Consumption of a Gelatin Supplemented with the Probiotic Strain Limosilactobacillus fermentum UCO-979C Prevents Helicobacter pylori Infection in a Young Adult Population Achieved
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Supervision
2.2. Eligibility and Recruiting of Participants
2.3. Procedures
2.4. Culture of the Probiotic Bacterial Strain, Production of the Probiotic Biomass and Preparation of Gelatin
2.5. Data Collection
2.6. Statistical Analysis
3. Results
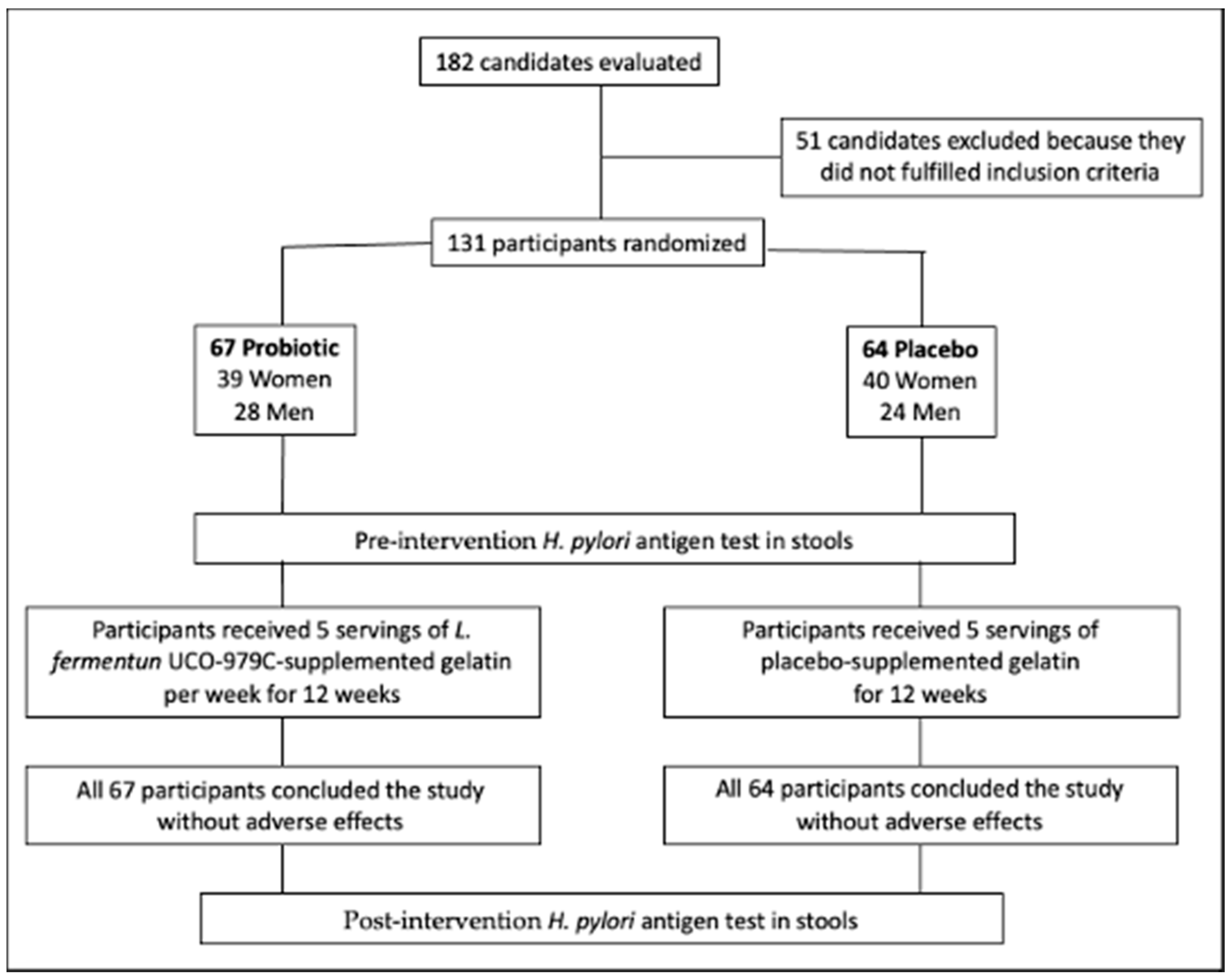
3.1. Recruiting and Participants
3.2. Evaluation of Participants
3.3. Viability of the Probiotic in the Gelatin
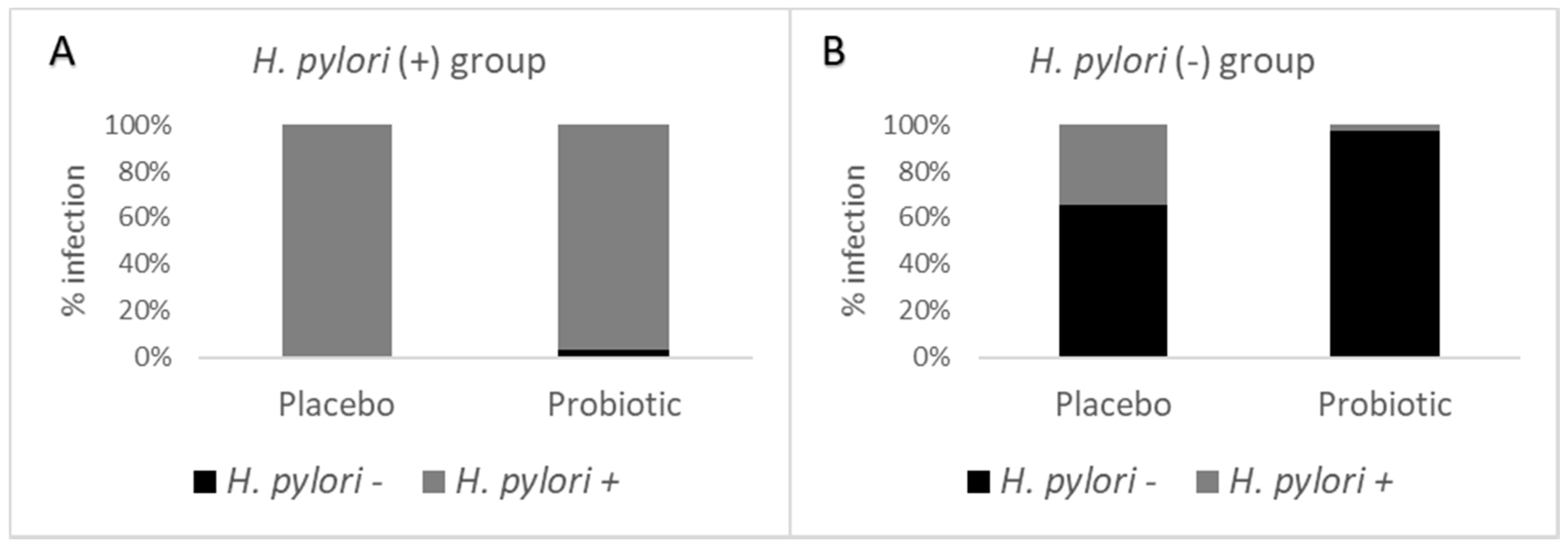
3.4. Detection of H. pylori Using an Immunoassay in Stools before and after Consuming the Probiotic
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Razik, A.; Mousa, N.; Shabana, W.; Refaey, M.; Elhelaly, R.; Elzehery, R.; Abdelsalam, M.; Elgamal, A.; Nassar, M.R.; Abu El-Soud, A.; et al. Helicobacter pylori and Non-Alcoholic Fatty Liver Disease: A New Enigma? Helicobacter 2018, 23, e12537. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. World Health Organization Releases Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. JMS J. Med. Soc. 2018, 32, 76–77. [Google Scholar] [CrossRef]
- Csendes, A.; Figueroa, M. Situación Del Cáncer Gástrico En El Mundo y En Chile. Rev. Chil. Cir. 2017, 69, 502–507. [Google Scholar] [CrossRef]
- Hunt, R.H.; Xiao, S.D.; Megraud, F.; Leon-Barua, R.; Bazzoli, F.; Van der Merwe, S.; Krabshuis, J.H. Guías Prácticas de La Organización Mundial de Gastroenterología (WGO): Helicobacter pylori En Los Países En Desarrollo. Gastroenterol. Lat. 2010, 21, 165–181. [Google Scholar]
- Ferreccio, C.; Rollán, A.; Harris, P.R.; Serrano, C.; Gederlini, A.; Margozzini, P.; Gonzalez, C.; Aguilera, X.; Venegas, A.; Jara, A. Gastric Cancer Is Related to Early Helicobacter pylori Infection in a High-Prevalence Country. Cancer Epidemiol. Biomark. Prev. 2007, 16, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Jaime, F.; Villagrán, A.; Serrano, C.; Cerda, J.; Harris, P.R. Frequency of Helicobacter pylori Infection in 144 School Age Chilean Children. Rev. Med. Chil. 2013, 141, 1249–12454. [Google Scholar] [CrossRef]
- Rollan, A.; Arab, J.P.; Camargo, M.C.; Candia, R.; Harris, P.; Ferreccio, C.; Rabkin, C.S.; Gana, J.C.; Cortés, P.; Herrero, R.; et al. Management of Helicobacter pylori Infection in Latin America: A Delphi Technique-Based Consensus. World J. Gastroenterol. 2014, 20, 10969–10983. [Google Scholar] [CrossRef]
- Guevara, B.; Cogdill, A.G. Helicobacter pylori: A Review of Current Diagnostic and Management Strategies. Dig. Dis. Sci. 2020, 65, 1917–1931. [Google Scholar] [CrossRef]
- Nutrition Division. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria-Joint FAO/WHO Expert Consultation. FAO/WHO. 2006. Available online: https://www.fao.org/publications/card/es/c/7c102d95-2fd5-5b22-8faf-f0b2e68dfbb6/ (accessed on 15 May 2022).
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Küster-Boluda, I.; Vidal-Capilla, I. Consumer Attitudes in the Election of Functional Foods. Span. J. Mark. ESIC 2017, 21, 65–79. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Bacillus Coagulans MTCC 5856 Supplementation in the Management of Diarrhea Predominant Irritable Bowel Syndrome: A Double Blind Randomized Placebo Controlled Pilot Clinical Study. Nutr. J. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.E.; Eliakim, R.; Fedorak, R.; Gangl, A.; Garisch, J.; Kaufmann, P.; Karakan, T.; Khan, A.; Kim, N.; et al. Probióticos y Prebióticos; World Gastroenterology Organisation: Milwaukee, WI, USA, 2017; pp. 1–35. [Google Scholar]
- Restrepo, Y.A.R.; Rojas, A.F.; Rodríguez-Barona, S. Encapsulación De Probióticos Para Aplicaciones Alimenticias. Biosalud 2016, 15, 106–115. [Google Scholar] [CrossRef]
- Miranda, J.S.; Costa, B.V.; de Oliveira, I.V.; de Lima, D.C.N.; Martins, E.M.F.; de Castro Leite Júnior, B.R.; Almeida do Nascimento Benevenuto, W.C.; Campelo de Queiroz, I.; Ribeiro da Silva, R.; Martins, M.L. Probiotic Jelly Candies Enriched with Native Atlantic Forest Fruits and Bacillus coagulans GBI-30 6086. LWT 2020, 126, 109275. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.; Te Chuan, L.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Abedinia, A.; Alimohammadi, F.; Teymori, F.; Razgardani, N.; Saeidi Asl, M.R.; Ariffin, F.; Mohammadi Nafchi, A.; Huda, N.; Roslan, J. Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics. Foods 2021, 10, 1761. [Google Scholar] [CrossRef]
- Rabaioli Rama, G.; Dall’Agnol, W.; Esquerdo, V.; Lehn, D.; Dullius, D.; Souza, C. Ricotta Whey Supplemented with Gelatin and Collagen for the Encapsulation of Probiotic Lactic Acid Bacteria. Food Sci. Technol. 2020, 41, 576–586. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi Esfahani, Z.; Lacroix, M. Gelatin and Low Methoxyl Pectin Films Containing Probiotics: Film Characterization and Cell Viability. Food Biosci. 2020, 36, 100660. [Google Scholar] [CrossRef]
- Salas-Jara, M.J.; Sanhueza, E.A.; Retamal-Díaz, A.; González, C.; Urrutia, H.; García, A. Probiotic Lactobacillus fermentum UCO-979C Biofilm Formation on AGS and Caco-2 Cells and Helicobacter pylori Inhibition. Biofouling 2016, 32, 1245–1257. [Google Scholar] [CrossRef]
- Garcia-Castillo, V.; Komatsu, R.; Clua, P.; Indo, Y.; Takagi, M.; Salva, S.; Islam, M.A.; Alvarez, S.; Takahashi, H.; Garcia-Cancino, A.; et al. Evaluation of the Immunomodulatory Activities of the Probiotic Strain Lactobacillus fermentum UCO-979C. Front. Immunol. 2019, 10, 1376. [Google Scholar] [CrossRef]
- Valenzuela, M.; Albar, J.P.; Paradela, A.; Toledo, H. Helicobacter pylori Exhibits a Fur-Dependent Acid Tolerance Response. Helicobacter 2011, 16, 189–199. [Google Scholar] [CrossRef] [PubMed]
- García, C.A.; Henríquez, A.P.; Retamal, R.C.; Pineda, C.S.; Delgado Sen, C.; González, C.C. Propiedades Probióticas de Lactobacillus spp. Aislados de Biopsias Gástricas de Pacientes Con y Sin Infección Por Helicobacter pylori. Rev. Med. Chil. 2009, 137, 369–376. [Google Scholar] [CrossRef][Green Version]
- Merino, J.S.; Araneda, L.; Lincoñir-Campos, P.; Parra, C.; Sáez, K.; García, A. Dynamics of Helicobacter pylori Infection in Infants during the First Six Months of Life. Enferm. Infecc. Microbiol. Clin. 2019, 37, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castillo, V.; Marcial, G.; Albarracín, L.; Tomokiyo, M.; Clua, P.; Takahashi, H.; Kitazawa, H.; Garcia-Cancino, A.; Villena, J. The Exopolysaccharide of Lactobacillus fermentum UCO-979C Is Partially Involved in Its Immunomodulatory Effect and Its Ability to Improve the Resistance against Helicobacter pylori Infection. Microorganisms 2020, 8, 479. [Google Scholar] [CrossRef]
- Apablaza, P.C.; Corra, C.M.; Rojas, M.V.; Zamorano, C.G.; Alonso, K.S.; Sepúlveda, C.P.; Carrillo, K.S.; Cancino, A.G. Efecto Del Consumo Del Probiótico Lactobacillus fermentum UCO-979C En Peso, Masa Grasa, Masa Magra, Circunferencia de La Cintura y Hábitos Alimenticios Introducción Las Bacterias Gástricas Comensales Más Material y Métodos Ciento Ochenta y Dos Estudian. Span. J. Community Nutr. 2021, 27, 49–54. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Paucar-Carrión, C.; Espinoza-Monje, M.; Gutiérrez-Zamorano, C.; Sánchez-Alonzo, K.; Carvajal, R.I.; Rogel-Castillo, C.; Sáez-Carrillo, K.; García-Cancino, A. Incorporation of Limosilactobacillus fermentum UCO-979C with Anti-Helicobacter pylori and Immunomodulatory Activities in Various Ice Cream Bases. Foods 2022, 11, 333. [Google Scholar] [CrossRef]
- Doll, R.; Hill, A.B. Smoking and Carcinoma of the Lung; Preliminary Report. Br. Med. J. 1950, 2, 739–748. [Google Scholar] [CrossRef]
- Sachs, G.; Scott, D.R. Helicobacter pylori: Eradication or Preservation. F1000 Med. Rep. 2012, 4, 2–6. [Google Scholar] [CrossRef]
- Camargo, M.C.; García, A.; Riquelme, A.; Camargo, C.A.; Hernandez-García, T.; Candia, R.; Bruce, M.G.; Rabkin, C.S. The Problem of Helicobacter pylori Resistance to Antibiotics: A Systematic Review in Latin America. Am. J. Gastroenterol. 2014, 109, 485–495. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori Infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Savoldl, A.; Carrara, E.; Graham, D.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobcater pylori: Revisón Sistemática y Metaanálisis En Las Regiones de La OMS. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori Infection—The Maastricht IV/ Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Parra-Sepúlveda, C.; Merino, J.S.; Sáez-Carrillo, K.; González, C.; García-Cancino, A. Antibiotic Resistance Surveillance of Helicobacter pylori at the Biobío Region (Chile) in a Decade. Arq. Gastroenterol. 2019, 56, 361–366. [Google Scholar] [CrossRef]
- Ayala, G.; Escobedo-Hinojosa, W.I.; de La Cruz-Herrera, C.F.; Romero, I. Exploring Alternative Treatments for Helicobacter pylori Infection. World J. Gastroenterol. 2014, 20, 1450–1469. [Google Scholar] [CrossRef]
- Sutton, P.; Chionh, Y.T. Why Can’t We Make an Effective Vaccine against Helicobacter pylori? Expert Rev. Vaccines 2013, 12, 433–441. [Google Scholar] [CrossRef]
- Augusto Urrego, J.; Otero, W.; Trespalacios, A. Avances En La Búsqueda de La Vacuna Contra Helicobacter pylori. Rev. Médicas UIS 2017, 30, 111–120. [Google Scholar] [CrossRef][Green Version]
- Bielecka, M. Probiotics in Food. In Chemical and Functional Properties of Food Components, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 413–426. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R. Probiotics. Indian J. Med. Microbiol. 2009, 27, 202–209. [Google Scholar] [CrossRef]
- Alvarez-Calatayud, G.; Margolles, A. Dual-Coated Lactic Acid Bacteria: An Emerging Innovative Technology in the Field of Probiotics. Future Microbiol. 2016, 11, 467–475. [Google Scholar] [CrossRef]
- Bhatia, S.J.; Kochar, N.; Abraham, P.; Nair, N.G.; Mehta, A.P. Lactobacillus acidophilus Inhibits Growth of Campylobacter pylori in Vitro. J. Clin. Microbiol. 1989, 27, 2328–2330. [Google Scholar] [CrossRef]
- Aiba, Y.; Ishikawa, H.; Tokunaga, M.; Komatsu, Y. Anti-Helicobacter pylori Activity of Non-Living, Heat-Killed Form of Lactobacilli Including Lactobacillus johnsonii No.1088. FEMS Microbiol. Lett. 2017, 364, fnx102. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Kannan, S. Probiotics: The Marvelous Factor and Health Benefits. Biomed. Biotechnol. Res. J. BBRJ 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Mukai, T.; Asasaka, T.; Sato, E.; Mori, K.; Matsumoto, M.; Ohori, H. Inhibition of Binding of Helicobacter pylori to the Glycolipid Receptors by Probiotic Lactobacillus reuteri. FEMS Immunol. Med. Microbiol. 2002, 32, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Xiaoguang, S.; Junhong, Z.; Lingshan, M.; Jialing, S.; Mengbin, Q.; Xue, H. Efficacy and Safety of Probiotics in Eradicating Helicobacter pylori. Nature 2018, 388, 539–547. [Google Scholar]
- McFarland, L.V.; Huang, Y.; Wang, L.; Malfertheiner, P. Systematic Review and Meta-Analysis: Multi-Strain Probiotics as Adjunct Therapy for Helicobacter pylori Eradication and Prevention of Adverse Events. United Eur. Gastroenterol. J. 2016, 4, 546–561. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Sáez, K.; Delgado, C.; González, C.L. Low Co-Existence Rates of Lactobacillus spp. and Helicobacter pylori Detected in Gastric Biopsies from Patients with Gastrointestinal Symptoms. Rev. Esp. Enferm. Dig. 2012, 104, 473–478. [Google Scholar] [CrossRef][Green Version]
- García, A.; Navarro, K.; Sanhueza, E.; Pineda, S.; Pastene, E.; Quezada, M.; Henríquez, K.; Karlyshev, A.; Villena, J.; González, C. Characterization of Lactobacillus fermentum UCO-979C, a Probiotic Strain with a Potent Anti-Helicobacter pylori Activity. Electron. J. Biotechnol. 2017, 25, 75–83. [Google Scholar] [CrossRef]
- Lorca, G.L.; Wadström, T.; Font de Valdez, G.; Ljungh, Å. Lactobacillus acidophilus Autolysins Inhibit Helicobacter pylori in Vitro. Curr. Microbiol. 2001, 42, 39–44. [Google Scholar] [CrossRef]
- Lebeer, S.; Verhoeven, T.L.A.; Vélez, M.P.; Vanderleyden, J.; De Keersmaecker, S.C.J. Impact of Environmental and Genetic Factors on Biofilm Formation by the Probiotic Strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2007, 73, 6768–6775. [Google Scholar] [CrossRef]
- Sgouras, D.N.; Panayotopoulou, E.G.; Martinez-Gonzalez, B.; Petraki, K.; Michopoulos, S.; Mentis, A. Lactobacillus johnsonii La1 Attenuates Helicobacter pylori-Associated Gastritis and Reduces Levels of Proinflammatory Chemokines in C57BL/6 Mice. Clin. Diagn. Lab. Immunol. 2005, 12, 1378–1386. [Google Scholar] [CrossRef]
- Butel, M.J. Probiotics, Gut Microbiota and Health. Med. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R. Genetics of Bacteriocins Produced by Lactic Acid Bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef]
- Ryan, K.A.; O’Hara, A.M.; Van Pijkeren, J.P.; Douillard, F.P.; O’Toole, P.W. Lactobacillus salivarius Modulates Cytokine Induction and Virulence Factor Gene Expression in Helicobacter pylori. J. Med. Microbiol. 2009, 58, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Speranza, B.; Corbo, M.R.; Sinigaglia, M. Effects of Nutritional and Environmental Conditions on Salmonella Sp. Biofilm Formation. J. Food Sci. 2011, 76, 12–16. [Google Scholar] [CrossRef]
- Terraf, M.C.L.; Juárez Tomás, M.S.; Nader-Macías, M.E.F.; Silva, C. Screening of Biofilm Formation by Beneficial Vaginal Lactobacilli and Influence of Culture Media Components. J. Appl. Microbiol. 2012, 113, 1517–1529. [Google Scholar] [CrossRef]
- Cats, A.; Kuipers, E.J.; Bosschaert, M.A.R.; Pot, R.G.J.; Vandenbroucke-Grauls, C.M.J.E.; Kusters, J.G. Effect of Frequent Consumption of a Lactobacillus casei-Containing Milk Drink in Helicobacter Pylori-Colonized Subjects. Aliment. Pharmacol. Ther. 2003, 17, 429–435. [Google Scholar] [CrossRef]
- Wendakoon, C.N.; Thomson, A.B.R.; Ozimek, L. Lack of Therapeutic Effect of a Specially Designed Yogurt for the Eradication of Helicobacter pylori Infection. Digestion 2002, 65, 16–20. [Google Scholar] [CrossRef]
- Lee, C.Y.; Shih, H.C.; Yu, M.C.; Lee, M.Y.; Chang, Y.L.; Lai, Y.Y.; Lee, Y.C.; Kuan, Y.H.; Lin, C.C. Evaluation of the Potential Inhibitory Activity of a Combination of L. Acidophilus, L. rhamnosus and L. sporogenes on Helicobacter pylori: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Chin. J. Integr. Med. 2017, 23, 176–182. [Google Scholar] [CrossRef]
- Gotteland, M.; Brunser, O.; Cruchet, S. Systematic Review: Are Probiotics Useful in Controlling Gastric Colonization by Helicobacter pylori? Aliment. Pharmacol. Ther. 2006, 23, 1077–1086. [Google Scholar] [CrossRef]
- Hauser, G.; Salkic, N.; Vukelic, K.; JajacKnez, A.; Stimac, D. Probiotics for Standard Triple Helicobacter pylori Eradication: A Randomized, Double-Blind, Placebo-Controlled Trial. Medicine 2015, 94, e685. [Google Scholar] [CrossRef]
- Lu, C.; Sang, J.; He, H.; Wan, X.; Lin, Y.; Li, L.; Li, Y.; Yu, C. Probiotic Supplementation Does Not Improve Eradication Rate of Helicobacter pylori Infection Compared to Placebo Based on Standard Therapy: A Meta-Analysis. Sci. Rep. 2016, 6, 23522. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, K.; Gaur, S. Role of Probiotics in Prophylaxis of Helicobacter pylori Infection. Curr. Pharm. Biotechnol. 2019, 20, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Zamorano, C.; González-Ávila, M.; Díaz-Blas, G.; Smith, C.T.; González-Correa, C.; García-Cancino, A. Increased Anti-Helicobacter pylori Effect of the Probiotic Lactobacillus fermentum UCO-979C Strain Encapsulated in Carrageenan Evaluated in Gastric Simulations under Fasting Conditions. Food Res. Int. 2019, 121, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.S.; García, A.; Pastene, E.; Salas, A.; Saez, K.; González, C.L. Lactobacillus fermentum UCO-979C Strongly Inhibited Helicobacter pylori SS1 in Meriones Unguiculatus. Benef. Microbes 2018, 9, 625–627. [Google Scholar] [CrossRef]
| Demographic and Clinical Characteristics | Placebo (N 64) | Probiotic (N 67) | p-Value |
|---|---|---|---|
| Age in years (average ± DS) | 22.34 ± 2.92 | 22.67 ± 2.84 | 0.3274 |
| Gender | 0.7213 | ||
| Women | 40 | 39 | |
| Men | 24 | 28 | |
| Pathology | |||
| Irritable bowel | 4 | 2 | 0.4399 |
| Diabetes | 3 | 1 | 0.3650 |
| Lupus | 0 | 1 | >0.9999 |
| Gastroesophageal refluxs | 0 | 1 | >0.9999 |
| Gastritis | 0 | 2 | 0.4960 |
| Lactose intolerance | 8 | 4 | 0.2354 |
| Alcohol consumption | 46 | 39 | 0.1426 |
| Asthma | 2 | 6 | 0.2732 |
| Allergy | 3 | 5 | 0.7176 |
| Intervention | Pre-Treatment | Post-Treatment | p-Value | |
|---|---|---|---|---|
| H. pylori (+)/% | H. pylori (−)/% | |||
| H. pylori (+) | >0.9999 | |||
| Placebo | 26 | 26/100 | 0/0 | |
| Probiotic | 30 | 29/96.7 | 1/3.3 | |
| Total | 56 | 55/98.2 | 1/1.8 | |
| H. pylori (−) | 0.0005 | |||
| Placebo | 38 | 13/34.2 | 25/65.8 | |
| Probiotic | 37 | 1/2.7 | 36/97.3 | |
| Total | 75 | 14/18.7 | 61/81.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Sepúlveda, C.; Sánchez-Alonzo, K.; Olivares-Muñoz, J.; Gutiérrez-Zamorano, C.; Smith, C.T.; Carvajal, R.I.; Sáez-Carrillo, K.; González, C.; García-Cancino, A. Consumption of a Gelatin Supplemented with the Probiotic Strain Limosilactobacillus fermentum UCO-979C Prevents Helicobacter pylori Infection in a Young Adult Population Achieved. Foods 2022, 11, 1668. https://doi.org/10.3390/foods11121668
Parra-Sepúlveda C, Sánchez-Alonzo K, Olivares-Muñoz J, Gutiérrez-Zamorano C, Smith CT, Carvajal RI, Sáez-Carrillo K, González C, García-Cancino A. Consumption of a Gelatin Supplemented with the Probiotic Strain Limosilactobacillus fermentum UCO-979C Prevents Helicobacter pylori Infection in a Young Adult Population Achieved. Foods. 2022; 11(12):1668. https://doi.org/10.3390/foods11121668
Chicago/Turabian StyleParra-Sepúlveda, Cristian, Kimberly Sánchez-Alonzo, Joaquín Olivares-Muñoz, Cristian Gutiérrez-Zamorano, Carlos T. Smith, Romina I. Carvajal, Katia Sáez-Carrillo, Carlos González, and Apolinaria García-Cancino. 2022. "Consumption of a Gelatin Supplemented with the Probiotic Strain Limosilactobacillus fermentum UCO-979C Prevents Helicobacter pylori Infection in a Young Adult Population Achieved" Foods 11, no. 12: 1668. https://doi.org/10.3390/foods11121668
APA StyleParra-Sepúlveda, C., Sánchez-Alonzo, K., Olivares-Muñoz, J., Gutiérrez-Zamorano, C., Smith, C. T., Carvajal, R. I., Sáez-Carrillo, K., González, C., & García-Cancino, A. (2022). Consumption of a Gelatin Supplemented with the Probiotic Strain Limosilactobacillus fermentum UCO-979C Prevents Helicobacter pylori Infection in a Young Adult Population Achieved. Foods, 11(12), 1668. https://doi.org/10.3390/foods11121668









