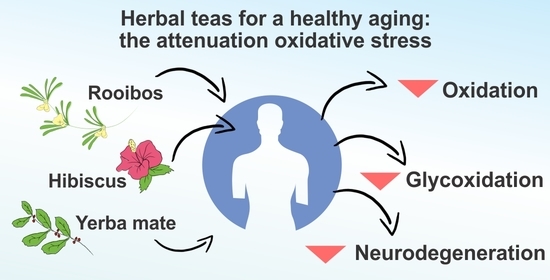
Hibiscus, Rooibos, and Yerba Mate for Healthy Aging: A Review on the Attenuation of In Vitro and In Vivo Markers Related to Oxidative Stress, Glycoxidation, and Neurodegeneration
Abstract
1. Introduction
2. Oxidative Stress Promoting Mechanism Related to Glycation and Neurodegeneration
2.1. Protein Glycation and Oxidative Stress

2.2. Oxidative Stress and Neurodegeneration: A Case of Alzheimer’s Disease
3. Hibiscus, Rooibos, Yerba Mate as Sources of Natural Bioactive Compounds
| Experimental Condition | Compound [Class] | Chemical Structure | Associated Bioactivity [Model] | Effect |
|---|---|---|---|---|
| In vitro | Caffeic acid [Phenolic compound] |  | Anti-glycation [Fluorescence 370/440 nm] | AGE formation Control: 180% Caffeic acid (0.2 mM): 80% [111] |
| Epicatechin [Polyphenol] | 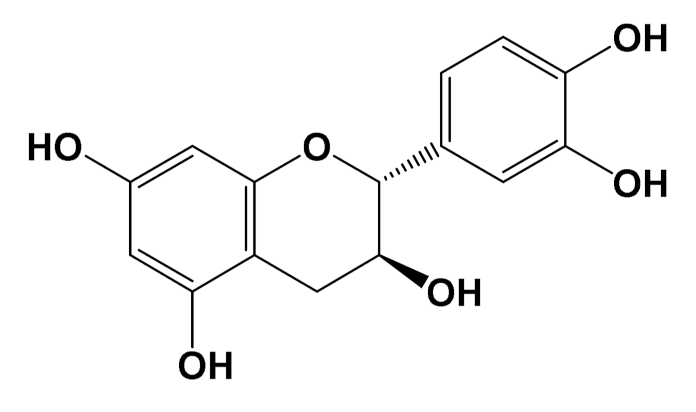 | Neuroprotective [SHSY5Y cells] | Parkin expression Rotenone (1 μM): 110 (a.u) Rotenone + Epicatechin (10 μM): 60 (a.u.) [112] | |
| Hibiscin [Polyphenol] | 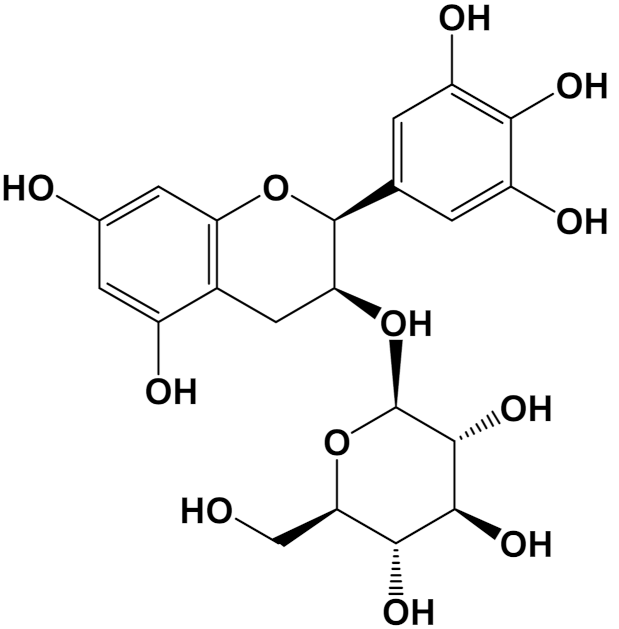 | Anti-inflammatory [RAW264.7 macrophage cells] | IL-6 expression Control (LPS 1 mg/kg): 1750 pg/mL LPS + Hibiscin (15 µM/kg): 750 pg/mL [102] | |
| Quercetin [Polyphenol] | 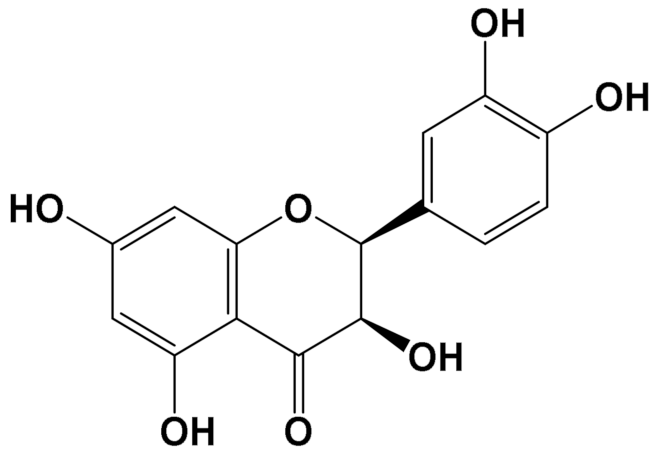 | Attenuation of mitophagy [Primary microglia] | MitoSox Control (LPS (100 ng/mL): 28 (a.u.) LPS + Quercetin (30 μM): 8 (a.u.) [113] | |
| Quinic acid [Polyol] | 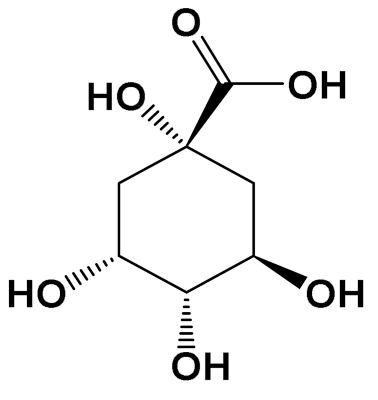 | Photoprotective [HaCaT keratinocytes] | UVB irradiation-induced ROS generation Control: 2000 (a.u.) Quinic acid: (10 uM): 1500 (a.u.) [114] | |
| Theobromine [Methylxanthine] | 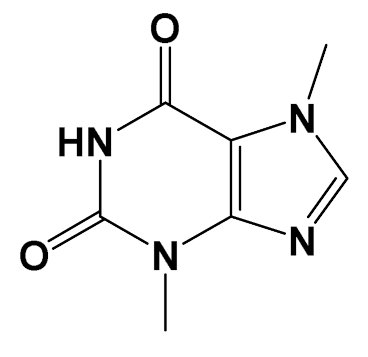 | Adipogenesis attenuation [SGBS cells] | Adipogenic differentiation Control: 100% Teobromine (100 µg/mL): 60% [115] | |
| In vivo | Acteoside [Polyphenol] | 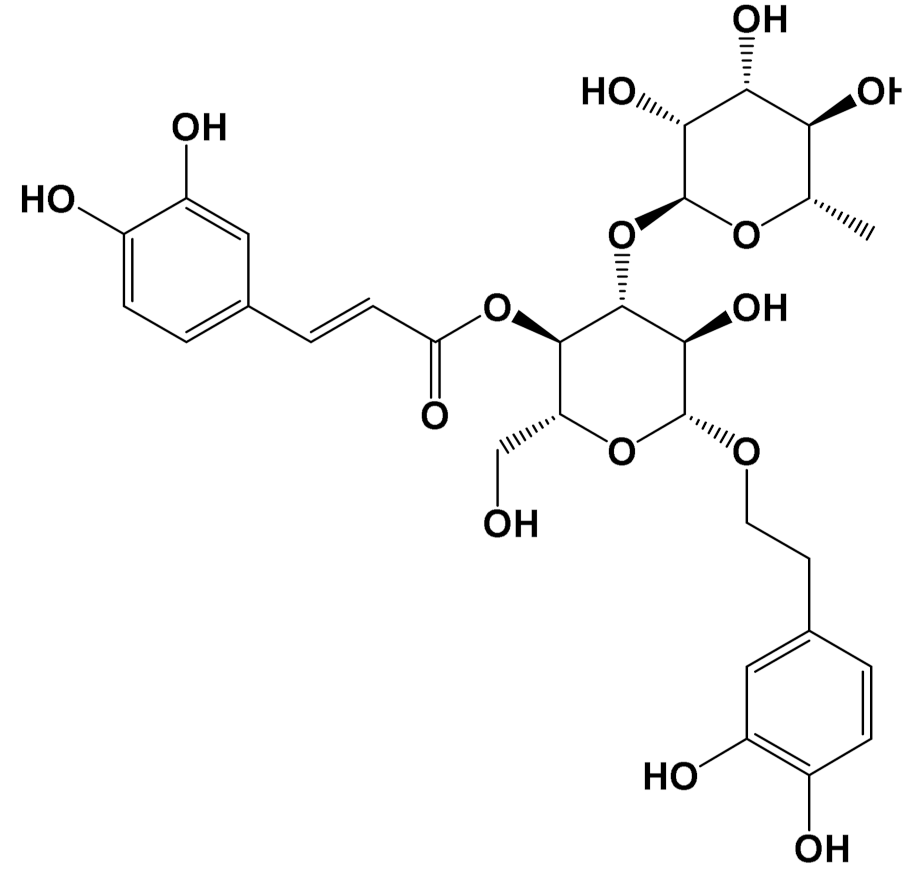 | Glucose metabolism [Male Wistar albino rats] | Blood glucose Control: 318 mg/dL Acteoside (40 mg/kg): 75 mg/dL (Oral) [116] |
| Aspalathin [Polyphenol] | 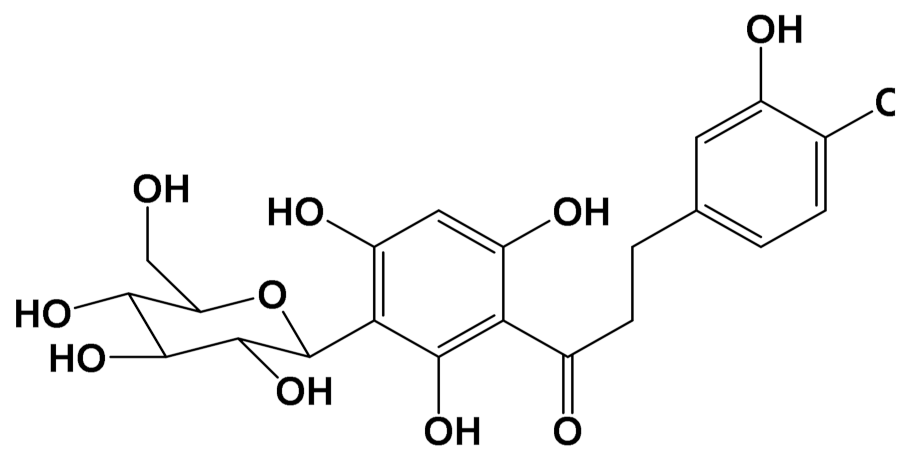 | Nephroprotective [Male C57BL/6 mice] | Creatinine levels Control: 0.47 mg/dL Aspalathin (1.00 mg/kg): 0.25 mg/dL (Intravenous injection) [117] | |
| Caffeine [Methylxanthine] | 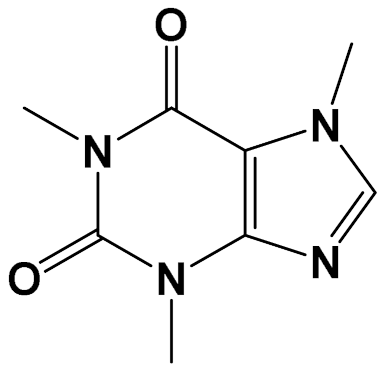 | Anti-AChE [Male albino rats] | AChE activity Control: 7 µmol/min/g Caffeine (20 mg/kg/day): 2 µmol/min/g (Oral) [118] | |
| Chlorogenic acid [Polyphenol] | 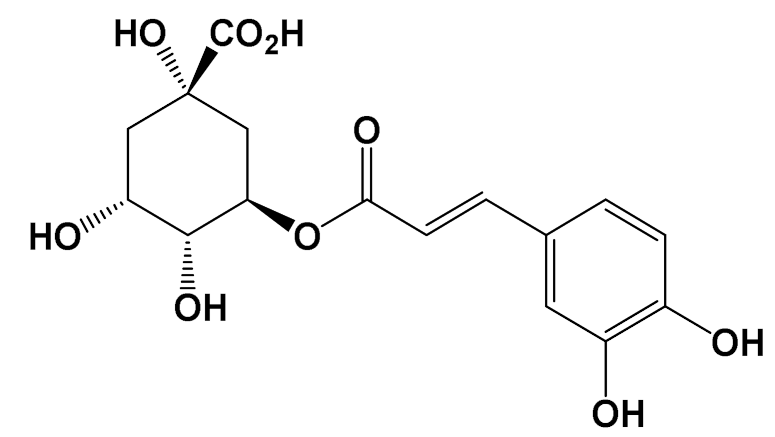 | Neuroprotective [Swiss albino mice] | Mitochondrial damage Control (MPTP): 3 nM Chlorogenic acid + MPTP: 5 nM (Oral) [119] |
4. The Potential of Hibiscus, Rooibos, and Yerba Mate in Glycoxidation and Neurodegeneration Attenuation
4.1. Antioxidant and Anti-Glycation Effects of Hibiscus, Rooibos, and Yerba Mate
| Assay | Species [Extract] | Measure | Dose or EC50 | Reference |
| Antioxidant | H. sabdariffa [Ethanolic] | Lipid peroxidation (SH-SY5Y cells) | Control: 800% Extract (100 µg/mL): 300% | [141] |
| ROS production (SH-SY5Y cells) | Control: 130% Extract (100 µg/mL): 100% | |||
| H. sabdariffa [Methanolic] | Malondialdehyde | EC50 22 μg/mL | [144] | |
| Monoamine Oxidase | EC50 44 μg/mL | |||
| ATPase activity | EC50 22 μg/mL | |||
| Anti-glycoxidation | A. linearis [Aqueous] | AGE formation inhibition (Fluorescence 340/420 nm) Glucose in BSA system | Control (aminoguanidine): 45% Green extract (200 μg/mL): 45% Fermented extract (200 μg/mL): 55% | [145] |
| H. rosa-sinensis [Aqueous] | AGE formation inhibition (Fluorescence 340/420 nm) Fructose in BSA system | Control (Aminoguanidine): IC50 6 μg/mL Extract: IC50 67 μg/mL | [146] | |
| I. paraguariensis [Aqueous] | AGE formation inhibition (Fluorescence 340/420 nm) Fructose in BSA system | Control (Fructose): 4000 a.u. Extract (2.5 µg/mL): 3000 a.u. | [147] | |
| AGE formation inhibition (Fluorescence 340/420 nm) Methylglyoxal in BSA system | Control (green tea): 65 a.u. Extract (20 µg/mL): 42 a.u. | [20] |
| Target Effect/Organ | Species [Extract] | Concentration | Animal Model | Measure | Effect | Tendency | Reference |
|---|---|---|---|---|---|---|---|
| Antioxidant/Brain | A. linearis [Aqueous] | 1 g/100 mL | Immobilization-induced oxidative stress Sprague Dawley rats | CAT | Control (Stress): 2 unit/mg Extract: 3 unit/mg | ↑ | [143] |
| FFA | Control (Stress): 700 µg/mL Extract: 650 µg/mL | ↓ | |||||
| GSH/GSSG | Control (Stress): 7.5 Extract: 9 | ↑ | |||||
| HIAA | Control (Stress): 400 mg/g tissue Extract: 350 mg/g tissue | ↓ | |||||
| Lipid peroxidation | Control (Stress): 50 nmol/g tissue Extract: 40 nmol/g tissue | ↓ | |||||
| SOD | Control (Stress): 1 unit/mg Extract: 1.7 unit/mg | ↑ | |||||
| H. rosa-sinensis [Aqueous] | 25 mg/kg body weight | STZ induced diabetic Male Sprague-Dawley | CAT | Control (Diabetic): 5 U/mg Extract: 10 U/mg | ↑ | [142] | |
| SOD | Control (Diabetic): 7 U/mg Extract: 15 U/mg | ↑ | |||||
| H. sabdariffa [Aqueous] | 200 mg/kg body weight | Male Swiss albino mice | MDA | Control (STZ): 3 nmol/g White hibiscus extract: 0.5 nmol/g Red hibiscus extract: 0.5 nmol/g | ↓ | [25] | |
| MPO | Control (STZ): 75 µg/mg tissue White hibiscus extract: 20 µg/mg tissue Red hibiscus extract: 20 µg/mg tissue | ↓ | |||||
| Cox-2 | Control (STZ): 4 (fold change) White hibiscus extract: 1 (fold change) Red hibiscus extract: 1 (fold change) | ↓ | |||||
| H. sabdariffa [Ethanolic] | 500 mg/kg body weight | Cypermethrin oxidative stress male mice (Mus musculus) | AChE | Control (Cypermethrin): 0.5 µmol/min/mg Extract: 2.5 µmol/min/mg | ↓ | [163] | |
| CAT | Control (Cypermethrin): 0.04 µmol/min/mg Extract: 0.06 µmol/min/mg | ↓ | |||||
| H2O2 | Control (Cypermethrin): 1.2 µmol/mg Extract: 0.3 µmol/mg | ↓ | |||||
| MDA | Control (Cypermethrin): 2 µmol/mg Extract: 0.5 µmol/mg | ↓ | |||||
| I. paraguariensis [Aqueous] | 200 mg/mL | Chronic immobilization stress male Wistar rats | GSH/GSSG | Control: 0.48 Extract: 0.50 | → | [154] | |
| Lipid peroxidation | Control: 2.1 TBA/mg Extract: 1.3 TBA/mg | ↓ | |||||
| 200 mg/mL | Male Wistar rats | GSH/GSSG | Control: 4.7 Extract: 16.6 | ↑ | [153] | ||
| Lipid peroxidation | Control: 1.3 MDA eq/mg Extract: 0.3 MDA eq/mg | ↓ | |||||
| 50 mg/kg BW | PTZ-induced seizure male Wistar rats | CAT | Control (PTZ): 5 mmol/min/mg Extract: 9 mmol/min/mg | ↑ | [164] | ||
| SOD | Control (PTZ): 15.50 U/mg Extract: 23 U/mg | ↑ | |||||
| Sulfhydryl protein | Control (PTZ): 0.09 nmol DTNB/mg Extract: 0.31 nmol DTNB/mg | ↑ | |||||
| Anti-glycoxidation | H. rosa-sinensis [Ethanolic] | 25 mg/kg BW | STZ induced diabetic Male Sprague-Dawley | Glycated hemoglobin | Control: 13% Extract: 6% | ↓ | [142] |
| H. sabdariffa [Methanolic] | 200 mg/kg BW | STZ induced diabetic Male Sprague-Dawley | Serum glucose | Diabetic control: 400 mg/dL Extract: 100 mg/dL | ↓ | [162] | |
| AGE levels | Diabetic control: 4.5 mg/mL Extract: 3 mg/dL | ↓ |
4.2. Neuroprotective Effects of Hibiscus, Rooibos, and Yerba Mate
| Species [Extract] | Concentration | Animal Model | Measure | Effect | Tendency | Reference |
|---|---|---|---|---|---|---|
| A. linearis [Aqueous] | 100 mg/mL | Zebrafish larvae | Monoamine oxidase | Control (Clorgyline): 100% Extract: 60% | ↓ | [174] |
| Cell viability | Control: 100% Extract: 40% | ↓ | ||||
| 12.5 µg/mL | Zebrafish larvae | ROS production | Control: 600% (120 min) Extract: 200% (120 min) | ↓ | ||
| H. sabdariffa [Aqueous] | 200 mg/kg BW | Male Swiss albino mice | Moris water test | Control (STZ): 20 sExtract: 30 s | ↑ | [25] |
| BACE1 | Control (STZ): 5 (fold change) White hibiscus extract: 2 (fold change) Red hibiscus extract: 2 (fold change) | ↓ | ||||
| Aβ-42 | Control (STZ): 250 mg/mg tissue White hibiscus extract: 100 mg/mg tissue Red hibiscus extract: 100 mg/mg tissue | ↓ | ||||
| γ-secretase | Control (STZ): 3.5 (fold change) White hibiscus extract: 1 (fold change) Red hibiscus extract: 1 (fold change) | ↓ | ||||
| H. sabdariffa [Ethanolic] | 500 mg/kg BW | Swiss albino mice | AChE activity | Control (Scopolamin): 44 nM/min/g tissue Extract: 33 nM/min/g tissue | ↓ | [175] |
| I. paraguariensis [Aqueous] | 10.5 mg/L | Caenorhabditis elegans | Aluminum induced oxidative stress | Control: 0.6 µM/h/mg Extract: 0.4 µM/h/mg | ↓ | [176] |
| I. paraguariensis [Ethanolic] | 4 mg/mL | C. elegans | Aβ-42 expression | Control: 1 a.u. Extract: 0.6 a.u. | ↓ | [170] |
| AChE activity | Control: 100% Extract: 50% | ↓ | ||||
| Lifespan | Control: 15 days Extract: 17 days | ↑ | ||||
| ROS production | Control: 100% Extract: 50% | ↓ | ||||
| 500 mg/kg | Male C57Bl/6 mice | Catalepsy | Control (reserpine): 120 s Extract: 60 s | ↓ | [177] | |
| 300 mg/kg BW | Male Swiss mice | Elevated Plus Maze | Control: 17% Extract:40% | ↑ | [178] | |
| AChE | Control: 4.5 mmol/min/mg Extract: 8.0 mmol/min/mg | ↑ | ||||
| Step-down avoidance task | Control: 170 s Extract: 70 s | ↓ |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 3 February 2022).
- Lu, W.; Pikhart, H.; Sacker, A. Socioeconomic Determinants of Healthy Ageing: Evidence from the English Longitudinal Study of Ageing. Lancet 2018, 392, S54. [Google Scholar] [CrossRef]
- Kim, Y.; Huan, T.; Joehanes, R.; McKeown, N.M.; Horvath, S.; Levy, D.; Ma, J. Higher Diet Quality Relates to Decelerated Epigenetic Aging. Am. J. Clin. Nutr. 2022, 115, 163–170. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Yu, Z.; Chen, J.; Han, J.-D.J. Assessing the Rate of Aging to Monitor Aging Itself. Ageing Res. Rev. 2021, 69, 101350. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced Glycation End Products (AGEs) and Other Adducts in Aging-Related Diseases and Alcohol-Mediated Tissue Injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Ludhiadch, A.; Sharma, R.; Muriki, A.; Munshi, A. Role of Calcium Homeostasis in Ischemic Stroke: A Review. CNS Neurol. Disord.-Drug Targets 2022, 21, 52–61. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of ALS. Int. J. Mol. Sci. 2021, 22, 6352. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sak, K.; Garg, V.K.; Goel, N.; Punia, S.; Chaudhary, A. Role of Reactive Oxygen Species in Cancer Progression. Curr. Pharmacol. Rep. 2019, 5, 79–86. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase System: A Systematic Review of Its Biological Activity, Related-Diseases, Screening Methods and Small Molecule Regulators. Biomed. Pharmacother. 2020, 131, 110663. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Baiamonte, E.; Guarrera, M.; Parisi, A.; Ruffolo, C.; Tagliaferri, F.; Barbagallo, M. Healthy Aging and Dietary Patterns. Nutrients 2022, 14, 889. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef]
- Luthra, R.; Roy, A. Role of Medicinal Plants against Neurodegenerative Diseases. Curr. Pharm. Biotechnol. 2022, 23, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Rasul, A.; Shah, M.A.; Hussain, G.; Asrar, M.; Riaz, A.; Sarfraz, I.; Hussain, A.; Khorsandi, K.; Lai, N.S.; et al. Radioprotective Role of Natural Polyphenols: From Sources to Mechanisms. Anti Cancer Agents Med. Chem. 2022, 22, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, P.; Banerjee, A.; Banerjee, A.; Murugesan, R.; Marotta, F.; Pathak, S. Chapter 17—An Overview of Dietary Polyphenols and Their Therapeutic Effects. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 221–235. ISBN 978-0-12-813006-3. [Google Scholar]
- Ahmed, J.U.; Waziri, M.; Dauda, A.; Bida, K.M. A Short Review of Medicinal Plants Extract Accompanied by Potential Antidepressant Activity. J. Chem. Rev. 2021, 3, 307–319. [Google Scholar] [CrossRef]
- Akkol, E.K.; Tatlı Çankaya, I.; Şeker Karatoprak, G.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural Compounds as Medical Strategies in the Prevention and Treatment of Psychiatric Disorders Seen in Neurological Diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef]
- Lunceford, N.; Gugliucci, A. Ilex paraguariensis Extracts Inhibit AGE Formation More Efficiently than Green Tea. Fitoterapia 2005, 76, 419–427. [Google Scholar] [CrossRef]
- Chen, W.; Sudji, I.R.; Wang, E.; Joubert, E.; van Wyk, B.-E.; Wink, M. Ameliorative Effect of Aspalathin from Rooibos (Aspalathus linearis) on Acute Oxidative Stress in Caenorhabditis Elegans. Phytomedicine 2013, 20, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Deolindo, C.T.P.; Hoffmann, J.F.; Chaves, F.C.; Prado-Silva, L.D.; Sant’Ana, A.S.; Azevedo, L.; Carmo, M.A.V.D.; Granato, D. Optimized Camellia sinensis Var. Sinensis, Ilex paraguariensis, and Aspalathus linearis Blend Presents High Antioxidant and Antiproliferative Activities in a Beverage Model. Food Chem. 2018, 254, 348–358. [Google Scholar] [CrossRef]
- Cittadini, M.C.; Albrecht, C.; Miranda, A.R.; Mazzuduli, G.M.; Soria, E.A.; Repossi, G. Neuroprotective Effect of Ilex paraguariensis Intake on Brain Myelin of Lung Adenocarcinoma-Bearing Male Balb/c Mice. Nutr. Cancer 2019, 71, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Sarima; Astuti, R.I.; Meryandini, A. Modulation of Aging in Yeast Saccharomyces Cerevisiae by Roselle Petal Extract (Hibiscus sabdariffa L.). Am. J. Biochem. Biotechnol. 2019, 15, 23–32. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Ashour, R.M.; Abd El-Haleim, E.A.; Ahmed, K.A.; Abdel-Sattar, E. Hibiscus sabdariffa L.: A Potent Natural Neuroprotective Agent for the Prevention of Streptozotocin-Induced Alzheimer’s Disease in Mice. Biomed. Pharmacother. 2020, 128, 110303. [Google Scholar] [CrossRef]
- Gorisse, L.; Pietrement, C.; Vuiblet, V.; Schmelzer, C.E.H.; Köhler, M.; Duca, L.; Debelle, L.; Fornès, P.; Jaisson, S.; Gillery, P. Protein Carbamylation Is a Hallmark of Aging. Proc. Natl. Acad. Sci. USA 2016, 113, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Tessier, F.J. The Maillard Reaction in the Human Body. The Main Discoveries and Factors That Affect Glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef]
- Teissier, T.; Quersin, V.; Gnemmi, V.; Daroux, M.; Howsam, M.; Delguste, F.; Lemoine, C.; Fradin, C.; Schmidt, A.-M.; Cauffiez, C.; et al. Knockout of Receptor for Advanced Glycation End-Products Attenuates Age-Related Renal Lesions. Aging Cell 2019, 18, e12850. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, É. The Receptor for Advanced Glycation End-Products (RAGE) Is an Important Pattern Recognition Receptor (PRR) for Inflammaging. Biogerontology 2019, 20, 279–301. [Google Scholar] [CrossRef]
- Naik, R.R.; Wang, Y.; Selomulya, C. Improvements of Plant Protein Functionalities by Maillard Conjugation and Maillard Reaction Products. Crit. Rev. Food Sci. Nutr. 2021, 0, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Browning and Pigmentation in Food through the Maillard Reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, F.; Wang, B.; Chen, L.; Liu, W.; Tan, S. A Literature Review on Maillard Reaction Based on Milk Proteins and Carbohydrates in Food and Pharmaceutical Products: Advantages, Disadvantages, and Avoidance Strategies. Foods 2021, 10, 1998. [Google Scholar] [CrossRef]
- Guibourdenche, M.; Haug, J.; Chevalier, N.; Spatz, M.; Barbezier, N.; Gay-Quéheillard, J.; Anton, P.M. Food Contaminants Effects on an In Vitro Model of Human Intestinal Epithelium. Toxics 2021, 9, 135. [Google Scholar] [CrossRef]
- Kim, C.-S.; Park, S.; Kim, J. The Role of Glycation in the Pathogenesis of Aging and Its Prevention through Herbal Products and Physical Exercise. J. Exerc. Nutr. Biochem. 2017, 21, 55–61. [Google Scholar] [CrossRef]
- Alexiou, P.; Chatzopoulou, M.; Pegklidou, K.; Demopoulos, V.J. RAGE: A Multi-Ligand Receptor Unveiling Novel Insights in Health and Disease. Curr. Med. Chem. 2010, 17, 2232–2252. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, E.; Grossin, N.; Wautier, M.-P.; Taamma, R.; Wautier, J.-L. Mesothelial RAGE Activation by AGEs Enhances VEGF Release and Potentiates Capillary Tube Formation. Kidney Int. 2007, 71, 126–133. [Google Scholar] [CrossRef]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Dinischiotu, A. AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism? Int. J. Mol. Sci. 2015, 16, 20100–20117. [Google Scholar] [CrossRef]
- Bakala, H.; Hamelin, M.; Mary, J.; Borot-Laloi, C.; Friguet, B. Catalase, a Target of Glycation Damage in Rat Liver Mitochondria with Aging. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2012, 1822, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Suravajjala, S.; Cohenford, M.; Frost, L.R.; Pampati, P.K.; Dain, J.A. Glycation of Human Erythrocyte Glutathione Peroxidase: Effect on the Physical and Kinetic Properties. Clin. Chim. Acta 2013, 421, 170–176. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.Y.; Rafi, Z.; Khan, H.; Siddiqui, Z.; Rehman, S.; Shahab, U.; Khan, M.S.; Saeed, M.; Alouffi, S.; et al. Oxidation, Glycation and Glycoxidation—The Vicious Cycle and Lung Cancer. Semin. Cancer Biol. 2018, 49, 29–36. [Google Scholar] [CrossRef]
- Glomb, M.A.; Monnier, V.M. Mechanism of Protein Modification by Glyoxal and Glycolaldehyde, Reactive Intermediates of the Maillard Reaction (∗). J. Biol. Chem. 1995, 270, 10017–10026. [Google Scholar] [CrossRef]
- Rau, R.; Glomb, M.A. Novel Pyridinium Cross-Link Structures Derived from Glycolaldehyde and Glyoxal. J. Agric. Food Chem. 2022, 70, 4434–4444. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.A.; Khalifah, R.G.; Hudson, B.G. Thiamine Pyrophosphate and Pyridoxamine Inhibit the Formation of Antigenic Advanced Glycation End-Products: Comparison with Aminoguanidine. Biochem. Biophys. Res. Commun. 1996, 220, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Nakamura, J.; Naruse, K.; Komori, T.; Kato, K.; Kasuya, Y.; Nagai, R.; Horiuchi, S.; Hotta, N. Epalrestat, an Aldose Reductase Ihibitor, Reduces the Levels of Nepsilon-(Carboxymethyl)Lysine Protein Adducts and Their Precursors in Erythrocytes from Diabetic Patients. Diabetes Care 2000, 23, 1539–1544. [Google Scholar] [CrossRef][Green Version]
- Hartog, J.W.L.; Willemsen, S.; van Veldhuisen, D.J.; Posma, J.L.; van Wijk, L.M.; Hummel, Y.M.; Hillege, H.L.; Voors, A.A.; for the BENEFICIAL investigators. Effects of Alagebrium, an Advanced Glycation Endproduct Breaker, on Exercise Tolerance and Cardiac Function in Patients with Chronic Heart Failure. Eur. J. Heart Fail. 2011, 13, 899–908. [Google Scholar] [CrossRef]
- NephroGenex, Inc. A Phase 3 Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Evaluate the Safety and Efficacy of Pyridorin (Pyridoxamine Dihydrochloride) in Subjects With Nephropathy Due to Type 2 Diabetes (PIONEER). 2016. Available online: clinicaltrials.gov (accessed on 23 May 2022).
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel Advances in Inhibiting Advanced Glycation End Product Formation Using Natural Compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A Review on Mechanism of Inhibition of Advanced Glycation End Products Formation by Plant Derived Polyphenolic Compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, S.; Foubert, K.; Pieters, L. Natural Products as a Source of Inspiration for Novel Inhibitors of Advanced Glycation Endproducts (AGEs) Formation. Planta Med. 2021, 87, 780–801. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal and Glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Sang, S. Dietary Quercetin Reduces Plasma and Tissue Methylglyoxal and Advanced Glycation End Products in Healthy Mice Treated with Methylglyoxal. J. Nutr. 2021, 151, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, W. Role of Quercetin in the Physicochemical Properties, Antioxidant and Antiglycation Activities of Bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, F.; Wang, M. Antioxidant and Antiglycation Activity of Selected Dietary Polyphenols in a Cookie Model. J. Agric. Food Chem. 2014, 62, 1643–1648. [Google Scholar] [CrossRef]
- Sergi, D.; Boulestin, H.; Campbell, F.; Williams, L. The Role of Dietary Advanced Glycation End Products (AGEs) in Metabolic Dysfunction. Mol. Nutr. Food Res. 2020, 65, e1900934. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, C.; Zhou, Y.; Qi, J.; Zhang, C.; Sun, B.; Wang, J.; Guan, Y. Progress of RAGE Molecular Imaging in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Noreen, H.; Rehman, S.; Kamal, M.A.; da Rocha, J.B.T. Association of Oxidative Stress with Neurological Disorders. Curr. Neuropharmacol. 2021, 20, 1046–1072. [Google Scholar] [CrossRef] [PubMed]
- Jagust, W. Imaging the Evolution and Pathophysiology of Alzheimer Disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- Kowalska, M.; Piekut, T.; Prendecki, M.; Sodel, A.; Kozubski, W.; Dorszewska, J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Cao, Q.; Tan, C.-C.; Xu, W.; Hu, H.; Cao, X.-P.; Dong, Q.; Tan, L.; Yu, J.-T. The Prevalence of Dementia: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between Amyloid-β and Tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Nuzzo, D.; Giacomazza, D.; Di Carlo, M. β-Amyloid Peptide: The Cell Compartment Multi-Faceted Interaction in Alzheimer’s Disease. Neurotox. Res. 2020, 37, 250–263. [Google Scholar] [CrossRef]
- Wong, K.Y.; Roy, J.; Fung, M.L.; Heng, B.C.; Zhang, C.; Lim, L.W. Relationships between Mitochondrial Dysfunction and Neurotransmission Failure in Alzheimer’s Disease. Aging Dis. 2020, 11, 1291–1316. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S.; Cholinergic System Working Group, and for the Alzheimer Precision Medicine Initiative (APMI). Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimers Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Sande, D.; da Silva Lima, G.; Fidelis e Moura, M.A.; Lima, M.T.N.S. Chapter 1—Fungal Metabolites as Promising New Drug Leads for the Treatment of Alzheimer’s Disease. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 62, pp. 1–39. [Google Scholar]
- Jangde, N.; Ray, R.; Rai, V. RAGE and Its Ligands: From Pathogenesis to Therapeutics. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 555–575. [Google Scholar] [CrossRef]
- Batkulwar, K.; Godbole, R.; Banarjee, R.; Kassaar, O.; Williams, R.J.; Kulkarni, M.J. Advanced Glycation End Products Modulate Amyloidogenic APP Processing and Tau Phosphorylation: A Mechanistic Link between Glycation and the Development of Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 988–1000. [Google Scholar] [CrossRef]
- Castellani, R.J.; Harris, P.L.; Sayre, L.M.; Fujii, J.; Taniguchi, N.; Vitek, M.P.; Founds, H.; Atwood, C.S.; Perry, G.; Smith, M.A. Active Glycation in Neurofibrillary Pathology of Alzheimer Disease: N(Epsilon)-(Carboxymethyl) Lysine and Hexitol-Lysine. Free Radic. Biol. Med. 2001, 31, 175–180. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.; Qiu, L.; Zeng, Y.; He, Y. Yunnan Black Tea Flavonoids Can Improve Cognitive Dysfunction in Septic Mice by Activating SIRT1. Evid. Based Complement. Altern. Med. 2021, 2021, e5775040. [Google Scholar] [CrossRef] [PubMed]
- Condezo-Hoyos, L.; Gazi, C.; Pérez-Jiménez, J. Design of Polyphenol-Rich Diets in Clinical Trials: A Systematic Review. Food Res. Int. 2021, 149, 110655. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules 2021, 11, 543. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Chapter 2—Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. ISBN 978-0-12-814774-0. [Google Scholar]
- Hamsalakshmi; Alex, A.M.; Marappa, M.A.; Joghee, S.; Chidambaram, S.B. Therapeutic Benefits of Flavonoids against Neuroinflammation: A Systematic Review. Inflammopharmacology 2022, 30, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of Plant Alkaloids on Human Health: A Review of Biological Activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Li, D.-D.; Zheng, C.-Q.; Zhang, F.; Shi, J.-S. Potential Neuroprotection by Dendrobium Nobile Lindl Alkaloid in Alzheimer’s Disease Models. Neural Regen. Res. 2021, 17, 972–977. [Google Scholar] [CrossRef]
- Plazas, E.; Avila M, M.C.; Muñoz, D.R.; Cuca S, L.E. Natural Isoquinoline Alkaloids: Pharmacological Features and Multi-Target Potential for Complex Diseases. Pharmacol. Res. 2022, 177, 106126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, H.; Yang, K.; Yang, T.; Zhou, R.; Miao, R.; Zhan, G.; Guo, Z. New Phenylpropanoids and Monoterpene Alkaloids with Vasorelaxant Activities from the Branches of Alstonia Scholaris. Fitoterapia 2022, 158, 105143. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant Alkaloids: Structures and Bioactive Properties. In Plant-derived Bioactives: Chemistry and Mode of Action; Swamy, M.K., Ed.; Springer: Singapore, 2020; pp. 85–117. ISBN 9789811523618. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Bachute, M.; Kotecha, K. Identification and Classification of the Tea Samples by Using Sensory Mechanism and Arduino UNO. Inventions 2021, 6, 94. [Google Scholar] [CrossRef]
- Tea Association of the USA. Tea Fact Sheet. Available online: http://www.teausa.com/14655/tea-fact-sheet (accessed on 27 February 2022).
- Bortolini, D.G.; Haminiuk, C.W.I.; Pedro, A.C.; Fernandes, I.d.A.A.; Maciel, G.M. Processing, Chemical Signature and Food Industry Applications of Camellia sinensis Teas: An Overview. Food Chem. X 2021, 12, 100160. [Google Scholar] [CrossRef]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Nutr. Assoc. 2020, 41, 65–93. [Google Scholar] [CrossRef]
- Silva, A.P.; Franco, M.I.; Mady, C.; Pallet, D.; Tomlins, K.; Bennett, B.; Pintado, M.; Sottomayor, M. Drivers of Acceptance of a New Beverage in Europe. Beverages 2016, 2, 12. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef]
- Biénabe, E.; Marie-Vivien, D. Institutionalizing Geographical Indications in Southern Countries: Lessons Learned from Basmati and Rooibos. World Dev. 2017, 98, 58–67. [Google Scholar] [CrossRef]
- Gregianini, T.; Winge, H. Storage Protein Variability in Natural Populations of Maté (Ilex paraguariensis) in Brazil. Ciênc. Rural 2019, 49, e20180451. [Google Scholar] [CrossRef]
- Huang, S.-H.; Kao, Y.-H.; Muller, C.J.F.; Joubert, E.; Chuu, C.-P. Aspalathin-Rich Green Aspalathus linearis Extract Suppresses Migration and Invasion of Human Castration-Resistant Prostate Cancer Cells via Inhibition of YAP Signaling. Phytomedicine 2020, 69, 153210. [Google Scholar] [CrossRef] [PubMed]
- Orlando, P.; Chellan, N.; Louw, J.; Tiano, L.; Cirilli, I.; Dludla, P.; Joubert, E.; Muller, C.J.F. Aspalathin-Rich Green Rooibos Extract Lowers LDL-Cholesterol and Oxidative Status in High-Fat Diet-Induced Diabetic Vervet Monkeys. Molecules 2019, 24, 1713. [Google Scholar] [CrossRef]
- Gebara, K.S.; Gasparotto Junior, A.; Palozi, R.A.; Morand, C.; Bonetti, C.I.; Gozzi, P.T.; de Mello, M.R.; Costa, T.A.; Cardozo Junior, E.L. A Randomized Crossover Intervention Study on the Effect a Standardized Mate Extract (Ilex paraguariensis A. St.-Hil.) in Men Predisposed to Cardiovascular Risk. Nutrients 2021, 13, 14. [Google Scholar] [CrossRef]
- Yerba Mate Market Size 2022–2027|Key Players, Regional Analysis, Segmentation by Types and Applications|Opportunities, Challenges, Trends, Drivers|Shares, Revenue, and Sales. Available online: https://finance.yahoo.com/news/yerba-mate-market-size-2022-120900894.html (accessed on 2 June 2022).
- Heiberg, T.; Hutchings, M. Unique Regional Status to South Africa’s Rooibos Tea Can Turn Fortunes for Crop. Reuters. 2021. Available online: https://www.reuters.com/world/africa/unique-regional-status-south-africas-rooibos-tea-can-turn-fortunes-crop-2021-08-12/ (accessed on 28 April 2022).
- Elisha, I.L.; Viljoen, A. Trends in Rooibos Tea (Aspalathus linearis) Research (1994–2018): A Scientometric Assessment. South Afr. J. Bot. 2021, 137, 159–170. [Google Scholar] [CrossRef]
- FAO. Intergovernmental Group on Tea|FAO|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/markets-and-trade/commodities/tea/fao-intergovernmental-group-on-tea/en/ (accessed on 2 June 2022).
- Islam, M. Food and Medicinal Values of Roselle (Hibiscus sabdariffa L. Linne Malvaceae) Plant Parts: A Review. Rev. Artic. 2019, 1, 1003. [Google Scholar]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.; Ferreira, I.C. Hibiscus sabdariffa L. as a Source of Nutrients, Bioactive Compounds and Colouring Agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef]
- Tsai, P.-J.; McIntosh, J.; Pearce, P.; Camden, B.; Jordan, B.R. Anthocyanin and Antioxidant Capacity in Roselle (Hibiscus sabdariffa L.) Extract. Food Res. Int. 2002, 35, 351–356. [Google Scholar] [CrossRef]
- Hou, D.-X.; Tong, X.; Terahara, N.; Luo, D.; Fujii, M. Delphinidin 3-Sambubioside, a Hibiscus Anthocyanin, Induces Apoptosis in Human Leukemia Cells through Reactive Oxygen Species-Mediated Mitochondrial Pathway. Arch. Biochem. Biophys. 2005, 440, 101–109. [Google Scholar] [CrossRef]
- Sogo, T.; Terahara, N.; Hisanaga, A.; Kumamoto, T.; Yamashiro, T.; Wu, S.; Sakao, K.; Hou, D.-X. Anti-Inflammatory Activity and Molecular Mechanism of Delphinidin 3-Sambubioside, a Hibiscus Anthocyanin. BioFactors 2015, 41, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Stander, M.A.; Joubert, E.; De Beer, D. Revisiting the Caffeine-Free Status of Rooibos and Honeybush Herbal Teas Using Specific MRM and High Resolution LC-MS Methods. J. Food Compos. Anal. 2019, 76, 39–43. [Google Scholar] [CrossRef]
- Muller, C.J.F.; Joubert, E.; Chellan, N.; Miura, Y.; Yagasaki, K. New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes—A Review. Int. J. Mol. Sci. 2022, 23, 356. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Beelders, T.; de Beer, D.; Malherbe, C.J.; de Villiers, A.J.; Sigge, G.O. Variation in Phenolic Content and Antioxidant Activity of Fermented Rooibos Herbal Tea Infusions: Role of Production Season and Quality Grade. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf302583r (accessed on 27 February 2022).
- Cardozo, A.G.L.; da Rosa, R.L.; Novak, R.S.; Folquitto, D.G.; Schebelski, D.J.; Brusamarello, L.C.C.; Ribeiro, D.T.B. Erva-mate (Ilex paraguariensis A. St.–hil.): Uma revisão abrangente sobre composição química, benefícios à saúde e recentes avanços. Res. Soc. Dev. 2021, 10, e590101120036. [Google Scholar] [CrossRef]
- Teselkin, Y.O.; Babenkova, I.V.; Pavlova, L.A.; Lee, A.; Kochetova, A.A.; Osipov, A.N.; Vladimirov, Y.A. The Antioxidant Capacity of Aqueous Extracts from Yerba Mate (Ilex paraguariensis). Biophysics 2021, 66, 125–132. [Google Scholar] [CrossRef]
- Jang, S.-H.; Hossain, M.A.; Lee, J.S.; Reza, M.A.; Lee, S.-P.; Kang, J.; Park, S.-C. Hepatoprotective Effects of Ilex paraguariensis St. Hilaire (Yerba Mate) Extract in Rats. Indian J. Tradit. Knowl. 2018, 17, 707–715. [Google Scholar]
- Paluch, E.; Okińczyc, P.; Zwyrzykowska-Wodzińska, A.; Szperlik, J.; Żarowska, B.; Duda-Madej, A.; Bąbelewski, P.; Włodarczyk, M.; Wojtasik, W.; Kupczyński, R.; et al. Composition and Antimicrobial Activity of Ilex Leaves Water Extracts. Molecules 2021, 26, 7442. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.J.; Othman, L.; Elchoueiry, M.; Ghanem, R.; Bazzi, S.; El-Sabban, M.; Abdel-Massih, R.M. Anti-Proliferative Activity of Yerba Mate (Ilex paraguariensis) Aqueous Extracts on Human Colorectal Cancer Cell Lines. Funct. Foods Health Dis. 2021, 11, 499–511. [Google Scholar] [CrossRef]
- Cao, X.; Xia, Y.; Zeng, M.; Wang, W.; He, Y.; Liu, J. Caffeic Acid Inhibits the Formation of Advanced Glycation End Products (AGEs) and Mitigates the AGEs-Induced Oxidative Stress and Inflammation Reaction in Human Umbilical Vein Endothelial Cells (HUVECs). Chem. Biodivers. 2019, 16, e1900174. [Google Scholar] [CrossRef]
- Luo, S.; Sun, X.; Huang, M.; Ma, Q.; Du, L.; Cui, Y. Enhanced Neuroprotective Effects of Epicatechin Gallate Encapsulated by Bovine Milk-Derived Exosomes against Parkinson’s Disease through Antiapoptosis and Antimitophagy. J. Agric. Food Chem. 2021, 69, 5134–5143. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin Hinders Microglial Activation to Alleviate Neurotoxicity via the Interplay between NLRP3 Inflammasome and Mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C.-S. Protective Effect of 3,5-dicaffeoyl-epi-quinic Acid against UVB-induced Photoaging in Human HaCaT Keratinocytes. Mol. Med. Rep. 2019, 20, 763–770. [Google Scholar] [CrossRef]
- Fuggetta, M.P.; Zonfrillo, M.; Villivà, C.; Bonmassar, E.; Ravagnan, G. Inflammatory Microenvironment and Adipogenic Differentiation in Obesity: The Inhibitory Effect of Theobromine in a Model of Human Obesity In Vitro. Mediat. Inflamm. 2019, 2019, e1515621. [Google Scholar] [CrossRef] [PubMed]
- El-Marasy, S.; El-Shenawy, S.M.; Moharram, F.; El-Sherbeeny, N. Antidiabetic and Antioxidant Effects of Acteoside from Jacaranda Mimosifolia Family Biognoniaceae in Streptozotocin–Nicotinamide Induced Diabetes in Rats. Open Access Maced. J. Med. Sci. 2020, 8, 125–133. [Google Scholar] [CrossRef]
- Yang, S.; Lee, C.; Lee, B.-S.; Park, E.K.; Kim, K.-M.; Bae, J.-S. Renal Protective Effects of Aspalathin and Nothofagin from Rooibos (Aspalathus linearis) in a Mouse Model of Sepsis. Pharmacol. Rep. 2018, 70, 1195–1201. [Google Scholar] [CrossRef]
- Hosny, E.N.; Sawie, H.G.; Elhadidy, M.E.; Khadrawy, Y.A. Evaluation of Antioxidant and Anti-Inflammatory Efficacy of Caffeine in Rat Model of Neurotoxicity. Nutr. Neurosci. 2019, 22, 789–796. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of DA Neurons in a Parkinsonian Mouse Model. Oxidative Med. Cell. Longev. 2020, 2020, e6571484. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Itharat, A.; Khonsung, P.; Lertprasertsuke, N.; Jaijoy, K. Toxicity Studies of the Water Extract from the Calyces of Hibiscus sabdariffa L. in Rats. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 122–127. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, F.; de Albuquerque, C.A.C.; Maraschin, M.; da Silva, E.L. Safety Assessment of Yerba Mate (Ilex paraguariensis) Dried Extract: Results of Acute and 90days Subchronic Toxicity Studies in Rats and Rabbits. Food Chem. Toxicol. 2012, 50, 328–334. [Google Scholar] [CrossRef]
- Engels, M.; Wang, C.; Matoso, A.; Maidan, E.; Wands, J. Tea Not Tincture: Hepatotoxicity Associated with Rooibos Herbal Tea. ACG Case Rep. J. 2013, 1, 58–60. [Google Scholar] [CrossRef]
- Carrier, P.; Debette-Gratien, M.; Jacques, J.; Grau, M.; Loustaud-Ratti, V. Rooibos, a Fake Friend. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101499. [Google Scholar] [CrossRef]
- Tsukahara, H.; Shibata, R.; Ohta, N.; Sato, S.; Hiraoka, M.; Ito, S.; Noiri, E.; Mayumi, M. High Levels of Urinary Pentosidine, an Advanced Glycation End Product, in Children with Acute Exacerbation of Atopic Dermatitis: Relationship with Oxidative Stress. Metabolism 2003, 52, 1601–1605. [Google Scholar] [CrossRef]
- Shaw, J.N.; Baynes, J.W.; Thorpe, S.R. N ε-(Carboxymethyl) Lysine (CML) as a Biomarker of Oxidative Stress in Long-Lived Tissue Proteins. In Oxidative Stress Biomarkers and Antioxidant Protocols; Springer: Berlin/Heidelberg, Germany, 2002; pp. 129–137. [Google Scholar]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s Disease: Current Status and Prospects for the Future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef]
- Paolillo, F.R.; Mattos, V.S.; Borghi-Silva, A.; Bagnato, V.S.; de Castro Neto, J.C. Advanced Glycation Endproducts as Biomarkers for Risk of Diabetes and Cardiovascular Diseases by Skin Autofluorescence: A Noninvasive Optical Screening. Photobiomodulation Photomed. Laser Surg. 2019, 37, 168–174. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, e9613090. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Shigi, K.; Fukuda, K. Reactive Oxygen Species Induce Cox-2 Expression via TAK1 Activation in Synovial Fibroblast Cells. FEBS Open Bio 2015, 5, 492–501. [Google Scholar] [CrossRef]
- Sentellas, S.; Morales-Ibanez, O.; Zanuy, M.; Albertí, J.J. GSSG/GSH Ratios in Cryopreserved Rat and Human Hepatocytes as a Biomarker for Drug Induced Oxidative Stress. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2014, 28, 1006–1015. [Google Scholar] [CrossRef]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen Peroxide: A Metabolic by-Product or a Common Mediator of Ageing Signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Niki, E. Biomarkers of Lipid Peroxidation in Clinical Material. Biochim. Biophys. Acta 2014, 1840, 809–817. [Google Scholar] [CrossRef]
- Uzbekov, M.G. Monoamine Oxidase as a Potential Biomarker of the Efficacy of Treatment of Mental Disorders. Biochem. Biokhimiia 2021, 86, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Lasola, J.J.M.; Kamdem, H.; McDaniel, M.W.; Pearson, R.M. Biomaterial-Driven Immunomodulation: Cell Biology-Based Strategies to Mitigate Severe Inflammation and Sepsis. Front. Immunol. 2020, 11, 1726. [Google Scholar] [CrossRef]
- Costa, N.A.; Gut, A.L.; Azevedo, P.S.; Tanni, S.E.; Cunha, N.B.; Magalhães, E.S.; Silva, G.B.; Polegato, B.F.; Zornoff, L.A.M.; de Paiva, S.A.R.; et al. Erythrocyte Superoxide Dismutase as a Biomarker of Septic Acute Kidney Injury. Ann. Intensive Care 2016, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Jayamohananan, H.; Manoj Kumar, M.K.; Aneesh, T.P. 5-HIAA as a Potential Biological Marker for Neurological and Psychiatric Disorders. Adv. Pharm. Bull. 2019, 9, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Yi, R.; Wei, Y.; Tan, F.; Mu, J.; Long, X.; Pan, Y.; Liu, W.; Zhao, X. Antioxidant Capacity-Related Preventive Effects of Shoumei (Slightly Fermented Camellia sinensis) Polyphenols against Hepatic Injury. Oxidative Med. Cell. Longev. 2020, 2020, e9329356. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, F.; Li, C.; Li, W.; Liao, W.; Li, Q.; Qin, G.; Liu, W.; Zhao, X. White Peony (Fermented Camellia sinensis) Polyphenols Help Prevent Alcoholic Liver Injury via Antioxidation. Antioxidants 2019, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Shalgum, A.; Govindarajulu, M.; Majrashi, M.; Ramesh, S.; Collier, W.E.; Griffin, G.; Amin, R.; Bradford, C.; Moore, T.; Dhanasekaran, M. Neuroprotective Effects of Hibiscus sabdariffa against Hydrogen Peroxide-Induced Toxicity. J. Herb. Med. 2019, 17–18, 100253. [Google Scholar] [CrossRef]
- Pillai, S.S.; Mini, S. Polyphenols Rich Hibiscus Rosa Sinensis Linn. Petals Modulate Diabetic Stress Signalling Pathways in Streptozotocin-Induced Experimental Diabetic Rats. J. Funct. Foods 2016, 20, 31–42. [Google Scholar] [CrossRef]
- Hong, I.-S.; Lee, H.-Y.; Kim, H.-P. Anti-Oxidative Effects of Rooibos Tea (Aspalathus linearis) on Immobilization-Induced Oxidative Stress in Rat Brain. PLoS ONE 2014, 9, e87061. [Google Scholar] [CrossRef]
- Oboh, G.; Adewuni, T.M.; Ademiluyi, A.O.; Olasehinde, T.A.; Ademosun, A.O. Phenolic Constituents and Inhibitory Effects of Hibiscus sabdariffa L. (Sorrel) Calyx on Cholinergic, Monoaminergic, and Purinergic Enzyme Activities. J. Diet. Suppl. 2018, 15, 910–922. [Google Scholar] [CrossRef]
- Pringle, N.; Koekemoer, T.; Holzer, A.; Young, C.; Venables, L.; van de Venter, M. Potential Therapeutic Benefits of Green and Fermented Rooibos (Aspalathus linearis) in Dermal Wound Healing. Planta Med. 2018, 84, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, A.; Veeresham, C.; Rama Rao, A. Aldose Reductase and Advanced Glycation End Products Formation Inhibitory Activity of Standardized Extracts of Picrorhiza Kurroa (Royle Ex Benth) and Hibiscus Rosa-Sinensis (Linn.). Pharm. Biol. Eval. 2017, 4, 198–206. [Google Scholar] [CrossRef]
- Pereira, D.F.; Kappel, V.D.; Cazarolli, L.H.; Boligon, A.A.; Athayde, M.L.; Guesser, S.M.; Da Silva, E.L.; Silva, F.R.M.B. Influence of the Traditional Brazilian Drink Ilex paraguariensis Tea on Glucose Homeostasis. Phytomedicine 2012, 19, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Sánchez, A.; Martinez, P.; Benedito, S.; López-Oliva, M.-E.; García-Sacristán, A.; Hernández, M.; Prieto, D. COX-2 Is Involved in Vascular Oxidative Stress and Endothelial Dysfunction of Renal Interlobar Arteries from Obese Zucker Rats. Free Radic. Biol. Med. 2015, 84, 77–90. [Google Scholar] [CrossRef]
- Sturza, A.; Popoiu, C.M.; Ionică, M.; Duicu, O.M.; Olariu, S.; Muntean, D.M.; Boia, E.S. Monoamine Oxidase-Related Vascular Oxidative Stress in Diseases Associated with Inflammatory Burden. Oxidative Med. Cell. Longev. 2019, 2019, e8954201. [Google Scholar] [CrossRef]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, e5028181. [Google Scholar] [CrossRef]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.E.; Colpo, A.C.; Maya-López, M.; Rosa, H.; Túnez, I.; Galván-Arzate, S.; Santamaría, A.; Folmer, V. Protective Effect of Yerba Mate (Ilex paraguariensis St. Hill.) against Oxidative Damage in Vitro in Rat Brain Synaptosomal/Mitochondrial P2 Fractions. J. Funct. Foods 2017, 34, 447–452. [Google Scholar] [CrossRef]
- Colpo, A.C.; de Lima, M.E.; Maya-López, M.; Rosa, H.; Márquez-Curiel, C.; Galván-Arzate, S.; Santamaría, A.; Folmer, V. Compounds from Ilex paraguariensis Extracts Have Antioxidant Effects in the Brains of Rats Subjected to Chronic Immobilization Stress. Appl. Physiol. Nutr. Metab. 2017, 42, 1172–1178. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.; Mackenzie, T.; Corstjens, H.; Muizzuddin, N.; Matsui, M.S. Two Fluorescent Wavelengths, 440ex/520em Nm and 370ex/440em Nm, Reflect Advanced Glycation and Oxidation End Products in Human Skin Without Diabetes. Diabetes Technol. Ther. 2012, 14, 285–292. [Google Scholar] [CrossRef]
- Raposeiras-Roubín, S.; Rodiño-Janeiro, B.K.; Paradela-Dobarro, B.; Grigorian-Shamagian, L.; García-Acuña, J.M.; Aguiar-Souto, P.; Jacquet-Hervet, M.; Reino-Maceiras, M.V.; González-Juanatey, J.R.; Álvarez, E. Fluorescent Advanced Glycation End Products and Their Soluble Receptor: The Birth of New Plasmatic Biomarkers for Risk Stratification of Acute Coronary Syndrome. PLoS ONE 2013, 8, e74302. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.M.; Schulze, J.; Souza, M.F.F. Caffeic and Chlorogenic Acids in Ilex paraguariensis Extracts Are the Main Inhibitors of AGE Generation by Methylglyoxal in Model Proteins. Fitoterapia 2009, 80, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Bains, Y.; Gugliucci, A. Ilex paraguariensis and Its Main Component Chlorogenic Acid Inhibit Fructose Formation of Advanced Glycation Endproducts with Amino Acids at Conditions Compatible with Those in the Digestive System. Fitoterapia 2017, 117, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Incani, M.; Sentinelli, F.; Perra, L.; Pani, M.G.; Porcu, M.; Lenzi, A.; Cavallo, M.G.; Cossu, E.; Leonetti, F.; Baroni, M.G. Glycated Hemoglobin for the Diagnosis of Diabetes and Prediabetes: Diagnostic Impact on Obese and Lean Subjects, and Phenotypic Characterization. J. Diabetes Investig. 2015, 6, 44–50. [Google Scholar] [CrossRef]
- Chinchansure, A.A.; Korwar, A.M.; Kulkarni, M.J.; Joshi, S.P. Recent Development of Plant Products with Anti-Glycation Activity: A Review. RSC Adv. 2015, 5, 31113–31138. [Google Scholar] [CrossRef]
- Cai, R.; Chen, S.; Jiang, S. Chlorogenic acid inhibits non-enzymatic glycation and oxidation of low density lipoprotein. Zhejiang Xue Xue Bao Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2018, 47, 27–34. [Google Scholar]
- Peng, C.-H.; Chyau, C.-C.; Chan, K.-C.; Chan, T.-H.; Wang, C.-J.; Huang, C.-N. Hibiscus sabdariffa Polyphenolic Extract Inhibits Hyperglycemia, Hyperlipidemia, and Glycation-Oxidative Stress While Improving Insulin Resistance. Available online: https://pubs.acs.org/doi/pdf/10.1021/jf2022379 (accessed on 11 February 2022).
- Mezni, A.; Mhadhbi, L.; Khazri, A.; Sellami, B.; Dellali, M.; Mahmoudi, E.; Beyrem, H. The Protective Effect of Hibiscus sabdariffa Calyxes Extract against Cypermethrin Induced Oxidative Stress in Mice. Pestic. Biochem. Physiol. 2020, 165, 104463. [Google Scholar] [CrossRef] [PubMed]
- Branco, C.D.S.; Scola, G.; Rodrigues, A.D.; Cesio, V.; Laprovitera, M.; Heinzen, H.; dos Santos, M.T.; Fank, B.; de Freitas, S.C.V.; Coitinho, A.S.; et al. Anticonvulsant, Neuroprotective and Behavioral Effects of Organic and Conventional Yerba Mate (Ilex paraguariensis St. Hil.) on Pentylenetetrazol-Induced Seizures in Wistar Rats. Brain Res. Bull. 2013, 92, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, L.; Huang, L.; Tekliye, M.; Xia, X.; Li, J.; Dong, M. Composition, Antioxidant Activity, and Neuroprotective Effects of Anthocyanin-Rich Extract from Purple Highland Barley Bran and Its Promotion on Autophagy. Food Chem. 2021, 339, 127849. [Google Scholar] [CrossRef]
- Bakhtiari, E.; Hosseini, A.; Mousavi, S.H. Protective Effect of Hibiscus sabdariffa against Serum/Glucose Deprivation-Induced PC12 Cells Injury. Avicenna J. Phytomed. 2015, 5, 231–237. [Google Scholar]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Askarova, S.; Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C.-M. Role of Aβ-Receptor for Advanced Glycation Endproducts Interaction in Oxidative Stress and Cytosolic Phospholipase A2 Activation in Astrocytes and Cerebral Endothelial Cells. Neuroscience 2011, 199, 375–385. [Google Scholar] [CrossRef]
- Lai, C.-H.; Chou, C.-Y.; Ch’Ang, L.-Y.; Liu, C.-S.; Lin, W. Identification of Novel Human Genes Evolutionarily Conserved in Caenorhabditis Elegans by Comparative Proteomics. Genome Res. 2000, 10, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.L.; Arantes, L.P.; da Silveira, T.L.; Zamberlan, D.C.; Cordeiro, L.M.; Obetine, F.B.B.; da Silva, A.F.; da Cruz, I.B.M.; Soares, F.A.A.; de Oliveira, R.P. Ilex paraguariensis Extract Provides Increased Resistance against Oxidative Stress and Protection against Amyloid Beta-Induced Toxicity Compared to Caffeine in Caenorhabditis Elegans. Nutr. Neurosci. 2021, 24, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Borba Filho, G.L.; Zenki, K.C.; Kalinine, E.; Baggio, S.; Pettenuzzo, L.; Zimmer, E.R.; Weis, S.N.; Calcagnotto, M.E.; Onofre de Souza, D. A New Device for Step-Down Inhibitory Avoidance Task—Effects of Low and High Frequency in a Novel Device for Passive Inhibitory Avoidance Task That Avoids Bioimpedance Variations. PLoS ONE 2015, 10, e0116000. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris Water Maze: Procedures for Assessing Spatial and Related Forms of Learning and Memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- López, V.; Cásedas, G.; Petersen-Ross, K.; Powrie, Y.; Smith, C. Neuroprotective and Anxiolytic Potential of Green Rooibos (Aspalathus linearis) Polyphenolic Extract. Food Funct. 2022, 13, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Alshabi, A.M.; Shaikh, I.A.; Habeeb, M.S. Nootropic and Neuroprotective Effects of Ethanol Extract of Hibiscus sabdariffa L. on Scopolamine- Induced Cognitive Deficit in Mice. Curr. Top. Nutraceutical Res. 2021, 19, 9. [Google Scholar]
- Bortoli, P.M.; Alves, C.; Costa, E.; Vanin, A.P.; Sofiatti, J.R.; Siqueira, D.P.; Dallago, R.M.; Treichel, H.; Vargas, G.D.L.P.; Kaizer, R.R. Ilex paraguariensis: Potential Antioxidant on Aluminium Toxicity, in an Experimental Model of Alzheimer’s Disease. J. Inorg. Biochem. 2018, 181, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Milioli, E.M.; Cologni, P.; Santos, C.C.; Marcos, T.D.; Yunes, V.M.; Fernandes, M.S.; Schoenfelder, T.; Costa-Campos, L. Effect of Acute Administration of Hydroalcohol Extract of Ilex paraguariensis St Hilaire (Aquifoliaceae) in Animal Models of Parkinson’s Disease. Phytother. Res. 2007, 21, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.C.S.; Bicca, M.A.; Blum-Silva, C.H.; Costa, A.P.R.; dos Santos, A.A.; Schenkel, E.P.; Farina, M.; Reginatto, F.H.; de Lima, T.C.M. Anxiolytic-like, Stimulant and Neuroprotective Effects of Ilex paraguariensis Extracts in Mice. Neuroscience 2015, 292, 13–21. [Google Scholar] [CrossRef] [PubMed]
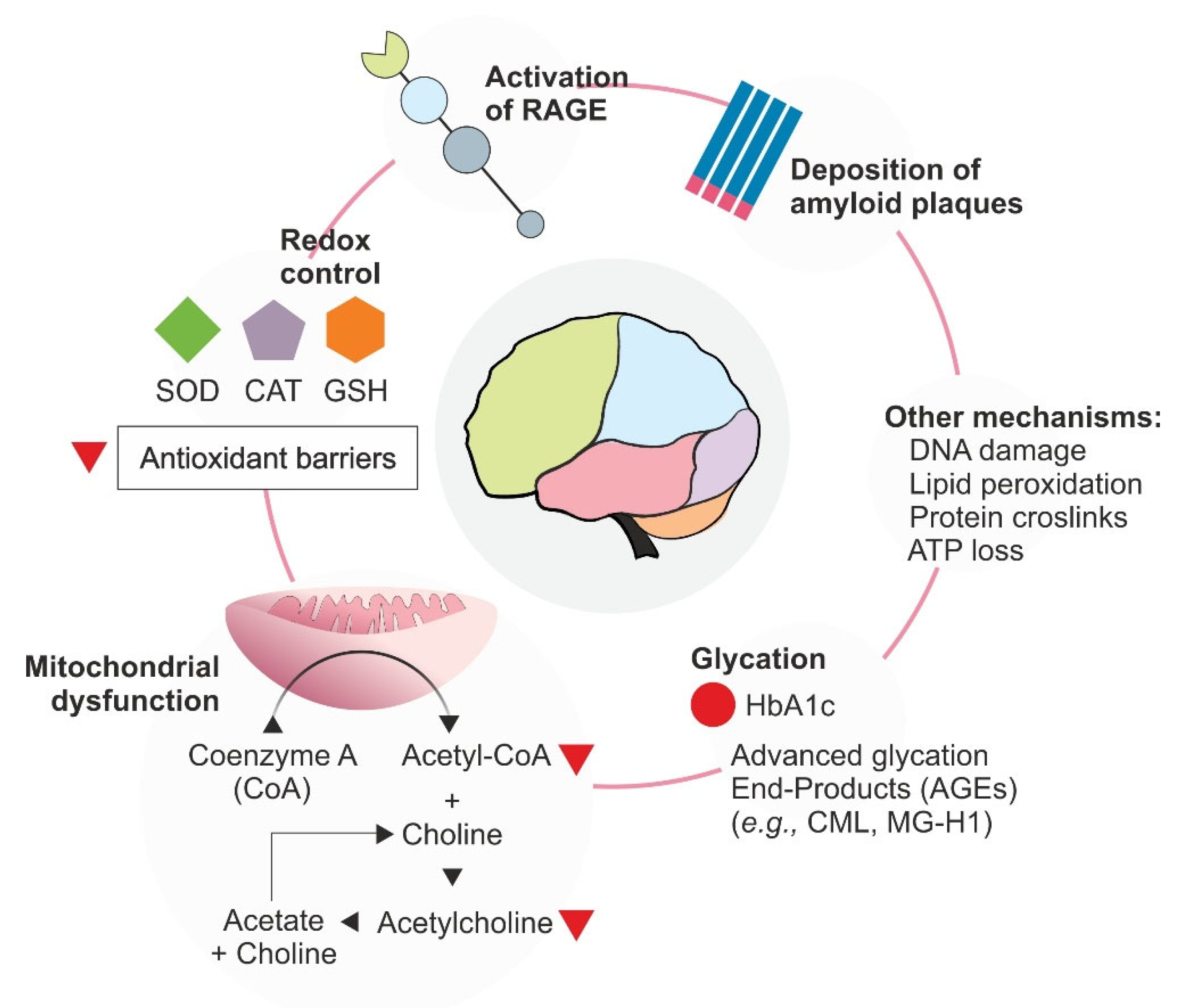

| Physiological Target | Biomarker | Pathological Implication | Reference |
|---|---|---|---|
| Neurodegeneration | Aβ-peptides (Amyloid beta-peptide) | Aβ cerebral deposition increases with AD progression | [126] |
| AChE (Acetylcholinesterase) | Participates in acetylcholine level decline in the genesis of AD | ||
| γ-secretase (Gamma secretase) | γ-secretase participates in Aβ-protein processing | ||
| Glycoxidation | Fluorescence (355/460 nm) | Marker of AGE occurrence (e.g., skin) | [127] |
| Oxidative stress | CAT (Catalase) | Takes part in cellular oxidative stress mitigation | [128] |
| COX-2 (Cyclooxygenase-2) | Inflammation and inflammation mediator | [129] | |
| GSH/GSSG (Reduced glutathione/oxidized glutathione ratio) | Redox balance indicator | [130] | |
| H2O2 (Hydrogen peroxide) | Mitochondrial dysfunction | [131] | |
| LDH (Lactate dehydrogenase) | Energy metabolism and cell senescence control | [132] | |
| Lipid peroxidation | Cellular lipid integrity biomarker | [133] | |
| MAO-A (Monoamine oxidase A) | Regulates amine metabolism, especially important for neurophysiology, associated with anxiety or depression studies | [134] | |
| MPO (Myeloperoxidase) | MPO is mostly produced by immune cells, especially neutrophils, being involved with both inflammation and oxidative stress | [135] | |
| SOD (Superoxide dismutase) | Plays a role in oxidative stress and cell injury indication | [136] | |
| HIAA (5-Hydroxyindoleacetic acid) | Product of serotonin metabolism pathway used as a biomarker of neurological injury | [137] |
| Extract | Measure | Dose or EC50 | Reference |
|---|---|---|---|
| Aqueous | AChE inhibition | Control (galantamine): IC50 7 μg/mL White hibiscus extract: IC50 123 μg/mL Red hibiscus extract: IC50 106 μg/mL | [25] |
| Ethanolic | PC12 cells Inhibition of cell apoptosis | Control (SGD): 65 apoptotic cells Extract (60 µg/mL): 30 apoptotic cells | [166] |
| Methanolic | AChE inhibition | IC50 46.96 μg/mL | [144] |
| BChE inhibition | EC50 40.38 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira Silva Lima, M.T.; Boulanger, E.; Tessier, F.J.; Takahashi, J.A. Hibiscus, Rooibos, and Yerba Mate for Healthy Aging: A Review on the Attenuation of In Vitro and In Vivo Markers Related to Oxidative Stress, Glycoxidation, and Neurodegeneration. Foods 2022, 11, 1676. https://doi.org/10.3390/foods11121676
Nogueira Silva Lima MT, Boulanger E, Tessier FJ, Takahashi JA. Hibiscus, Rooibos, and Yerba Mate for Healthy Aging: A Review on the Attenuation of In Vitro and In Vivo Markers Related to Oxidative Stress, Glycoxidation, and Neurodegeneration. Foods. 2022; 11(12):1676. https://doi.org/10.3390/foods11121676
Chicago/Turabian StyleNogueira Silva Lima, Matheus Thomaz, Eric Boulanger, Frédéric J. Tessier, and Jacqueline Aparecida Takahashi. 2022. "Hibiscus, Rooibos, and Yerba Mate for Healthy Aging: A Review on the Attenuation of In Vitro and In Vivo Markers Related to Oxidative Stress, Glycoxidation, and Neurodegeneration" Foods 11, no. 12: 1676. https://doi.org/10.3390/foods11121676
APA StyleNogueira Silva Lima, M. T., Boulanger, E., Tessier, F. J., & Takahashi, J. A. (2022). Hibiscus, Rooibos, and Yerba Mate for Healthy Aging: A Review on the Attenuation of In Vitro and In Vivo Markers Related to Oxidative Stress, Glycoxidation, and Neurodegeneration. Foods, 11(12), 1676. https://doi.org/10.3390/foods11121676