Development of a natural food preservative. Mater. Sci. Eng. 2020, 771, 012037. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Khan, I.; Nkufi, C.; Deog-Hwan, T. Development and evaluation of chitosan and its derivative for the shelf life extension of beef meat under refrigeration storage. Int. J. Food Sci. Technol. 2017, 52, 1111–1121. [Google Scholar] [CrossRef]
- Hernandez, M.D.; Lopez, M.B.; Alvarez, A.; Ferrandini, E.; Garcia, B.; Garrido, M.D. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 2009, 114, 237–245. [Google Scholar] [CrossRef]
- Selmi, S.; Sadok, S. The effect of natural antioxidant (Thymus vulgaris Linnaeus) on flesh quality of tuna (Thunnus thynnus (Linnaeus)) during chilled storage. Pan-Am. J. Aquat. Sci. 2008, 3, 36–45. [Google Scholar]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Chitosan coatings enriched with active shrimp waste for shrimp preservation. Food Control 2015, 54, 259–266. [Google Scholar] [CrossRef]
- Khalafalla, F.A.; Ali, F.; Hassan, A. Quality improvement and shelf-life extension of refrigerated Nile tilapia (Oreochromis niloticus) fillets using natural herbs. Beni-Suef. Univ. J. Basic Appl. Sci. 2015, 4, 33–40. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Sci. 2015, 110, 174–179. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, M.J.; Zhang, B.S.; Lei, C.G.; Meng, Y.Z. Combined effect of cinnamon essential oil-chitosan coating and modified atmosphere packaging on the quality of chilled meat. J. Zhejiang Univ. (Agric. Life Sci.) 2019, 45, 723–735. [Google Scholar]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef]
- Pellicer, K. Behavior of Listeria monocytogenes type1 355/98 (85) in meat emulsions as affected by temperature, pH, water activity, fat and microbial preservatives. Food Control 2011, 22, 1573–1581. [Google Scholar]
- Li, M.; Wang, H.; Sun, L.; Zhao, G.; Huang, X. Application of electronic nose for measuring total volatile basic nitrogen and total viable counts in packaged pork during refrigerated storage. J. Food Sci. 2016, 81, M906–M912. [Google Scholar] [CrossRef]
- Cao, Y.; Warner, R.D.; Fang, Z. Effect of chitosan/nisin/gallic acid coating on preservation of pork loin in high oxygen modified atmosphere packaging. Food Control 2019, 101, 9–16. [Google Scholar] [CrossRef]
| Score Metrics | Specific Rules | Scores |
|---|---|---|
| Color (20 points) | pale yellow, shiny, and mildew free | 13–20 points |
| pale yellow, with lost luster and mildew free | 7–12 points | |
| grayish or darker yellow with mildew | 0–6 points | |
| Form (35 points) | great elasticity, moderate hardness, with obvious meat fiber, no mucus | 27–35 points |
| good elasticity, too hard or too soft, without obvious meat fiber, no mucus | 14–16 points | |
| poor elasticity, unacceptable hardness, no meat fiber, mucus | 0–13 points | |
| Odor (35 points) | strong fragrance of specific products, no odor, no acidic taste | 27–35 points |
| fragrance of a specific product, no odor, no acidic taste | 14–16 points | |
| weak fiber, smelly, sour | 0–13 points | |
| Impurities (10 points) | no impurities visible to the naked eye | 10 points |
| Preservation Types | Storage Time/d | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 28 | 42 | 56 | 70 | |
| CK | 6.52 ± 0.00 a | 6.34 ± 0.00 b | 6.11 ± 0.00 c | ||||
| 1% CS | 6.67 ± 0.01 a | 6.51 ± 0.00 b | 6.48 ± 0.01 bc | 6.40 ± 0.00 d | 6.45 ± 0.06 cd | ||
| 2.5% TPs | 6.42 ± 0.00 a | 6.28 ± 0.00 c | 6.28 ± 0.00 c | 6.39 ± 0.00 b | 6.23 ± 0.00 d | - | |
| 0.04% Nisin | 6.65 ± 0.00 a | 6.57 ± 0.00 b | 6.38 ± 0.00 c | 6.22 ± 0.00 d | 6.21 ± 0.03 d | ||
| 1% CS + 2.5% TPs | 7.47 ± 0.01 a | 6.97 ± 0.00 b | 6.90 ± 0.00 b | 6.81 ± 0.00 c | 6.65 ± 0.00 d | 6.51 ± 0.00 e | 6.07 ± 0.00 f |
| 1% CS + 0.04% Nisin | 7.48 ± 0.00 a | 7.42 ± 0.03 ab | 7.36 ± 0.00 b | 6.50 ± 0.01 c | 6.59 ± 0.09 c | 6.37 ± 0.00 d | 6.29 ± 0.00 d |
| 2.5% TPs + 0.04% Nisin | 7.35 ± 0.01 a | 6.92 ± 0.02 b | 6.86 ± 0.00 c | 6.73 ± 0.01 d | 6.46 ± 0.00 e | 6.31 ± 0.01 f | 6.17 ± 0.01 g |
| 1% CS + 2.5% TPs + 0.04% Nisin | 7.45 ± 0.00 a | 6.82 ± 0.00 b | 6.80 ± 0.01 b | 6.58 ± 0.06 c | 6.49 ± 0.00 d | 6.37 ± 0.02 e | 6.10 ± 0.04 f |
| Preservation Types | Storage Time/d | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 28 | 42 | 56 | 70 | |
| CK | 0.000 ± 0.000 c | 0.232 ± 0.013 b | 0.268 ± 0.011 a | ||||
| 1% CS | 0.000 ± 0.000 e | 0.018 ± 0.000 d | 0.132 ± 0.000 c | 0.160 ± 0.001 b | 0.170 ± 0.000 a | ||
| 2.5% TPs | 0.102 ± 0.001 e | 0.126 ± 0.002 d | 0.243 ± 0.001 b | 0.498 ± 0.000 a | 0.208 ± 0.002 c | ||
| 0.04% Nisin | 0.098 ± 0.000 e | 0.122 ± 0.000 d | 0.265 ± 0.000 c | 0.324 ± 0.000 a | 0.322 ± 0.004 b | ||
| 1% CS + 2.5% TPs | 0.083 ± 0.029 e | 0.221 ± 0.045 d | 0.257 ± 0.011 cd | 0.290 ± 0.000 c | 0.490 ± 0.017 a | 0.367 ± 0.000 b | 0.319 ± 0.013 bc |
| 1% CS + 0.04% Nisin | 0.002 ± 0.004 d | 0.030 ± 0.046 cd | 0.126 ± 0.095 bc | 0.152 ± 0.013 b | 0.261 ± 0.027 a | 0.188 ± 0.006 ab | 0.188 ± 0.006 ab |
| 2.5% TPs + 0.04% Nisin | 0.000 ± 0.000 e | 0.019 ± 0.027 e | 0.126 ± 0.039 d | 0.243 ± 0.013 c | 0.352 ± 0.013 a | 0.327 ± 0.004 ab | 0.283 ± 0.013 bc |
| 1% CS + 2.5% TPs + 0.04% Nisin | 0.000 ± 0.000 d | 0.011 ± 0.010 d | 0.035 ± 0.013 d | 0.146 ± 0.019 c | 0.250 ± 0.025 b | 0.381 ± 0.006 a | 0.241 ± 0.025 b |
| Preservation Types | Storage Time/d | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 28 | 42 | 56 | 70 | |
| CK | - | 1.99 ± 0.77 b | 5.16 ± 1.47 a | ||||
| 1% CS | - | 0.38 ± 0.07 d | 1.78 ± 0.70 c | 2.71 ± 0.28 b | 3.47 ± 0.14 a | ||
| 2.5% TPs | - | 0.71 ± 0.07 d | 1.10 ± 0.21 c | 1.92 ± 0.84 b | 3.61 ± 0.56 a | ||
| 0.04% Nisin | - | 0.58 ± 0.21 d | 1.85 ± 0.42 c | 2.98 ± 0.14 b | 4.53 ± 0.49 a | ||
| 1% CS + 2.5% TPs | - | 0.04 ± 0.00 f | 0.51 ± 0.21 e | 1.14 ± 0.63 d | 2.73 ± 0.56 c | 3.26 ± 0.91 b | 5.55 ± 1.33 a |
| 1% CS + 0.04% Nisin | - | 0.23 ± 0.14 f | 0.66 ± 0.49 e | 1.43 ± 0.63 d | 2.98 ± 0.28 c | 3.65 ± 0.63 b | 5.93 ± 0.91 a |
| 2.5% TPs + 0.04% Nisin | - | 0.31 ± 0.21 f | 0.81 ± 0.42 e | 1.59 ± 0.70 d | 3.15 ± 0.84 c | 3.89 ± 0.56 b | 5.06 ± 0.91 a |
| 1% CS + 2.5% TPs + 0.04% Nisin | - | 0.02 ± 0.00 e | 0.30 ± 0.07 e | 0.97 ± 0.35 d | 1.48 ± 0.28 c | 2.83 ± 0.84 b | 3.97 ± 1.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Han, L.; Li, Z.; Gu, M.; Xiao, Z.; Lu, F. Combination of Chitosan, Tea Polyphenols, and Nisin on the Bacterial Inhibition and Quality Maintenance of Plant-Based Meat. Foods 2022, 11, 1524. https://doi.org/10.3390/foods11101524
Dai Z, Han L, Li Z, Gu M, Xiao Z, Lu F. Combination of Chitosan, Tea Polyphenols, and Nisin on the Bacterial Inhibition and Quality Maintenance of Plant-Based Meat. Foods. 2022; 11(10):1524. https://doi.org/10.3390/foods11101524
Chicago/Turabian StyleDai, Zenghui, Linna Han, Zhe Li, Mengqing Gu, Zhigang Xiao, and Fei Lu. 2022. "Combination of Chitosan, Tea Polyphenols, and Nisin on the Bacterial Inhibition and Quality Maintenance of Plant-Based Meat" Foods 11, no. 10: 1524. https://doi.org/10.3390/foods11101524
APA StyleDai, Z., Han, L., Li, Z., Gu, M., Xiao, Z., & Lu, F. (2022). Combination of Chitosan, Tea Polyphenols, and Nisin on the Bacterial Inhibition and Quality Maintenance of Plant-Based Meat. Foods, 11(10), 1524. https://doi.org/10.3390/foods11101524