Ingredients from Climate Resilient Crops to Enhance the Nutritional Quality of Gluten-Free Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Breadmaking
2.3. Instrumental Analysis
2.4. Sensory Analysis
2.4.1. Sensory Focus Group
2.4.2. Focus Group Training
2.4.3. Sample Analysis
2.5. Statistical Analysis
3. Results
3.1. Instrumental Analysis
3.1.1. Long Fermentation (1 H)
3.1.2. Short Fermentation (30 Min)
3.2. Descriptive Sensory Analysis
4. Discussion
4.1. The Effect of Sourdough Fermentation on the Quality of the Tapioca–Brown Rice Bread
4.2. The Effect of Alternative Starter Cultures on the Quality of the Tapioca–Brown Rice Bread
4.3. The Effect of the Addition of Aquafaba on the Quality of the Tapioca–Brown Rice Bread
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Ingredients of Commercial Breads Used for Focus Group Training and Descriptor Generation
| Ingredients | ||
| Sunny Crust Wholemeal | Vogel’s Gluten Free White | Gluten Freedom Sweet Potato Sourdough |
| Wheat flour (wholemeal and white) Water Wheat gluten Yeast Iodized salt Canola oil Soy flour Emulsifiers (471, 481) Acidity regulator (263) Vtamin (folic acid) | Water Modified tapioca starch (1442) Flour (rice, soy) Maize starch Canola oil Sugar gg white powder Yeast Iodized salt Psyllium Cultured dextrose White vinegar Stabilizers (412, 464) | Water Organic Sourdough (Brown Rice Flour, Water, Vegetable Gum (Guar Gum)) Modified Tapioca Starch (1442) Corn Starch Coconut Sugar Coconut Oil Kumara Powder (2.9%) (Sweet Potato) Psyllium Husk Polenta Yeast Iodized Salt Stabilizer (464) Vegetable Gum (Guar Gum) Emulsifier (Sunflower Lecithin) |
References
- Amelework, A.B.; Bairu, M.W.; Maema, O.; Venter, S.L.; Laing, M. Adoption and promotion of resilient crops for climate risk mitigation and import substitution: A case analysis of cassava for South African agriculture. Front. Sustain. Food Syst. 2021, 5, 617783. [Google Scholar] [CrossRef]
- Isaac, N. Cassava Is an Important Food Crop in Africa. Alliance for Science. (Internet Site). 2017. Available online: https://allianceforscience.cornell.edu/blog/2017/09/gmo-cassava-could-help-nigerian-farmers-prolong-crop-storage/ (accessed on 5 May 2022).
- Bourekoua, H.; Różyło, R.; Benatallah, L.; Wójtowicz, A.; Łysiak, G.; Zidoune, M.N.; Sujak, A. Characteristics of gluten-free bread: Quality improvement by the addition of starches/hydrocolloids and their combinations using a definitive screening design. Eur. Food Res. Technol. 2018, 244, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Yun, Y.; Jeong, Y. Effects of corn, potato, and tapioca starches on the quality of gluten-free rice bread. Food Sci. Biotechnol. 2015, 24, 913–919. [Google Scholar] [CrossRef]
- Serventi, L.; Jensen, S.; Skibsted, L.H.; Kidmose, U. Addition of enzymes to improve sensory quality of composite wheat–cassava bread. Eur. Food Res. Technol. 2016, 242, 1245–1252. [Google Scholar] [CrossRef]
- Horstmann, S.W.; Belz, M.C.; Heitmann, M.; Zannini, E.; Arendt, E.K. Fundamental study on the impact of gluten-free starches on the quality of gluten-free model breads. Foods 2016, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Plessas, S. Innovations in Sourdough Bread Making. Fermentation 2021, 7, 29. [Google Scholar] [CrossRef]
- Aguiar, E.V.; Santos, F.G.; Krupa-Kozak, U.; Capriles, V.D. Nutritional facts regarding commercially available gluten-free bread worldwide: Recent advances and future challenges. Crit. Rev. Food Sci. Nutr. 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Ramos, L.; Alonso-Hernando, A.; Martínez-Castro, M.; Morán-Pérez, J.A.; Cabrero-Lobato, P.; Pascual-Maté, A.; Mujico, J.R. Sourdough biotechnology applied to gluten-free baked goods: Rescuing the tradition. Foods 2021, 10, 1498. [Google Scholar] [CrossRef]
- Livsmedelsverket. Riksmaten 1997–1998 (Dietary Habits and Nutrient Intake in Sweden. Methods and Results). 2002. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0278691503001881 (accessed on 4 May 2022).
- Saa, D.T.; Di Silvestro, R.; Dinelli, G.; Gianotti, A. Effect of sourdough fermentation and baking process severity on dietary fibre and phenolic compounds of immature wheat flour bread. LWT-Food Sci. Technol. 2017, 83, 26–32. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ruzzi, M. Current and forward-looking approaches to technological and nutritional improvements of gluten-free bread with legume flours: A critical review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1101–1122. [Google Scholar] [CrossRef]
- İspirli, H.; Özmen, D.; Yılmaz, M.T.; Sağdıç, O.; Dertli, E. Impact of glucan type exopolysaccharide (EPS) production on technological characteristics of sourdough bread. Food Control 2020, 107, 106812. [Google Scholar] [CrossRef]
- Thierry, A.; Deutsch, S.M.; Falentin, H.; Dalmasso, M.; Cousin, F.J.; Jan, G. New insights into physiology and metabolism of Propionibacteriumbacterium freudenreichii. Int. J. Food Microbiol. 2011, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Lv, J.P.; Chu, Z.Q.; Cui, Y.Y.; Ren, X.H. Production of conjugated linoleic acid by Propionibacteriumbacterium freudenreichii. Food Chem. 2007, 103, 313–318. [Google Scholar] [CrossRef]
- Xie, C.; Coda, R.; Chamlagain, B.; Edelmann, M.; Deptula, P.; Varmanen, P.; Piironen, V.; Katina, K. In situ fortification of vitamin B12 in wheat flour and wheat bran by fermentation with Propionibacteriumbacterium freudenreichii. J. Cereal Sci. 2018, 81, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Brede, D.A.; Faye, T.; Johnsborg, O.; Ødegård, I.; Nes, I.F.; Holo, H. Molecular and genetic characterization of Propionibacteriumcin F, a bacteriocin from Propionibacteriumbacterium freudenreichii. Appl. Environ. Microbiol. 2004, 70, 7303–7310. [Google Scholar] [CrossRef] [Green Version]
- Assis, D.A.D.; Matte, C.; Aschidamini, B.; Rodrigues, E.; Zachia Ayub, M.A. Biosynthesis of vitamin B12 by Propionibacteriumbacterium freudenreichii subsp. shermanii ATCC 13673 using liquid acid protein residue of soybean as culture medium. Biotechnol. Prog. 2020, 36, e3011. [Google Scholar] [CrossRef]
- Stantiall, S.E.; Dale, K.J.; Calizo, F.S.; Serventi, L. Application of pulses cooking water as functional ingredients: The foaming and gelling abilities. Eur. Food Res. Technol. 2018, 244, 97–104. [Google Scholar] [CrossRef]
- Do Nascimento, K.D.O.; Paes, S.D.N.D.; de Oliveira, I.R.; Reis, I.P.; Augusta, I.M. Teff: Suitability for different food applications and as a raw material of gluten-free, a literature review. J. Food Nutr. Res. 2018, 6, 74–81. [Google Scholar] [CrossRef]
- Saleh, A.S.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [Green Version]
- Xie, C. In Situ Fortification of Vitamin B12 in Grain Materials by Fermentation with Propionibacteriumbacterium freudenreichii. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2020. [Google Scholar]
- American Association of Cereal Chemists; Approved Methods Committee. Approved Methods of the American Association of Cereal Chemists; Amer Assn of Cereal Chemists: St. Paul, MN, USA, 2000; Volume 1. [Google Scholar]
- AACC International. Approved Methods of Analysis, 11th ed.; Method 44-19.01. Moisture—Air-Oven Method, Drying at 135°; AACC International: St. Paul, MN, USA, 2001. [Google Scholar]
- Alderson, H.; Liu, C.; Mehta, A.; Gala, H.S.; Mazive, N.R.; Chen, Y.; Serventi, L. Sensory profile of kombucha brewed with New Zealand ingredients by focus group and word clouds. Fermentation 2021, 7, 100. [Google Scholar] [CrossRef]
- Crucean, D.; Debucquet, G.; Rannou, C.; Le-Bail, A.; Le-Bail, P. Vitamin B4 as a salt substitute in bread: A challenging and successful new strategy. Sensory perception and acceptability by French consumers. Appetite 2019, 134, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Heiniö, R.L.; Autio, K.; Poutanen, K. Optimization of sourdough process for improved sensory profile and texture of wheat bread. LWT-Food Sci. Technol. 2006, 39, 1189–1202. [Google Scholar] [CrossRef]
- Tinzl-Malang, S.K.; Grattepanche, F.; Rast, P.; Fischer, P.; Sych, J.; Lacroix, C. Purified exopolysaccharides from Weissella confusa 11GU-1 and Propionibacteriumbacterium freudenreichii JS15 act synergistically on bread structure to prevent staling. LWT 2020, 127, 109375. [Google Scholar] [CrossRef]
- Aleman, R.S.; Paz, G.; Morris, A.; Prinyawiwatkul, W.; Moncada, M.; King, J.M. High protein brown rice flour, tapioca starch & potato starch in the development of gluten-free cupcakes. LWT—Food Sci. Technol. 2021, 152, 112326. [Google Scholar]
- Serventi, L.; Gao, C.; Chen, M.; Chelikani, V. Cooking Water Functional Properties. In Upcycling Legume Water: From Wastewater to Food Ingredients; Springer Nature: Cham, Switzerland, 2020; pp. 87–103. [Google Scholar]
- Serventi, L. Upcycling Legume Water: From Wastewater to Food Ingredients; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Bird, L.G.; Pilkington, C.L.; Saputra, A.; Serventi, L. Products of chickpea processing as texture improvers in gluten-free bread. Food Sci. Technol. Int. 2017, 23, 690–698. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallagher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Technol. 2010, 230, 437–445. [Google Scholar] [CrossRef]
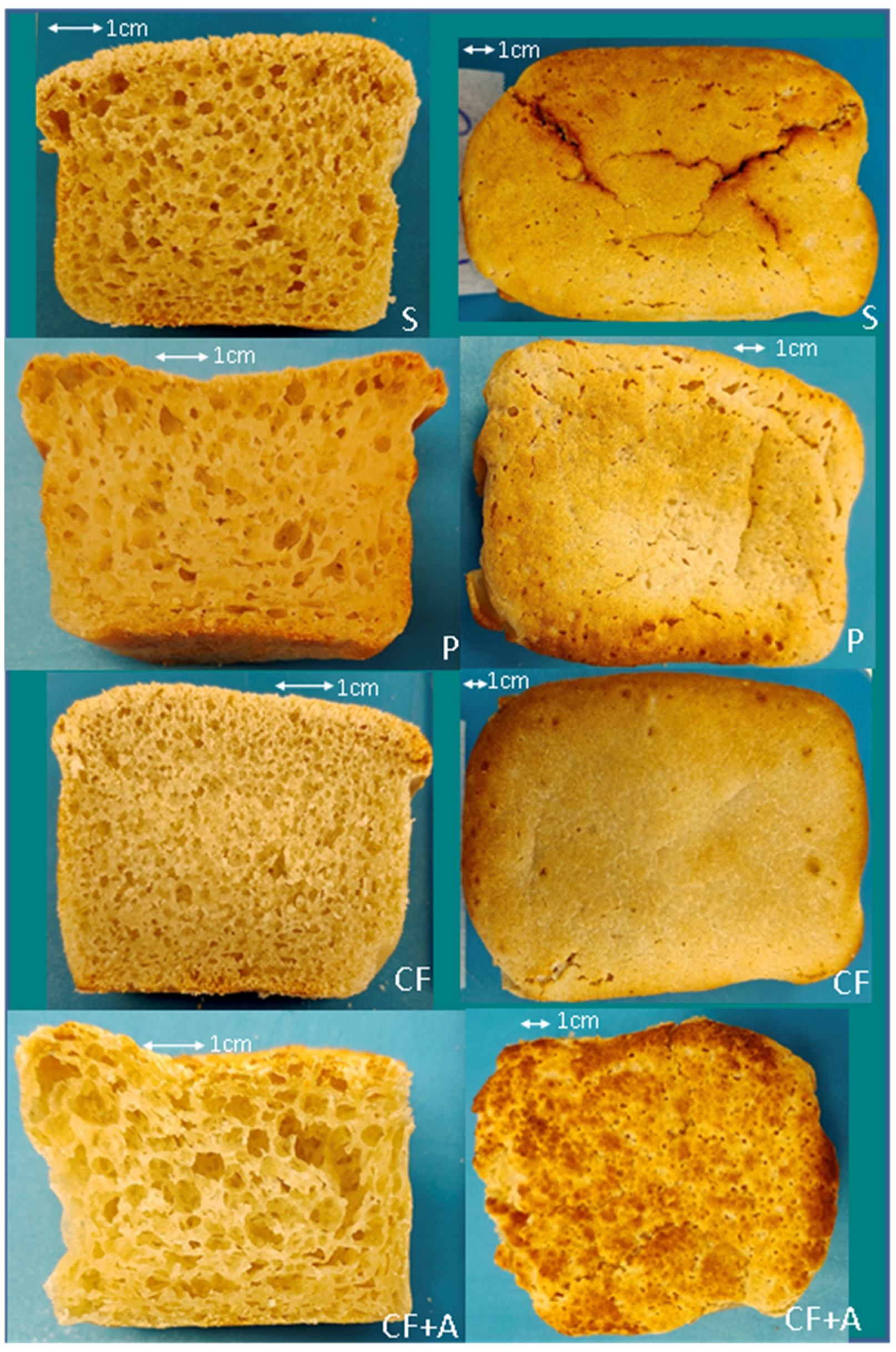
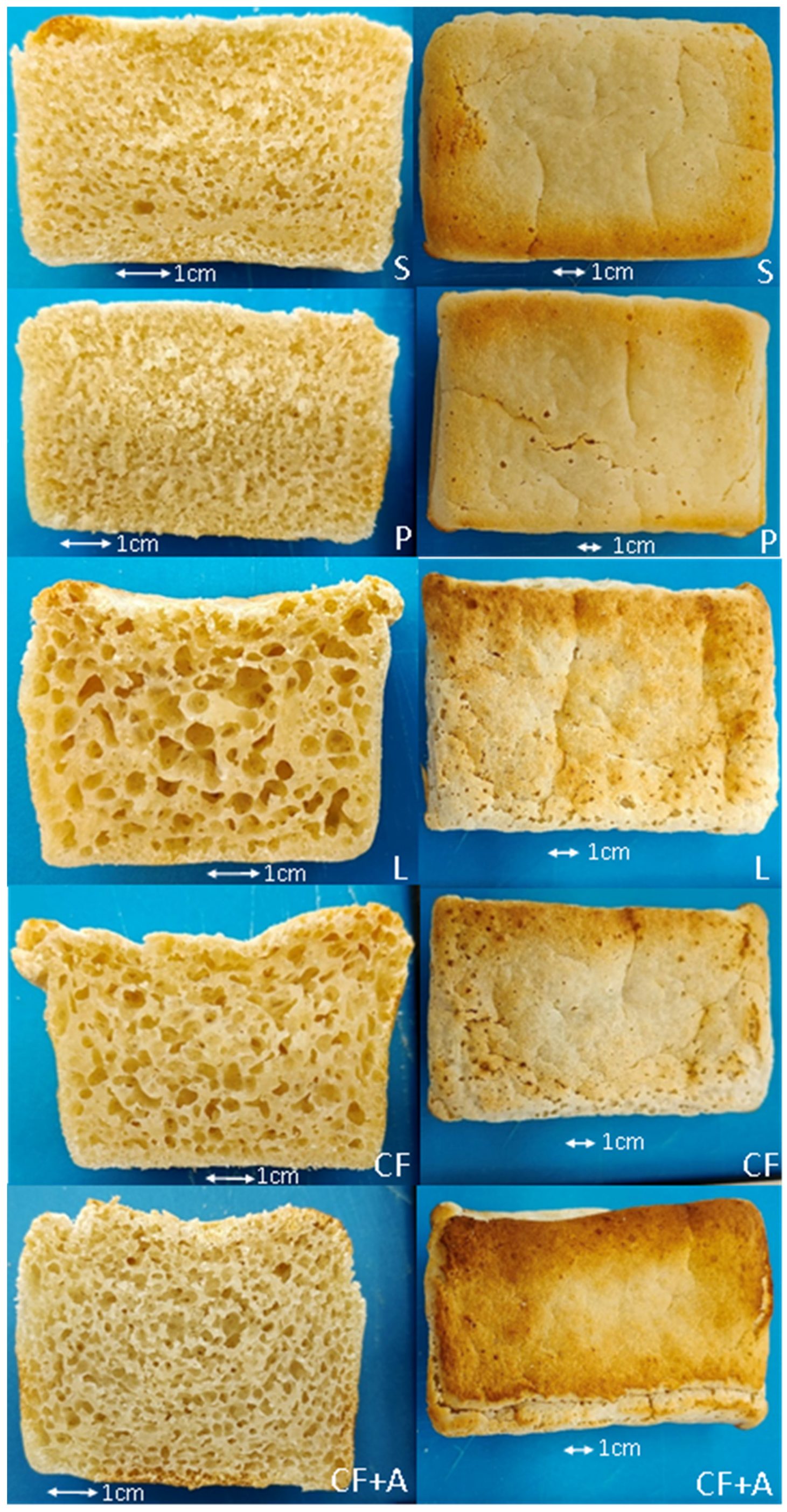
| Ingredients (g) | S | P | L | CF | CF + A |
|---|---|---|---|---|---|
| Tapioca flour | 100 | 100 | 100 | 100 | 100 |
| Brown rice flour | 100 | 100 | 100 | 100 | 100 |
| Water | 160 | 160 | 160 | 160 | 20 |
| Propionibacterium freudenreichii subsp. globosum | 0 | 0.3 | 0 | 0.3 | 0.3 |
| Lactobacillus lactis | 0 | 0 | 1.0 | 1.0 | 1.0 |
| Aquafaba | 0 | 0 | 0 | 0 | 148 |
| Bread Formulation | Hardness (g) | Specific Volume (cm3/g) | Moisture Content (%) |
|---|---|---|---|
| S | 747 ± 116 B | 2.50 ± 0.12 B | 44.7 ± 2.0 A |
| L | Nonmeasurable | Nonmeasurable | Nonmeasurable |
| P | 256 ± 37 C | 2.10 ± 0.11 C | 45.2 ± 1.3 A |
| CF | 1394 ± 190 A | 1.82 ± 0.10 D | 44.5 ± 3.1 A |
| CF + A | 38 ± 38 D | 2.86 ± 0.10 A | 42.0 ± 3.28 A |
| Yeast only (reference) | 1490 ± 235 | Not measured | Not measured |
| Bread Formulation | Hardness (g) | Specific Volume (cm3/g) | Moisture Content (%) |
|---|---|---|---|
| S | 943 ± 90 B | 1.86 ± 0.24 AB | 46.0 ± 2.2 A |
| L | 750 ± 128 C | 2.31 ± 0.33 A | 46.7 ± 0.8 A |
| P | 1461 ± 191 A | 1.56 ± 0.16 B | 46.8 ± 1.5 A |
| CF | 853 ± 127 BC | 2.07 ± 0.06 AB | 47.0 ± 1.5 A |
| CF + A | 577 ± 93 D | 2.39 ± 0.29 A | 50.0 ± 2.8 A |
| Sensory Characteristic | Wholemeal | Gluten-Free White | Gluten-Free Sweet Potato Sourdough |
| Appearance | Spotty Grainy | Uniform Processed | Brown Attractive Holey (air bubbles) |
| Aroma | Bready Familiar Wheaty | Neutral | Sour Yeasty Bready Fermented |
| Taste | Pleasant Bready Familiar Sweet Yeasty | Stale Bland Lingering | Sweet Sour Vinegar |
| Texture | Soft Fluffy Grainy | Dry Mushy Gritty Stale Kitchen Sponge Sticky | Dry Hard Uniform Crumbly |
| Sensory Characteristic | S | L | P | CF | CF + A |
|---|---|---|---|---|---|
| Appearance | Dense 9 Grey 6 Undercooked 5 | Pale 8 Tan Crust 5 Attractive 4 Yellower 4 Rustic 1 | Dense 9 Pale 9 Undercooked 1 | Rustic 9 Holey Crumb 9 Pale 8 Flaky Crust 2 | Uniform 9 Attractive 7 Bready 6 Less Crumbly 1 Less Holes 1 |
| Aroma | Sour 7 Beer Like 6 | Sour 8 Yeasty 5 | Fermented 7 Sour Fruit 1 Kombucha 1 Unpleasant 1 | Sour 9 Apple Cider 5 Acetic 4 Vinegar 3 | Sour 9 Roast Potato 1 |
| Taste | Neutral 9 Bitter 4 Tasteless 3 Salty 1 | Sour 8 Acidic Aftertaste 6 Sweet 3 Pleasant 3 | Bitter Aftertaste 9 Less Sour 2 | Salty 6 Too Yeasty 2 A Little Bitter 2 | Bitter Crust 8 Sour 6 Baking Soda 1 Bitter Aftertaste 1 |
| Texture | Chewy 9 Dense 9 Chalky 6 Dry 5 | Crumpet 9 Springy 9 Sticky 7 | Heavy 9 Chewy 9 Gluey 9 Sticky 9 Dense 7 | Doughy 9 Chewy 9 Sticky 8 | Sticky 9 Gluey 6 Chewy 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roozen, M.; Serventi, L. Ingredients from Climate Resilient Crops to Enhance the Nutritional Quality of Gluten-Free Bread. Foods 2022, 11, 1628. https://doi.org/10.3390/foods11111628
Roozen M, Serventi L. Ingredients from Climate Resilient Crops to Enhance the Nutritional Quality of Gluten-Free Bread. Foods. 2022; 11(11):1628. https://doi.org/10.3390/foods11111628
Chicago/Turabian StyleRoozen, Megan, and Luca Serventi. 2022. "Ingredients from Climate Resilient Crops to Enhance the Nutritional Quality of Gluten-Free Bread" Foods 11, no. 11: 1628. https://doi.org/10.3390/foods11111628
APA StyleRoozen, M., & Serventi, L. (2022). Ingredients from Climate Resilient Crops to Enhance the Nutritional Quality of Gluten-Free Bread. Foods, 11(11), 1628. https://doi.org/10.3390/foods11111628







