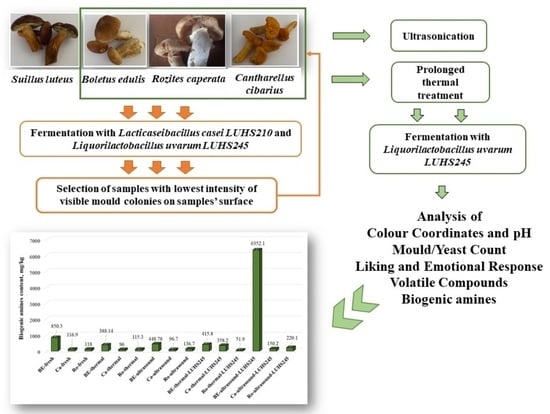
Biopreservation of Wild Edible Mushrooms (Boletus edulis, Cantharellus, and Rozites caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Edible Mushrooms and LAB Strains Used for Fermentation
2.2. Analysis of the Mushrooms’ Colour Characteristics, pH, and Mould/Yeast Count
2.3. Determination of the Mushrooms’ Volatile Compounds (VC)
2.4. Evaluation of the Overall Acceptability and Emotions Induced in Consumers by the Edible Mushrooms
2.5. Analysis of Biogenic Amine (BA) Content in Fermented and Untreated Edible Mushrooms
2.6. Statistical Analysis
3. Results and Discussion
3.1. Selection of the Most Appropriate LAB Strain for Edible Mushroom Fermentation
3.2. Colour Characteristics, pH, and Mould and Yeast Count in Edible Mushrooms
3.3. VC Profile of Mushrooms
3.4. Overall Acceptability and Emotions Induced in Consumers by the Edible Mushrooms
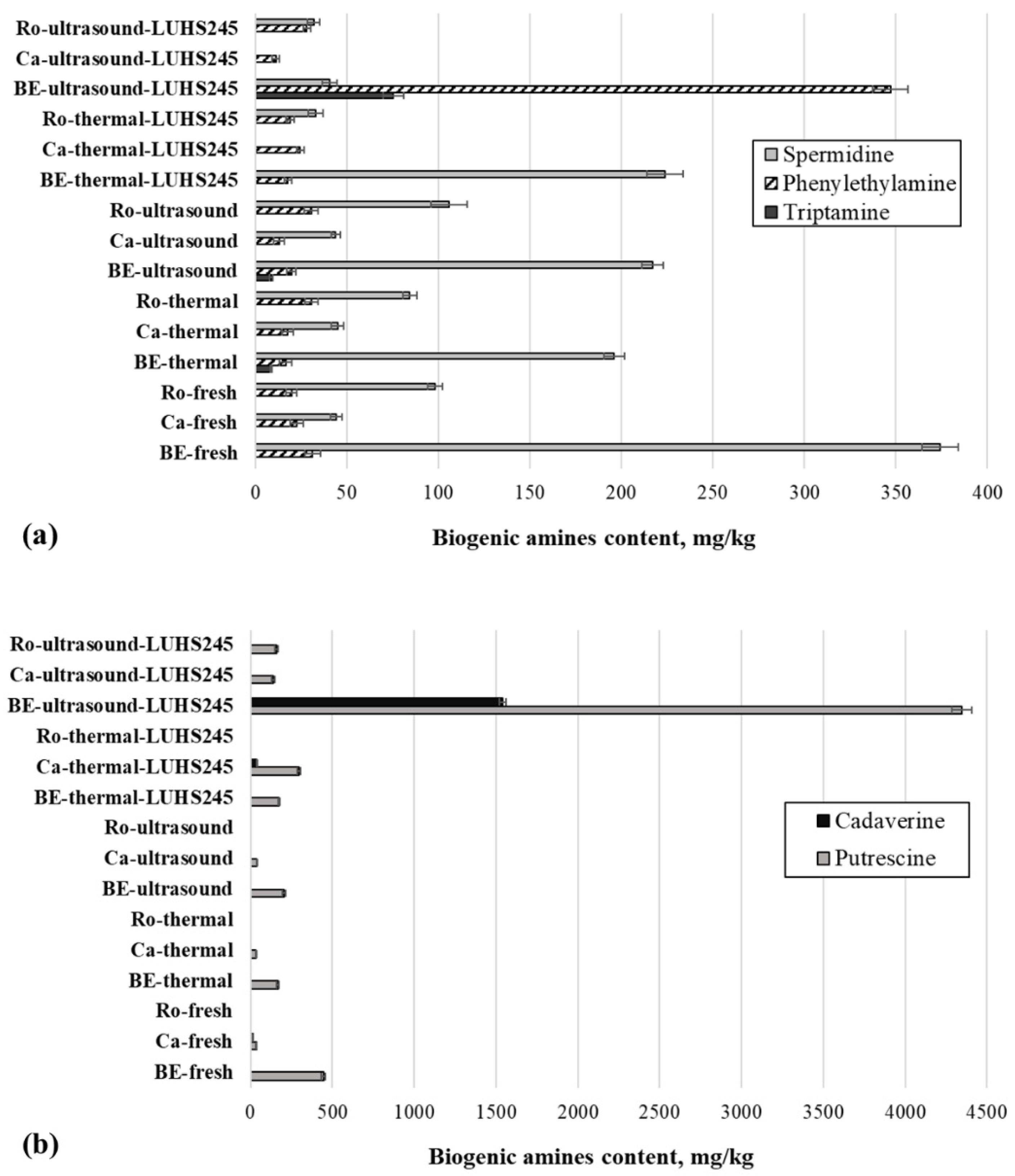
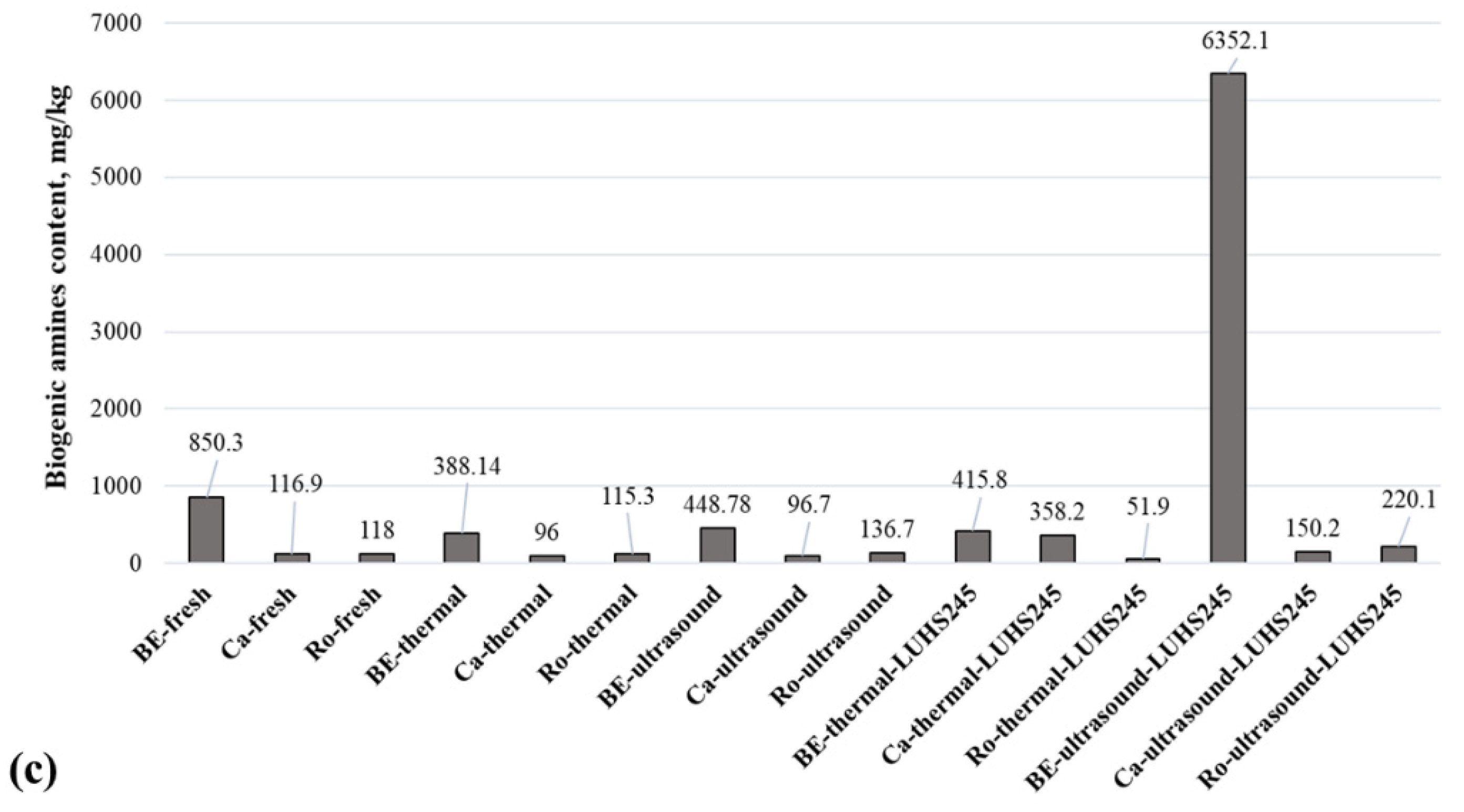
3.5. BA Content in Edible Mushrooms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Review article edible mushrooms: Improving human health and promoting edible mushrooms: Improving human health and promoting. Int. J. Microbiol. 2015, 99, 100. [Google Scholar]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible mushrooms: Role in the prevention of cardiovascular diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Painuli, S.; Semwal, P.; Egbuna, C. Mushroom: Nutraceutical, mineral, proximate constituents and bioactive component. In Functional Foods and Nutraceuticals; Egbuna, C., Dable Tupas, G., Eds.; Springer: Cham, Switzerland, 2020; pp. 307–336. [Google Scholar]
- Zhang, K.; Pu, Y.; Sun, D. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Skrzypczak, K.; Sławińska, A.; Radzki, W.; Gustaw, W. Lactic acid fermentation of edible mush-rooms: Tradition, technology, current state of research: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 655–669. [Google Scholar] [CrossRef]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Starkute, V.; Zadeike, D.; Juodeikiene, G. The nutritional and safety challenges associated with lupin lacto-fermentation. Front. Plant Sci. 2016, 7, 951. [Google Scholar] [CrossRef]
- Rhee, S.J.; Lee, J.; Lee, C. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell. Fact. 2011, 10, S5. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Fermentations in world food processing. Compr. Rev. Food Sci. Food Saf. 2002, 1, 23–32. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Dabadé, D.S.; Jacxsens, L.; Miclotte, L.; Abatih, E.; Devlieghere, F.; De Meulenaer, B. Survey of multiple biogenic amines and correlation to microbiological quality and free amino acids in foods. Food Control 2021, 120, 107497. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Grootveld, M.; Percival, B.C.; Zhang, J. Extensive chemometric investigations of distinctive patterns and levels of 690 biogenic amines in fermented foods: Human health implications. Foods 2020, 9, 1807. [Google Scholar] [CrossRef] [PubMed]
- Özogul, F.; Hamed, I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Aytar, E.C.; Akata, İ.; Açık, L. Antioxidant, antimicrobial and anti-proliferative activity of Suillus luteus (L.). Ankara Ecz. Fak. Derg. 2020, 44, 373–387. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Fărcaș, A.C.; Tofană, M.; Semeniuc, C.A. Bioactive compounds and volatile profiles of five Transylvanian wild edible mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Mineral Composition of Three Popular Wild Mushrooms from Poland. Molecules 2020, 25, 3588. [Google Scholar] [CrossRef]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.; Naliwajko, S.K.; Moskwa, J.; Bielecka, J.; Grabia, M.; Borawska, M.; Socha, K. Mushrooms as potential therapeutic agents in the treatment of cancer: Evaluation of anti-glioma effects of Coprinus comatus, Cantharellus cibarius, Lycoperdon perlatum and Lactarius deliciosus extracts. Biomed. Pharmacother. 2021, 133, 111090. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mockus, E.; Mozuriene, E.; Klementaviciute, J.; Monstaviciute, E.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Cernauskas, D.; Klupsaite, D. The evaluation of dark chocolate-elicited emotions and their relation with physico chemical attributes of chocolate. Foods 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Ben-gigirey, B.; De Sousa, J.M.V.B.; Villa, T.G.; Barros-Velazquez, J. Chemical changes and visual appearance of albacore tuna as related to frozen storage. J. Food Sci. 1999, 64, 20–24. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Cernauskas, D.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I. Combination of Extrusion and Fermentation with Lactobacillus plantarum and L. uvarum Strains for Improving the Safety Characteristics of Wheat Bran. Toxins 2021, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, J.; Ström, K.; Roos, S.; Sjögren, J.; Schnürer, J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. Lett. 2003, 219, 129–135. [Google Scholar] [CrossRef]
- Castellanos-Reyes, K.; Villalobos-Carvajal, R.; Beldarrain-Iznaga, T. Fresh Mushroom Preservation Techniques. Foods 2021, 10, 2126. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Ryś, E.; Sławińska, A.; Radzki, W.; Gustaw, W. Evaluation of the potential use of probiotic strain Lactobacillus plantarum 299v in lactic fermentation of button mushroom fruiting bodies. Acta Sci. Pol. Technol. Aliment. 2016, 15, 399–407. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Xie, X.; Ibrahim, S.A.; Khaskheli, S.G.; Yang, H.; Wang, Y.; Huang, W. Characterization of Lactobacillus pentosus as a starter culture for the fermentation of edible oyster mushrooms (Pleurotus spp.). LWT-Food Sci. Technol. 2016, 68, 21–26. [Google Scholar] [CrossRef]
- Moosavi, M.H.; Khaneghah, A.M.; Javanmardi, F.; Hadidi, M.; Hadian, Z.; Jafarzadeh, S.; Huseyn, E.; Sant’Ana, A.S. A review of recent advances in the decontamination of mycotoxin and inactivation of fungi by ultrasound. Ultrason. Sonochem. 2021, 79, 105755. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.; Freitas, A.C.; Barros, L.; Ferreira, I.C.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, X.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Eissa, H.A.; Fouad, G.M.; Shouk, A.E.A. Effect of some thermal and chemical pre-treatments on smoked oyster mushroom quality. Int. J. Food Sci. Technol. 2009, 44, 251–261. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Malcata, F.X. Microbiological profile of maize and rye flours, and sourdough used for the manufacture of traditional Portuguese bread. Food Microbiol. 2012, 31, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Malcata, F.X. Behavior of the Complex Micro-Ecology in Maize and Rye Flour and Mother-Dough for B roa Throughout Storage. J. Food Qual. 2016, 39, 218–233. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. Microbial ecology dynamics in Portuguese broa sourdough. J. Food Qual. 2016, 39, 634–648. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Zadeike, D.; Juodeikiene, G. Application of antifungal lactobacilli in combination with coatings based on apple processing by-products as a bio-preservative in wheat bread production. J. Food Sci. Technol. 2019, 56, 2989–3000. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Mickiene, R.; Zadeike, D.; Juodeikiene, G.A. concept of mould spoilage prevention and acrylamide reduction in wheat bread: Application of lactobacilli in combination with a cranberry coating. Food Contr. 2018, 91, 284–293. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, S.Y.; Choi, H.; Kim, Y. Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing.). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef]
- Aisala, H.; Manninen, H.; Laaksonen, T.; Linderborg, K.M.; Myoda, T.; Hopia, A.; Sandell, M. Linking volatile and non-volatile compounds to sensory profiles and consumer liking of wild edible Nordic mushrooms. Food Chem. 2020, 304, 125403. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. TGSC Information System. Available online: http://www.thegoodscentscompany.com/search2.html (accessed on 18 December 2021).
- Zhuang, J.; Xiao, Q.; Feng, T.; Huang, Q.; Ho, C.; Song, S. Comparative flavor profile analysis of four different varieties of Boletus mushrooms by instrumental and sensory techniques. Food Res. Int. 2020, 136, 109485. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Sánchez-Rodríguez, L.; Figiel, A.; Szumny, A.; Grubor, M.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of oyster mushroom as affected by drying method. Dry. Technol. 2018, 36, 685–696. [Google Scholar] [CrossRef]
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the key aroma and phenolic compounds of champignon (Agaricus bisporus) and oyster (Pleurotus ostreatus) mushrooms after two cooking treatments as elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santaescolástica, C.; Carballo, J.; Fulladosa, E.; José, V.G.; Benedito, J.; Lorenzo, J.M. Application of temperature and ultrasound as corrective measures to decrease the adhesiveness in dry-cured ham. Influence on free amino acid and volatile compound profile. Food Res. Int. 2018, 114, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Bozok, F.; Zarifikhosroshahi, M.; Kafkas, E.; Taskin, H.; Buyukalaca, S. Comparison of volatile compounds of fresh Boletus edulis and B. pinophilus in Marmara region of Turkey. Not. Bot. Horti. Agrobot. Cluj Napoca 2015, 43, 192–195. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Y.; Zhang, L.; Shan, M.; Tang, Y.; Ding, A. Quantitative comparative analysis of the bio-active and toxic constituents of leaves and spikes of Schizonepeta tenuifolia at different harvesting times. Int. J. Mol. Sci. 2011, 12, 6635–6644. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Pogacic, T.; Weber, M.; Lortal, S. Production of flavor compounds by lactic acid bacteria in fermented foods. In Biotechnology of Lactic Acid Bacteria; Mozzi, F., Raya, R.R., Vignolo, G.M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 314–340. [Google Scholar]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, D.; Pu, D.; Zhang, Y.; Chen, H.; Sun, B.; Ren, F. Multivariate relationships among sensory attributes and volatile components in commercial dry porcini mushrooms (Boletus edulis). Food Res. Int. 2020, 133, 109112. [Google Scholar] [CrossRef]
- Low, J.Y.; Janin, N.; Traill, R.M.; Hort, J. The who, what, where, when, why and how of measuring emotional response to food. A systematic review. Food Qual. Prefer. 2022, 100, 104607. [Google Scholar] [CrossRef]
- Yang, Q.; Shen, Y.; Foster, T.; Hort, J. Measuring consumer emotional response and acceptance to sustainable food products. Food Res. Int. 2020, 131, 108992. [Google Scholar] [CrossRef]
- Tepsongkroh, B.; Jangchud, K.; Jangchud, A.; Chonpracha, P.; Ardoin, R.; Prinyawiwatkul, W. Consumer perception of extruded snacks containing brown rice and dried mushroom. Int. J. Food Sci. Technol. 2020, 55, 46–54. [Google Scholar] [CrossRef]
- Tittarelli, F.; Perpetuini, G.; Di Gianvito, P.; Tofalo, R. Biogenic amines producing and degrading bacteria: A snapshot from raw ewes’ cheese. LWT-Food Sci. Technol. 2019, 101, 1–9. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F. Biogenic Amines Formation, Toxicity, Regulations in Food. In Biogenic Amines in Food: Analysis, Occurrence and Toxicity; The Royal Society of Chemistry: London, UK, 2019; pp. 1–17. [Google Scholar]
- Wójciak, K.M.; Stasiak, D.M.; Stadnik, J.; Ferysiuk, K.; Kononiuk, A. The influence of sonication time on the biogenic amines formation as a critical point in uncured dry-fermented beef manufacturing. Int. J. Food Sci. Technol. 2019, 54, 75–83. [Google Scholar] [CrossRef]
- Makhamrueang, N.; Sirilun, S.; Sirithunyalug, J.; Chaiyana, W.; Wangcharoen, W.; Peerajan, S.; Chaiyasut, C. Effect of pretreatment processes on biogenic amines content and some bioactive compounds in Hericium erinaceus extract. Foods 2021, 10, 996. [Google Scholar] [CrossRef]
- Ladero, V.; Calles-Enríquez, M.; Fernández, M.; Alvarez, M. Toxicological effects of dietary biogenic amines. Curr. Res. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of biogenic amines in food and their public health implications: A review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Sławińska, A.; Stachniuk, A.; Stadnik, J. Determination of biogenic amines in processed and unprocessed mushrooms from the Polish market. J. Food Compost. Anal. 2020, 92, 103492. [Google Scholar] [CrossRef]
- Reis, G.C.; Custódio, F.B.; Botelho, B.G.; Guidi, L.R.; Gloria, M.B.A. Investigation of biologically active amines in some selected edible mushrooms. J. Food Compost. Anal. 2020, 86, 103375. [Google Scholar] [CrossRef]




| Edible Mushroom Samples | Colour Coordinates, NBS | pH | Mould and Yeast Count, log10 CFU/g | |||
|---|---|---|---|---|---|---|
| L* | a* | b* | BE-Fresh | 3.63 ± 0.28 A | ||
| Ca-Fresh | 2.98 ± 0.31 A | |||||
| Ro-Fresh | 3.13 ± 0.24 A | |||||
| Thermal Treated Non-Fermented | ||||||
| BE-thermal | 29.19 ± 0.22 a,A | 5.17 ± 0.21 b,C | 5.21 ± 0.10 b,B | 5.92 ± 0.26 a,F | 3.02 ± 0.21 a,C | |
| Ca-thermal | 43.25 ± 0.14 a,F | 7.19 ± 0.11 a,E | 21.76 ± 0.19 a,D | 7.11 ± 0.17 a,G | 2.55 ± 0.24 a,C,B | |
| Ro-thermal | 42.30 ± 0.19 a,E | 5.71 ± 0.09 a,D | 20.99 ± 0.18 a,C | 5.72 ± 0.15 a,E,F | 2.42 ± 0.18 b,C,B | |
| Ultrasonicated non-fermented | ||||||
| BE-ultrasound | 29.76 ± 0.21 b,B | 4.45 ± 0.11 a,B | 4.75 ± 0.05 a,A | 5.90 ± 0.04 a,F | 3.01 ± 0.15 a,C | |
| Ca-ultrasound | 43.10 ± 0.19 a,F | 15.61 ± 0.27 b,H | 31.34 ± 0.13 b,H | 7.15 ± 0.08 a,G | 2.83 ± 0.26 a,C | |
| Ro-ultrasound | 51.23 ± 0.24 b,H | 9.62 ± 0.10 b,F | 29.70 ± 0.21 b,G | 5.71 ± 0.04 a,E | 2.04 ± 0.19 a,B | |
| Thermal treated and fermented with L. uvarum LUHS245 strain | ||||||
| BE-thermal-LUHS245 | 32.14 ± 0.23 a,C | 5.19 ± 0.14 a,C | 5.05 ± 0.09 a,B | 3.97 ± 0.04 a,C | 2.46 ± 0.22 a,C,B | |
| Ca-thermal-LUHS245 | 51.98 ± 0.27 b,I | 14.66 ± 0.11 a,G | 38.07 ± 0.21 a,I | 4.09 ± 0.03 a,D | 2.13 ± 0.20 a,B | |
| Ro-thermal-LUHS245 | 56.59 ± 0.18 a,J | 4.27 ± 0.08 a,A | 23.67 ± 0.07 a,E | 3.41 ± 0.05 a,B | 1.97 ± 0.17 a,B | |
| Ultrasonicated and fermented with L. uvarum LUHS245 strain | ||||||
| BE-ultrasound-LUHS245 | 33.18 ± 0.22 b,D | 5.29 ± 0.08 a,C | 5.10 ± 0.04 a,B | 3.96 ± 0.05 a,C,D | 2.17 ± 0.21 a,B | |
| Ca-ultrasound-LUHS245 | 50.07 ± 0.15 a,G | 16.52 ± 0.11 b,I | 39.82 ± 0.18 b,J | 4.11 ± 0.06 a,D | 2.21 ± 0.23 a,B | |
| Ro-ultrasound-LUHS245 | 60.98 ± 0.18 b,K | 4.46 ± 0.06 b,B | 26.95 ± 0.21 b,F | 3.55 ± 0.04 b,A | 2.12 ± 0.13 a,B | |
| RT, Min | VC | BE—Non-Treated | Ca—Non-Treated | Ro—Non-Treated |
|---|---|---|---|---|
| 5.763 | Hexanal | nd | 1.89 ± 0.17 b | 0.445 ± 0.025 a |
| 9.949 | Benzaldehyde | 0.538 ± 0.027 a | 1.27 ± 0.13 b | 35.6 ± 2.5 c |
| 10.493 | 1-Octen-3-ol | 61.9 ± 2.5 b | 55.2 ± 0.51 a | 50.4 ± 3.8 a |
| 10.669 | 3-Octanone | 5.62 ± 0.34 b | nd | 1.81 ± 1.6 a |
| 10.885 | 3-Octanol | 8.81 ± 0.72 c | 0.525 ± 0.049 a | 3.71 ± 2.7 b |
| 11.091 | Octanal | 5.47 ± 0.61 c | 0.367 ± 0.028 a | 1.34 ± 0.21 b |
| 11.844 | 3-ethyl-2-methyl-1,3-hexadiene | nd | 1.82 ± 0.26 | nd |
| 12.476 | Oct-(2E)-enal | 4.55 ± 0.39 b | 16.3 ± 0.58 c | 2.25 ± 0.23 a |
| 12.728 | (E)-2-octen-1-ol | nd | 16.9 ± 0.18 b | 1.13 ± 0.11 a |
| 12.788 | 1-Octanol | 8.01 ± 0.78 | nd | nd |
| RT, Min | VC | BE—Thermal | Ca—Thermal | Ro—Thermal | BE—Ultrasound | Ca—Ultrasound | Ro—Ultrasound |
|---|---|---|---|---|---|---|---|
| 5.763 | Hexanal | 0.922 ± 0.054 B | 5.32 ± 0.49 b,C | 0.986 ± 0.074 b,B | nd | 3.46 ± 0.31 a | 0.618 ± 0.059 a,A |
| 8.11 | 2-Heptanone | 1.05 ± 0.09 | nd | nd | nd | nd | nd |
| 8.424 | Heptanal | 2.06 ± 0.18 a,B | 0.357 ± 0.031 a,A | nd | 1.90 ± 0.18 a,B | 0.323 ± 0.029 a,A | nd |
| 9.949 | Benzaldehyde | 4.53 ± 0.36 b,C | 1.97 ± 0.17 a,A | 44.5 ± 3.9 a,D | 1.53 ± 0.16 a,A | 2.22 ± 0.23 a,B | 51.6 ± 4.4 a,D |
| 10.493 | 1-Octen-3-ol | 57.9 ± 3.8 a,C | 51.1 ± 4.2 a,B | 35.9 ± 2.8 a,A | 63.6 ± 0.59 b,C | 50.9 ± 4.6 a,B | 31.7 ± 2.9 a,A |
| 10.669 | 3-Octanone | nd | nd | 4.57 ± 0.48 a,A | nd | nd | 4.35 ± 0.38 a,A |
| 10.885 | 3-Octanol | 7.00 ± 0.69 D | 1.29 ± 0.13 b,B | 6.08 ± 0.57 b,D | nd | 0.602 ± 0.058 a,A | 4.20 ± 0.31 a,C |
| 11.091 | Octanal | 1.27 ± 0.11 a,C | 0.651 ± 0.045 b,B | 1.51 ± 0.14 c,C,D | 1.35 ± 0.14 a,C | 0.436 ± 0.041 a,A | 1.49 ± 0.13 c,C |
| 11.673 | p-Cymene | 0.955 ± 0.043 a,A | nd | nd | 1.48 ± 0.13b,B | nd | nd |
| 11.788 | Limonene | 3.55 ± 0.28 a,B | nd | nd | 4.60 ± 0.36 b,C | nd | 0.409 ± 0.032 A |
| 11.844 | 3-ethyl-2-methyl-1,3-hexadiene | nd | 2.80 ± 0.27 a,A | nd | nd | 3.10 ± 0.028 b,B | nd |
| 12.476 | Oct-(2E)-enal | 3.16 ± 0.29 a,B | 22.2 ± 1.3 a,D | 2.21 ± 0.23 a,A | 4.45 ± 0.42 b,C | 20.7 ± 1.8 a,D | 2.50 ± 0.23 a,A |
| 12.728 | (E)-2-octen-1-ol | 9.85 ± 0.41 a,D | 4.54 ± 0.45 a,C | 0.519 ± 0.049 b,B | 12.0 ± 0.13 b,E | 11.28 ± 1.3 b,E | 0.213 ± 0.019 a,A |
| 13.341 | 6-Methyl-hept-2-en-4-ol | nd | 1.40 ± 0.15b,B | nd | nd | 0.877 ± 0.065 a,A | nd |
| 13.612 | Nonanal | 1.16 ± 0.12 a,C | 0.907 ± 0.073 b,B | 0.962 ± 0.081 b,B | 1.48 ± 0.15 a,C | 0.641 ± 0.047 a,A | 0.718 ± 0.053 a,A |
| 15.611 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran | 1.41 ± 0.13 a,A | nd | nd | 1.53 ± 0.16 a,A | nd | nd |
| 16.731 | Heptylidene acetone | 0.663 ± 0.059 a,A | nd | nd | 1.07 ± 0.09 b,B | nd | nd |
| RT, Min | VC | BE—Thermal-LUHS245 | Ca—Thermal-LUHS245 | Ro—Thermal-LUHS245 | BE—Ultrasound-LUHS245 | Ca—Ultrasound-LUHS245 | Ro—Ultrasound-LUHS245 |
|---|---|---|---|---|---|---|---|
| 2.375 | Acetic acid | nd | nd | 2.32 ± 0.22 | nd | nd | nd |
| 3.779 | Acetoin | nd | 14.8 ± 0.13 B | 2.68 ± 0.24 A | 2.76 ± 0.26 A | nd | nd |
| 4.355 | 3-methyl-1-butanol | nd | 13.0 ± 0.58 b,B | nd | 18.1 ± 0.52 C | 1.86 ± 0.19 a,A | 6.98 ± 0.65 |
| 5.277 | 2,3-Butanediol | nd | nd | nd | 3.73 ± 0.34 | nd | nd |
| 5.763 | Hexanal | nd | nd | 16.8 ± 0.17 b,C | nd | 3.16 ± 0.32 B | 1.69 ± 0.18 a,A |
| 7.164 | 3-methyl-butanoic acid ethyl ester | nd | nd | nd | nd | nd | 2.12 ± 0.23 |
| 7.567 | 1-Hexanol | nd | nd | 1.09 ± 0.10 a,A | nd | 2.72 ± 0.25 B | 5.08 ± 0.41 b,C |
| 8.11 | 2-Heptanone | 1.83 ± 0.16 B | nd | nd | nd | nd | 0.312 ± 0.24 A |
| 9.451 | Ethyl tiglate | nd | 3.31 ± 0.27 A | nd | 2.90 ± 0.28 A | nd | 14.1 ± 0.83 B |
| 9.631 | 2,7-dimethyl-4,5-octanediol | nd | 1.46 ± 0.12 | nd | nd | nd | nd |
| 9.865 | 2-Heptenal | nd | 0.545 ± 0.041 b,B | 9.02 ± 0.72 C | nd | 0.456 ± 0.041 a,A | nd |
| 9.949 | Benzaldehyde | 4.67 ± 0.39 B | 1.65 ± 0.15 a,A | nd | nd | 1.75 ± 0.16 a,A | 6.32 ± 0.59 C |
| 10.244 | Heptyl formate | nd | nd | nd | nd | 1.11 ± 0.12 | nd |
| 10.493 | 1-Octen-3-ol | 52.7 ± 0.48 b,F | 22.2 ± 1.3 a,C | 7.30 ± 0.59 a,A | 26.8 ± 1.3 a,D | 36.5 ± 2.8 b,E | 11.1 ± 0.12 b,B |
| 10.584 | 2,5-Octanedione | nd | nd | 3.39 ± 0.32 | nd | nd | nd |
| 10.669 | 3-Octanone | nd | 7.32 ± 0.56 a,A | nd | 26.4 ± 2.1 C | 12.8 ± 1.1 b,B | 12.5 ± 0.9 B |
| 10.812 | 2-pentylfuran | nd | 7.09 ± 0.48 B | 3.53 ± 0.32 A | nd | nd | nd |
| 10.885 | 3-Octanol | nd | nd | 3.26 ± 0.30 a,A | 9.12 ± 0.83 C | 7.46 ± 0.62 C | 5.46 ± 0.42 b,B |
| 10.988 | Hexanoic acid ethyl ester | nd | nd | nd | nd | nd | 5.24 ± 0.48 |
| 11.091 | Octanal | 1.50 ± 0.14 A | nd | 2.82 ± 0.25 B | nd | 1.72 ± 0.16 A | nd |
| 11.788 | Limonene | 3.64 ± 0.32 b,C | 0.587 ± 0.051 B | nd | 0.151 ± 0.11 a,A | nd | 0.188 ± 0.015 A |
| 11.844 | 3-ethyl-2-methyl-1,3-hexadiene | nd | nd | 2.03 ± 0.18 A | nd | 3.32 ± 0.29 B | nd |
| 11.883 | Benzyl alcohol | nd | nd | nd | nd | nd | 3.11 ± 0.31 |
| 12.476 | Oct-(2E)-enal | 4.32 ± 0.41 b,D | 1.52 ± 0.14 a,B | 9.94 ± 0.85 b,F | 0.320 ± 0.28 a,A | 7.66 ± 0.59 b,E | 2.06 ± 0.19 a,C |
| 12.728 | (E)-2-octen-1-ol | 9.62 ± 0.83 D | 3.58 ± 0.31 b,B | 4.23 ± 0.38 b,C | nd | 1.98 ± 0.17 a,A | 3.00 ± 0.28 a,B |
| 12.788 | 1-Octanol | nd | nd | nd | 2.53 ± 0.41A | 11.6 ± 0.12B | nd |
| 13.267 | 2-iodo-3-methyl-butane | nd | nd | nd | 1.07 ± 0.10 | nd | nd |
| 13.314 | 2-Nonanone | 4.14 ± 0.25 A | 4.97 ± 0.41 A | 4.16 ± 0.36 a,A | nd | nd | 4.98 ± 0.43 a,A |
| 13.612 | Nonanal | 1.44 ± 0.12 D | 1.10 ± 0.10 b,C | 4.39 ± 0.41 b,E | nd | 0.642 ± 0.059 a,A | 0.918 ± 0.058 a,B |
| 13.865 | Phenylethyl Alcohol | nd | 1.44 ± 0.13 b,C | 0.593 ± 0.042 a,A | 1.80 ± 0.17 D | 0.803 ± 0.065 a,B | 2.54 ± 0.25 b,E |
| 13.945 | N-hexyl-1-hexanamine | nd | nd | nd | 1.64 ± 0.15 | nd | nd |
| 14.922 | (E)-non-2-enal | 0.327 ± 0.029 B | 0.571 ± 0.041 a,C | 1.96 ± 0.18 b,E | nd | 0.844 ± 0.079 b,D | 0.252 ± 0.021 a,A |
| 15.611 | 3,6-Dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran | 1.04 ± 0.09 | nd | nd | nd | nd | nd |
| 15.753 | Octanoic acid ethyl ester | nd | 0.560 ± 0.052 B | nd | 0.154 ± 0.014 A | nd | 1.34 ± 0.11 C |
| 16.171 | (E,E)-2,4-nonadienal | 0.129 ± 0.11 b,B | nd | 2.27 ± 0.21 b,E | 0.029 ± 0.003 a,A | 0.179 ± 0.015 C | 0.248 ± 0.023 a,D |
| 16.416 | 2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde | nd | 1.26 ± 0.13 b,B | nd | nd | 0.370 ± 0.029 a,A | nd |
| 17.206 | Dec-(2E)-enal | nd | nd | 1.66 ± 0.15 b,B | nd | nd | 0.276 ± 0.026 a,A |
| 17.293 | Nonanoic acid | 2.37 ± 0.22 b,D | 3.41 ± 0.28 b,E | 1.96 ± 0.17 B | 0.170 ± 0.014 a,A | 0.300 ± 0.025 a,C | nd |
| 18.376 | Deca-(2E,4E)-dienal | nd | nd | 1.13 ± 0.10 b,D | 0.020 ± 0.03 A | 0.215 ± 0.019 B | 0.287 ± 0.027 a,C |
| 19.656 | trans-4,5-Epoxy-(E)-2-decenal | nd | nd | 1.33 ± 0.12 | nd | nd | nd |
| 21.48 | 9-Decen-1-yl acetate | 1.28 ± 0.13 C | 0.209 ± 0.019 A | 0.596 ± 0.046 B | nd | nd | nd |
| 21.88 | 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | nd | 2.29 ± 0.23 b,B | nd | nd | 0.725 ± 0.047 a,A | nd |
| 23.051 | Dodecanoic acid | nd | nd | 1.65 ± 0.14 B | 0.036 ± 0.004 A | nd | nd |
| Edible Mushroom Samples | OA | Emotions Induced by the Samples (from 0 to 1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | Happy | Sad | Angry | Surprised | Scared | Disgusted | Contempt | Valence | ||
| Thermal treated non-fermented | ||||||||||
| BE-thermal | 7.6 ± 1.2 a | 0.8068 ± 0.0096 d | 0.0106 ± 0.0015 d | 0.0675 ± 0.0028 c | 0.0224 ± 0.0018 c | 0.0086 ± 0.0017 d | 0.0037 ± 0.0006 d | 0.0156 ± 0.0016 a | 0.0014 ± 0.0004 c | 0.0852 ± 0.0075 b |
| Ca-thermal | 8.9 ± 2.3 a | 0.8010 ± 0.0048 d | 0.0177 ± 0.0019 c | 0.0135 ± 0.0011i | 0.0322 ± 0.0025 b | 0.0059 ± 0.0006 e | 0.0014 ± 0.0005 e | 0.0034 ± 0.0005 e | 0.0011 ± 0.0003 c | 0.0269 ± 0.0034 f |
| Ro-thermal | 7.6 ± 1.4 a | 0.8196 ± 0.0115 d | 0.0015 ± 0.0006 f | 0.0433 ± 0.0021 e | 0.0263 ± 0.0023 c | 0.0024 ± 0.0004 f | 0.0209 ± 0.0018 a | 0.0071 ± 0.0008 d | 0.0006 ± 0.0002 c,d | 0.0847 ± 0.0041 b |
| Ultrasonicated non-fermented | ||||||||||
| BE-ultrasound | 7.8 ± 2.1 a | 0.8700 ± 0.0086 b | 0.0005 ± 0.0004 f | 0.0231 ± 0.0019 f | 0.0217± 0.0021 c | 0.0130 ± 0.0015 c | 0.0087 ± 0.0009 b | 0.0029 ± 0.0003 e | 0.0009 ± 0.0004 c,d | 0.0440 ± 0.0029 d |
| Ca-ultrasound | 8.3 ± 1.6 a | 0.7926 ± 0.0108 d | 0.0544 ± 0.0031 b | 0.0485 ± 0.0024 d | 0.0364 ± 0.0027 b | 0.0110 ± 0.0009 c | 0.0049 ± 0.0006 c | 0.0097 ± 0.0011 c | 0.0027 ± 0.0004 b | 0.0782 ± 0.0063 c |
| Ro-ultrasound | 7.8 ± 1.3 a | 0.8822 ± 0.0147 b | 0.0019 ± 0.0005 f | 0.0162 ± 0.0018 h | 0.0144 ± 0.0015 d | 0.0057 ± 0.0018 e | 0.0059 ± 0.0007 c | 0.0072 ± 0.0013 d | 0.0005 ± 0.0002 c | 0.0356 ± 0.0027 e |
| Thermal treated and fermented with L. uvarumLUHS245 strain | ||||||||||
| BE-thermal-LUHS245 | 7.5 ± 0.9 a | 0.9442 ± 0.0132 a | 0.0006 ± 0.0005 f | 0.0120 ± 0.0015 i | 0.0059 ± 0.0009 a | 0.0057 ± 0.0011 e | 0.0001 ± 0.0001 f | 0.0006 ± 0.0004 f | 0.0001 ± 0.0001 e | 0.0166 ± 0.0017 g |
| Ca-thermal-LUHS245 | 7.4 ± 1.1 a | 0.8358 ± 0.0214 c | 0.0060 ± 0.0007 e | 0.0934 ± 0.0032 b | 0.0042 ± 0.0006 a,b | 0.0090 ± 0.0010 d | 0.0001 ± 0.0001 f | 0.0166 ± 0.0014 a | 0.0008 ± 0.0003 c,d | 0.0994 ± 0.0068 a |
| Ro-thermal-LUHS245 | 7.3 ± 1.4 a | 0.9385 ± 0.0219 a | 0.0008 ± 0.0004 f | 0.0278 ± 0.0019 g | 0.0047 ± 0.0009 a,b | 0.0053 ± 0.0006 e | 0.0001 ± 0.0001 f | 0.0108 ± 0.0011 b | 0.0004 ± 0.0002 e | 0.0399 ± 0.0029 e |
| Ultrasonicated and fermented with L. uvarumLUHS245 strain | ||||||||||
| BE-ultrasound-LUHS245 | 8.9 ± 1.3 a | 0.8230 ± 0.0143 d | 0.2702 ± 0.0097 a | 0.1419 ± 0.0076 a | 0.0060 ± 0.0007 a | 0.0701 ± 0.0008 a | 0.0017 ± 0.0002 e | 0.0070 ± 0.0009 d | 0.0288 ± 0.0019 ª | 0.0395 ± 0.0025 e |
| Ca-ultrasound-LUHS245 | 8.0 ± 1.2 a | 0.8697 ± 0.0157 b | 0.0013 ± 0.0005 f | 0.0232 ± 0.0021 f | 0.0217 ± 0.0019 c | 0.0129 ± 0.0013 c | 0.0086 ± 0.0014 b | 0.0032 ± 0.0004 e | 0.0007 ± 0.0004 c,d | 0.0446 ± 0.0032 d |
| Ro-ultrasound-LUHS24 | 9.1 ± 0.8 a | 0.8408 ± 0.0139 c | 0.0004 ± 0.0002 f | 0.0228 ± 0.0023 f | 0.0217 ± 0.0018 c | 0.0227 ± 0.0019 b | 0.0088 ± 0.0012 b | 0.0033 ± 0.0005 e | 0.0011 ± 0.0005 c,d | 0.0443 ± 0.0028 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkiene, E.; Zokaityte, E.; Starkute, V.; Mockus, E.; Klupsaite, D.; Lukseviciute, J.; Bogomolova, A.; Streimikyte, A.; Ozogul, F. Biopreservation of Wild Edible Mushrooms (Boletus edulis, Cantharellus, and Rozites caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties. Foods 2022, 11, 1800. https://doi.org/10.3390/foods11121800
Bartkiene E, Zokaityte E, Starkute V, Mockus E, Klupsaite D, Lukseviciute J, Bogomolova A, Streimikyte A, Ozogul F. Biopreservation of Wild Edible Mushrooms (Boletus edulis, Cantharellus, and Rozites caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties. Foods. 2022; 11(12):1800. https://doi.org/10.3390/foods11121800
Chicago/Turabian StyleBartkiene, Elena, Egle Zokaityte, Vytaute Starkute, Ernestas Mockus, Dovile Klupsaite, Justina Lukseviciute, Alina Bogomolova, Audrone Streimikyte, and Fatih Ozogul. 2022. "Biopreservation of Wild Edible Mushrooms (Boletus edulis, Cantharellus, and Rozites caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties" Foods 11, no. 12: 1800. https://doi.org/10.3390/foods11121800
APA StyleBartkiene, E., Zokaityte, E., Starkute, V., Mockus, E., Klupsaite, D., Lukseviciute, J., Bogomolova, A., Streimikyte, A., & Ozogul, F. (2022). Biopreservation of Wild Edible Mushrooms (Boletus edulis, Cantharellus, and Rozites caperata) with Lactic Acid Bacteria Possessing Antimicrobial Properties. Foods, 11(12), 1800. https://doi.org/10.3390/foods11121800