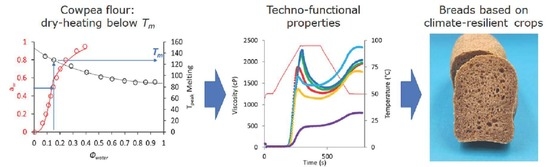
Dry Heating of Cowpea Flour below Biopolymer Melting Temperatures Improves the Physical Properties of Bread Made from Climate-Resilient Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cowpea Protein Isolation
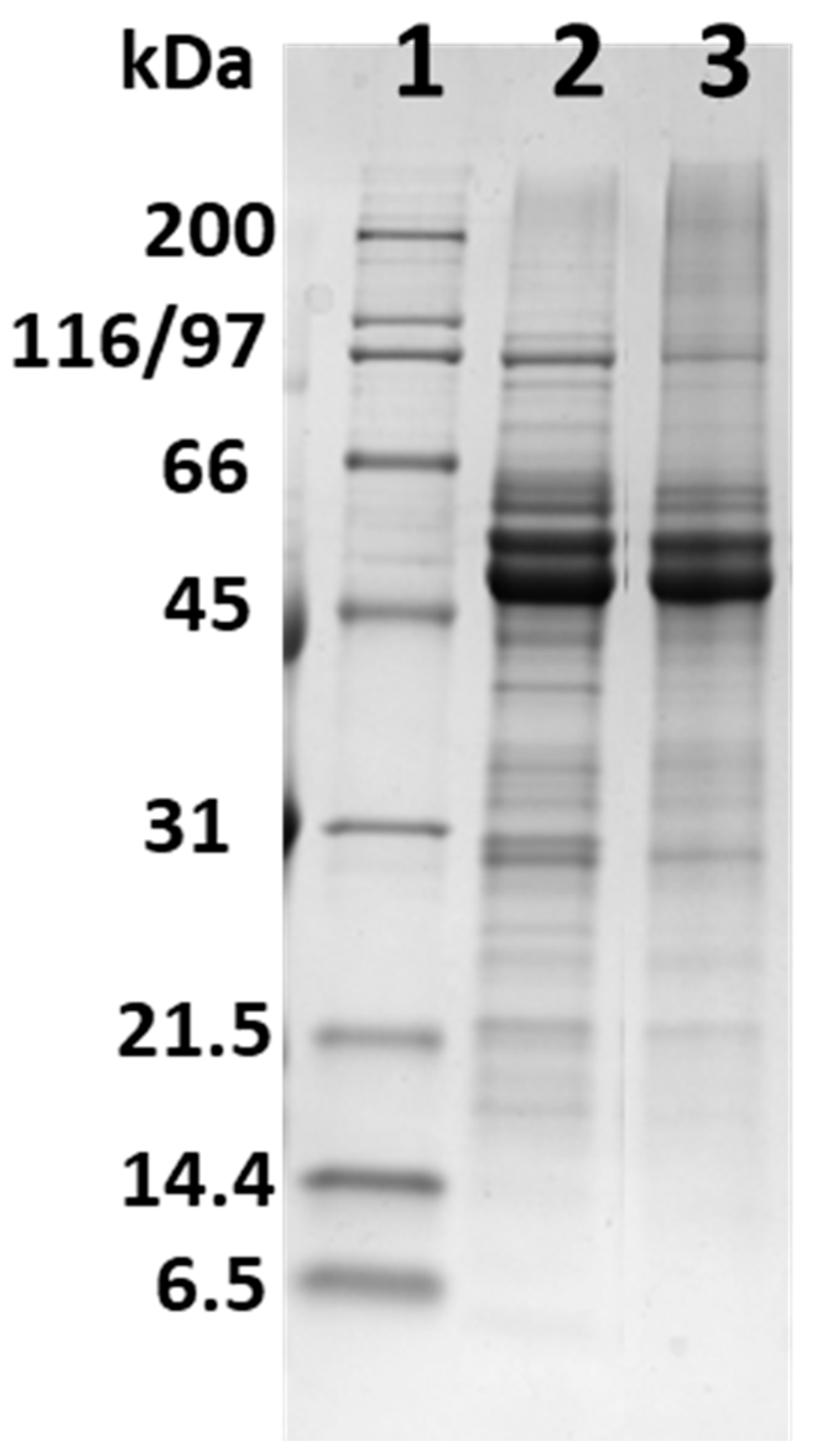
2.2.2. SDS-PAGE of CPF and CPPC
2.2.3. Melting Transitions of Native Cowpea Flour and Cowpea Protein Isolate at Different Levels of Hydration
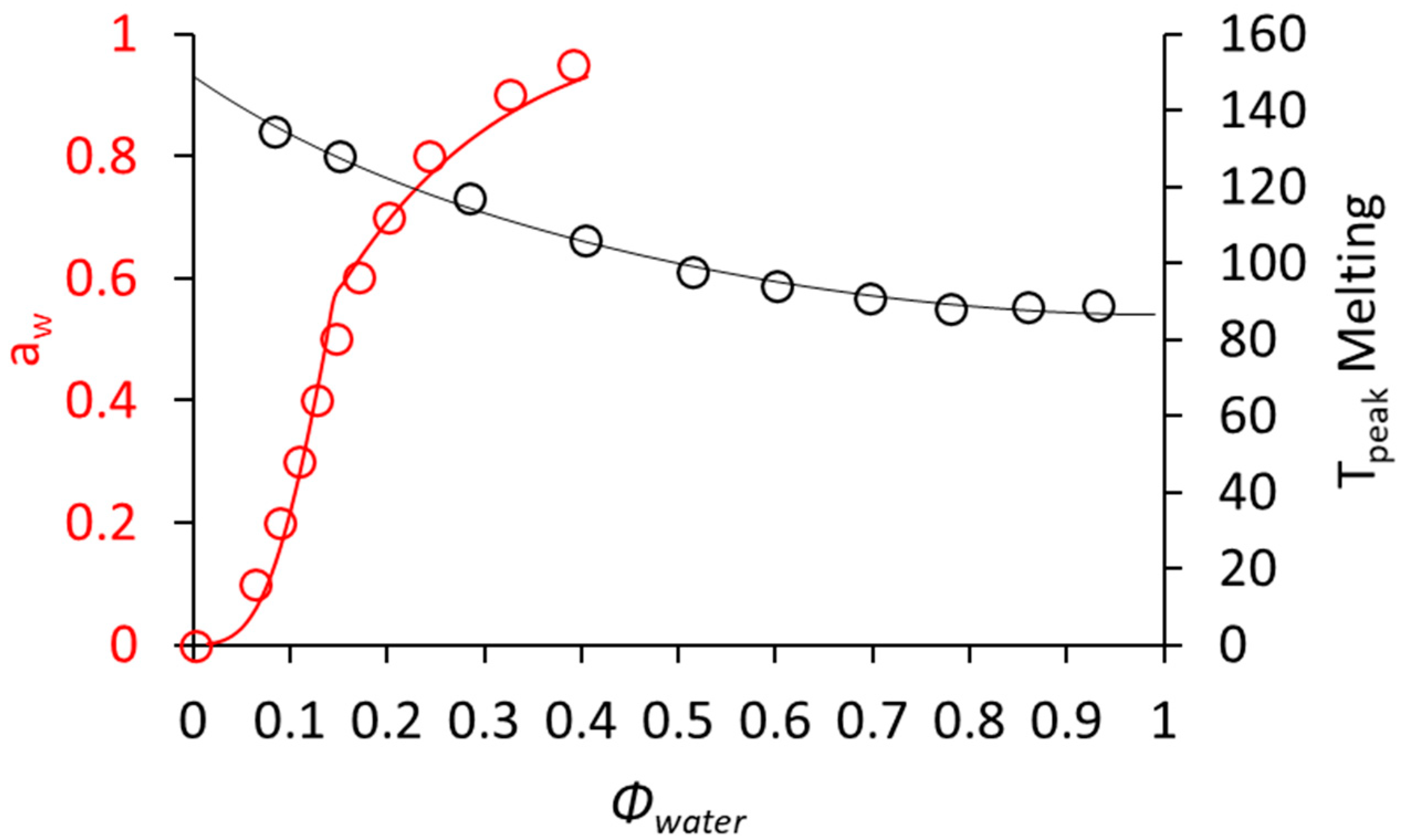
2.2.4. Moisture Sorption Behavior of Cowpea Protein Isolate and Cowpea Flour
2.2.5. Dry-Heating Treatments of Cowpea Flour
2.2.6. Thermal Analysis of Dry-Heated Cowpea Flours
2.2.7. Water Binding Capacity and Soluble Solids of Native and Dry-Heated CPF
2.2.8. Rapid Visco Analyzer (RVA) of Native and Treated Flours
2.2.9. Bread-Making Procedure
2.2.10. Bread Quality Evaluation
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Protein Profile of CPF and CPPC
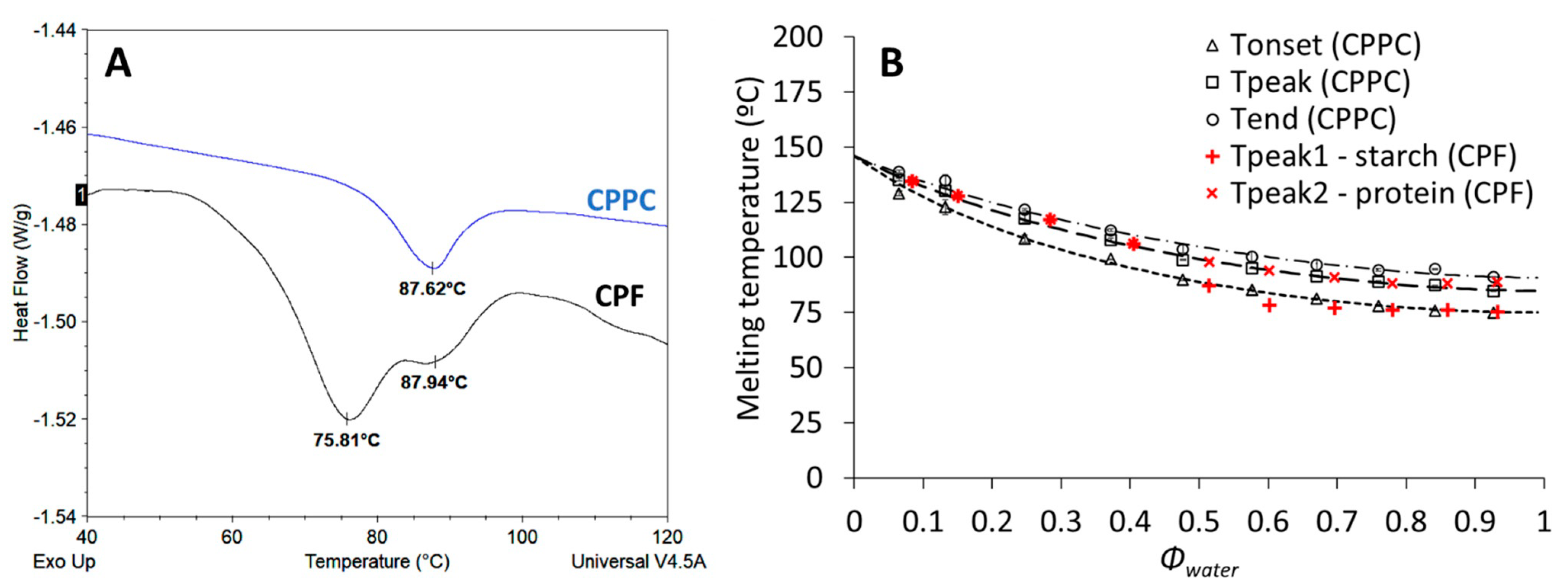
3.2. Characterization of the Thermal and Sorption Properties of CPF and CPPC
3.3. Characterization of Thermal Properties of Dry-Heated CPF
3.4. WBC and Solubility of Native and Dry-Heated CPF
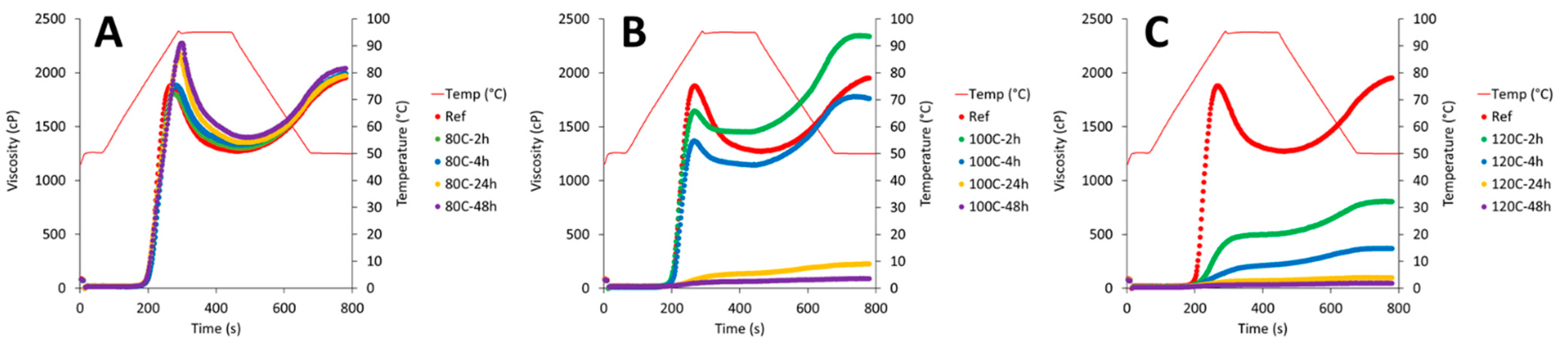
3.5. Pasting Properties of Native and Dry-Heated CPF
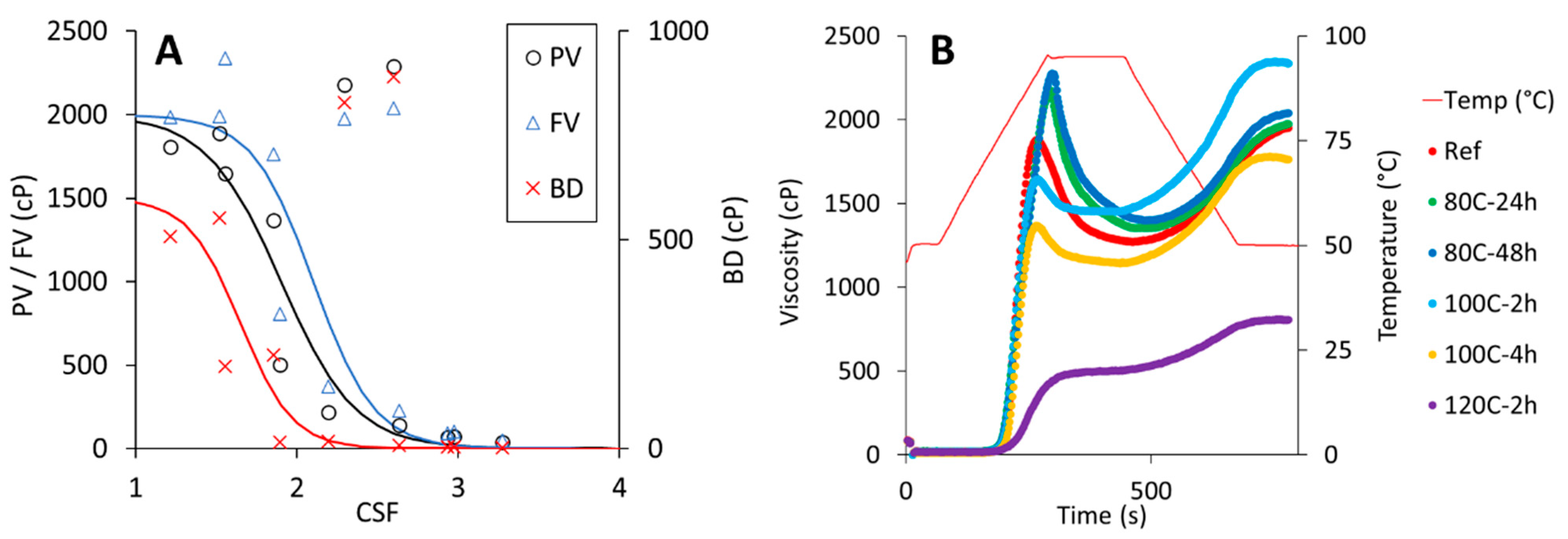
3.6. Selection of Dry-Heated CPF for Testing in a Tin-Bread Application
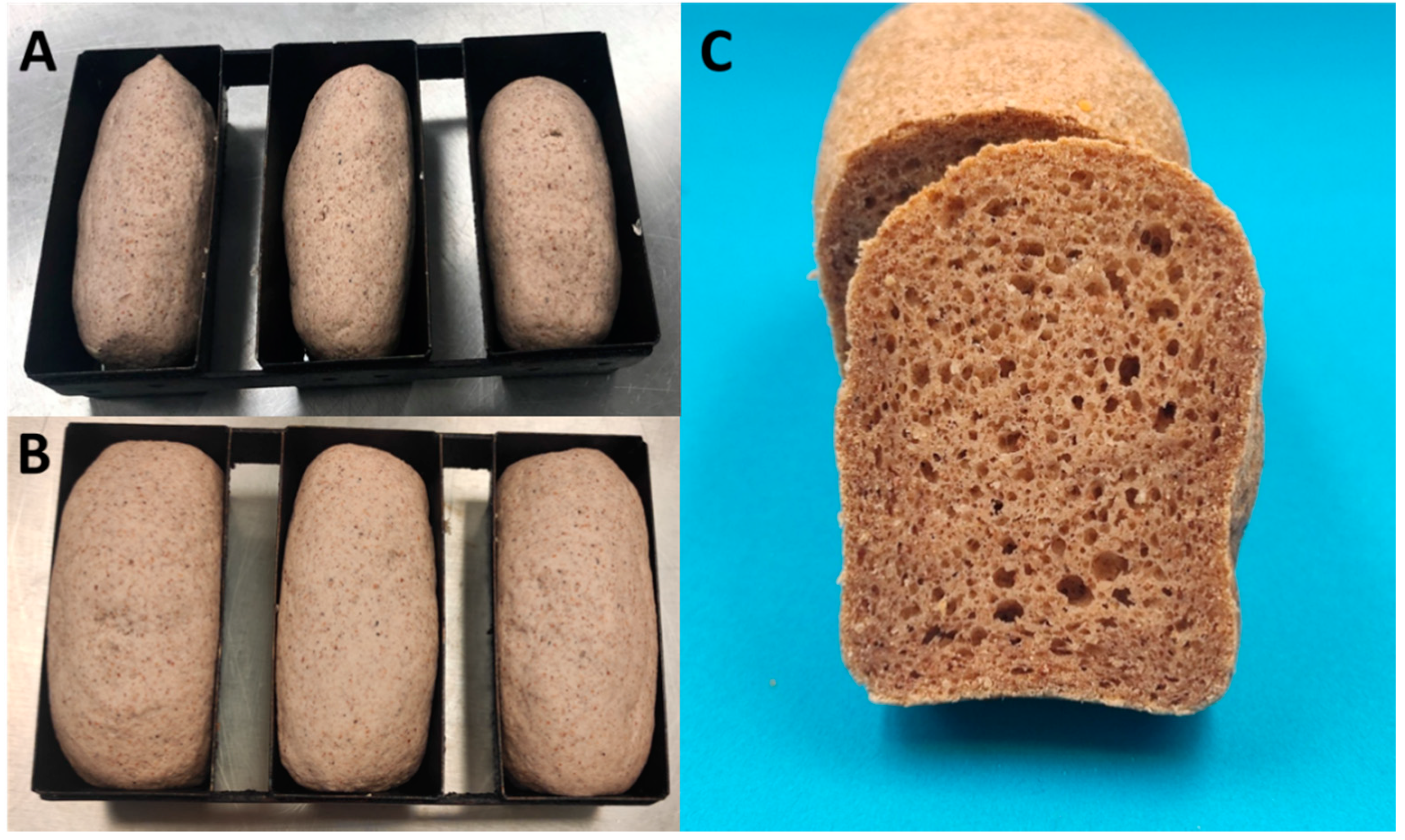
3.7. Properties of Bread with Selected Dry-Heated CPF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noort, M.W.J.; Renzetti, S.; Linderhof, V.; du Rand, G.E.; Marx-Pienaar, N.J.M.M.; de Kock, H.L.; Magano, N.; Taylor, J.R.N. Towards Sustainable Shifts to Healthy Diets and Food Security in Sub-Saharan Africa with Climate-Resilient Crops in Bread-Type Products: A Food System Analysis. Foods 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.M.; Jayne, T.S.; Shiferaw, B. Africa’s Rising Demand for Wheat: Trends, Drivers, and Policy Implications. Dev. Policy Rev. 2015, 33, 581–613. [Google Scholar] [CrossRef]
- Raheem, D.; Dayoub, M.; Birech, R.; Nakiyemba, A. The Contribution of Cereal Grains to Food Security and Sustainability in Africa: Potential Application of UAV in Ghana, Nigeria, Uganda, and Namibia. Urban Sci. 2021, 5, 8. [Google Scholar] [CrossRef]
- Acevedo, M.; Pixley, K.; Zinyengere, N.; Meng, S.; Tufan, H.; Cichy, K.; Bizikova, L.; Isaacs, K.; Ghezzi-Kopel, K.; Porciello, J. A scoping review of adoption of climate-resilient crops by small-scale producers in low- and middle-income countries. Nat. Plants 2020, 6, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Foyer, C.H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef]
- Prinyawiwatkul, W.; McWatters, K.H.; Beuchat, L.R.; Phillips, R.D. Cowpea Flour: A Potential Ingredient in Food Products. Crit. Rev. Food Sci. Nutr. 1996, 36, 413–436. [Google Scholar] [CrossRef]
- Ndiaye, M.; Termorshuizen, A.J.; van Bruggen, A.H.C. Effect of rotation of cowpea (Vigna unguiculata) with fonio (Digitaria exilis) and millet (Pennisetum glaucum) on Macrophomina phaseolina densities and cowpea yield. Afr. J Agric. Res. 2008, 3, 37–43. [Google Scholar]
- Zougmore, R.; Kambou, F.N.; Ouattara, K.; Guillobez, S. Sorghum-cowpea Intercropping: An Effective Technique Against Runoff and Soil Erosion in the Sahel (Saria, Burkina Faso). Arid Soil Res. Rehabil. 2010, 14, 329–342. Available online: https://www.tandfonline.com/doi/abs/10.1080/08903060050136441 (accessed on 22 May 2022). [CrossRef]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Cao, J.; Xiong, L.; Sun, Q. Differences in physicochemical, morphological, and structural properties between rice starch and rice flour modified by dry heat treatment. Starch Stärke 2015, 67, 756–764. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, M.; Li, Y.; Xiong, L. Effect of dry heat treatment on the physicochemical properties and structure of proso millet flour and starch. Carbohydr. Polym. 2014, 110, 128–134. [Google Scholar] [CrossRef]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; McCarthy, S.; Gallagher, E. Dough properties and baking characteristics of white bread, as affected by addition of raw, germinated and toasted pea flour. Innov. Food Sci. Emerg. Technol. 2019, 56, 102189. [Google Scholar] [CrossRef]
- Ouazib, M.; Garzon, R.; Zaidi, F.; Rosell, C.M. Germinated, toasted and cooked chickpea as ingredients for breadmaking. J. Food Sci. Technol. 2016, 53, 2664–2672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, G.; Bourré, L.; Frohlich, P.; Borsuk, Y.; Sarkar, A.; Sopiwnyk, E.; Jones, S.; Dyck, A.; Malcolmson, L. Effect of roasting as a premilling treatment on the functional and bread baking properties of whole yellow pea flour. Cereal Chem. 2020, 97, 183–195. [Google Scholar] [CrossRef]
- Khalid, I.I.; Elhardallou, S.B.; Elkhalifa, E.A. Composition and Functional Properties of Cowpea (Vigna ungiculata L. Walp) Flour and Protein Isolates. Am. J. Food Technol. 2021, 7, 113–122. [Google Scholar] [CrossRef]
- Renzetti, S.; van den Hoek, I.A.F.; van der Sman, R.G.M. Amino acids, polyols and soluble fibres as sugar replacers in bakery applications: Egg white proteins denaturation controlled by hydrogen bond density of solutions. Food Hydrocoll. 2020, 108, 106034. [Google Scholar] [CrossRef]
- Van Der Sman, R.G.M.; Meinders, M.B.J. Prediction of the state diagram of starch water mixtures using the Flory-Huggins free volume theory. Soft Matter. 2011, 7, 429–442. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M. Sugar and polyol solutions as effective solvent for biopolymers. Food Hydrocoll. 2016, 56, 144–149. [Google Scholar] [CrossRef]
- Renzetti, S.; Van Den Hoek, I.A.F.; Van Der Sman, R.G.M. Mechanisms controlling wheat starch gelatinization and pasting behaviour in presence of sugars and sugar replacers: Role of hydrogen bonding and plasticizer molar volume. Food Hydrocoll. 2021, 119, 106880. [Google Scholar] [CrossRef]
- Renzetti, S.; Theunissen, M.; Horrevorts, K. A systematic comparison of the intrinsic properties of wheat and oat bran fractions and their effects on dough and bread properties: Elucidation of chemical mechanisms, water binding, and steric hindrance. Foods 2021, 10, 2311. [Google Scholar] [CrossRef] [PubMed]
- Van der Sman, R.G.M. Moisture sorption in mixtures of biopolymer, Disaccharides and water. Food Hydrocoll. 2013, 32, 186–194. [Google Scholar] [CrossRef]
- Cornet, S.H.V.; van der Goot, J.A.; van der Sman, R.G.M. Current Research in Food Science Effect of mechanical interaction on the hydration of mixed soy protein and gluten gels. Curr. Res. Food Sci. 2020, 3, 134–145. [Google Scholar] [CrossRef]
- Zanoletti, M.; Marti, A.; Marengo, M.; Iametti, S.; Pagani, M.A.; Renzetti, S. Understanding the influence of buckwheat bran on wheat dough baking performance: Mechanistic insights from molecular and material science approaches. Food Res. Int. 2017, 102, 728–737. [Google Scholar] [CrossRef]
- Horax, R.; Hettiarachchy, N.S.; Chen, P.; Jalaluddin, M. Preparation and Characterization of Protein Isolate from Cowpea (Vigna unguiculata L. Walp.). J. Food. Sci. 2004, 69, 114–118. [Google Scholar] [CrossRef]
- Angel, A.L.R.; Omont, G.I.B.D.; Edrosa, C.R.P. Functional Properties of Purified Vicilins from Cowpea (Vigna unguiculata) and Pea (Pisum sativum) and Cowpea Protein Isolate. J. Agric. Food Chem. 2003, 51, 5792–5797. [Google Scholar] [CrossRef]
- Huang, J.; Schols, H.A.; van Soest, J.J.G.; Jin, Z.; Sulmann, E.; Voragen, A.G.J. Physicochemical properties and amylopectin chain profiles of cowpea, chickpea and yellow pea starches. Food Chem. 2007, 101, 1338–1345. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Renzetti, S. Understanding functionality of sucrose in biscuits for reformulation purposes. Crit. Rev. Food Sci. Nutr. 2019, 59, 2225–2239. [Google Scholar] [CrossRef]
- Morales, A.; Kokini, J.L. Glass Transition of Soy Globulins Using Differential Scanning Calorimetry and Mechanical Spectrometry. Biotechnol. Prog. 1997, 1984, 624–629. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, C.; Jiang, S.; Cao, J.; Xiong, L.; Sun, Q. Functional Properties of Glutinous Rice Flour by Dry-Heat Treatment. PloS ONE 2016, 11, e0160371. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.D.; Chinnan, M.S.; Branch, A.L.; Miller, J.; McWatters, K.H. Effects of Pretreatment Properties on Functional and Nutritional of Cowpea Meal. J. Food Sci. 1988, 53, 805–809. [Google Scholar] [CrossRef]
- Błaszczak, W.; Doblado, R.; Frias, J.; Vidal-Valverde, C.; Sadowska, J.; Fornal, J. Microstructural and biochemical changes in raw and germinated cowpea seeds upon high-pressure treatment. Food Res. Int. 2007, 40, 415–423. [Google Scholar] [CrossRef]
- Adjei-Fremah, S.; Worku, M.; De Erive, M.O.; He, F.; Wang, T.; Chen, G. Effect of microfluidization on microstructure, protein profile and physicochemical properties of whole cowpea flours. Innov. Food Sci. Emerg. Technol. 2019, 57, 102207. [Google Scholar] [CrossRef]
- Woodbury, T.J.; Grush, E.; Allan, M.C.; Mauer, L.J. The effects of sugars and sugar alcohols on the pasting and granular swelling of wheat starch. Food Hydrocoll. 2021, 126, 107433. [Google Scholar] [CrossRef]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser ( RVA ) as a Tool for Measuring Starch-Related Physiochemical Properties in Cereals: A Review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Renzetti, S.; Courtin, C.M.; Delcour, J.A.; Arendt, E.K. Oxidative and proteolytic enzyme preparations as promising improvers for oat bread formulations: Rheological, biochemical and microstructural background. Food Chem. 2010, 119, 1465–1473. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Griffin, V.K. Effect of disulfide bond-containing protein on rice starch gelatinization and pasting. Cereal Chem. 1993, 70, 377–380. [Google Scholar]
- Yano, H. Improvements in the bread-making quality of gluten-free rice batter by glutathione. J. Agric. Food Chem. 2010, 58, 7949–7954. [Google Scholar] [CrossRef]
- Derycke, V.; Veraverbeke, W.S.; Vandeputte, G.E.; De Man, W.; Hoseney, R.C.; Delcour, J.A. Impact of proteins on pasting and cooking properties of nonparboiled and parboiled rice. Cereal Chem. 2005, 82, 468–474. [Google Scholar] [CrossRef]
- Steeneken, P.A.M. Rheological properties of acqueous suspensions of swollen starch granules. Carbohydr. Polym. 1989, 11, 23–42. [Google Scholar] [CrossRef]
- De Farias Silva, C.E.; Bertucco, A. Severity factor as an efficient control parameter to predict biomass solubilization and saccharification during acidic hydrolysis of microalgal biomass. Bioenergy Res. 2018, 11, 491–504, Correction in Bioenergy Res. 2020, 13, 998. [Google Scholar] [CrossRef]
- Renzetti, S.; Arendt, E.K. Effect of protease treatment on the baking quality of brown rice bread: From textural and rheological properties to biochemistry and microstructure. J. Cereal Sci. 2009, 50, 22–28. [Google Scholar] [CrossRef]
- Kawamura-Konishi, Y.; Shoda, K.; Koga, H.; Honda, Y. Improvement in gluten-free rice bread quality by protease treatment. J. Cereal Sci. 2013, 58, 45–50. [Google Scholar] [CrossRef]
- Renzetti, S.; Rosell, C.M. Role of enzymes in improving the functionality of proteins in non-wheat dough systems. J. Cereal Sci. 2016, 67, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Verbauwhede, A.E.; Lambrecht, M.A.; Fierens, E.; Hermans, S.; Shegay, O.; Brijs, K.; Delcour, J.A. Thermo-reversible inhibition makes aqualysin 1 from Thermus aquaticus a potent tool for studying the contribution of the wheat gluten network to the crumb texture of fresh bread. Food Chem. 2018, 264, 118–125. [Google Scholar] [CrossRef]
- Roman, L.; Gomez, M.; Martinez, M.M. Mesoscale structuring of gluten-free bread with starch. Curr. Opin. Food Sci. 2021, 38, 189–195. [Google Scholar] [CrossRef]
- Roman, L.; Reguilon, M.P.; Gomez, M.; Martinez, M.M. Intermediate length amylose increases the crumb hardness of rice flour gluten-free breads. Food Hydrocoll. 2020, 100, 105451. [Google Scholar] [CrossRef]
- Roman, L.; de la Cal, E.; Gomez, M.; Martinez, M.M. Specific ratio of A-to B-type wheat starch granules improves the quality of gluten-free breads: Optimizing dough viscosity and pickering stabilization. Food Hydrocoll. 2018, 82, 510–518. [Google Scholar] [CrossRef]
| Samples | Solubility (%) | WBC (g/g) |
|---|---|---|
| Native | 29.1 e ± 0.4 | 4.1 e ± 0.1 |
| 80C-2h | 27.0 d ± 0.0 | 4.1 e ± 0.1 |
| 80C-4h | 26.2 d ± 0.4 | 3.8 de ± 0.2 |
| 80C-24h | 21.9 b ± 0.1 | 3.8 de ± 0.0 |
| 80C-48h | 21.2 b ± 0.3 | 3.6 d ± 0.0 |
| 100C-2h | 21.6 b ± 0.3 | 3.1 bc ± 0.1 |
| 100C-4h | 24.6 c ± 0.3 | 2.8 ab ± 0.3 |
| 100C-24h | 22.0 b ± 0.1 | 3.6 cd ± 0.2 |
| 100C-48h | 16.8 a ± 0.0 | 2.6 a ± 0.1 |
| 120C-2h | 16.4 a ± 0.6 | 3.0 ab ± 0.1 |
| 120C-4h | 16.6 a ± 0.0 | 2.9 ab ± 0.1 |
| 120C-24h | 16.4 a ± 0.1 | 2.7 a ± 0.0 |
| 120C-48h | 17.0 a ± 1.0 | 2.6 a ± 0.0 |
| Samples | PV (cP) | HV (cP) | FV (cP) | SB (cP) | Tpaste (°C) | Peak T. (°C) | BD (cP) |
|---|---|---|---|---|---|---|---|
| Native | 1881.0 f ± 76.2 | 1272.3 f ± 42.5 | 1953.0 f ± 67.1 | 680.7 gh ± 24.8 | 77.7 b ± 0.2 | 91.8 ± 0.5 a | 608.7 ± 44.5 c |
| 80C-2h | 1809.0 f ± 97.1 | 1300.0 fg ± 44.8 | 1983.7 f ± 49.0 | 683.7 h ± 5.5 | 77.5 b ± 0.0 | 93.7 ± 0.6 b | 509.0 ± 52.4 c |
| 80C-4h | 1888.0 f ± 84.3 | 1335.0 fgh ± 12.3 | 1990.3 f ± 5.5 | 655.3 fg ± 6.8 | 77.4 b ± 0.0 | 94.7 ± 0.2 c | 553.0 ± 96.5 c |
| 80C-24h | 2180.7 g ± 67.7 | 1351.3 gh ± 3.8 | 1974.7 f ± 5.8 | 623.3 e ± 2.1 | 76.5 a ± 0.2 | 94.6 ± 0.1 c | 829.3 ± 68.0 d |
| 80C-48h | 2289.3 g ± 26.6 | 1399.0 hi ± 16.1 | 2040.0 f ± 8.7 | 641.0 ef ± 10.4 | 76.7 a ± 0.1 | 94.72 ± 0.1 c | 890.3 ± 14.6 d |
| 100C-2h | 1647.0 e ± 84.5 | 1450.0 i ± 52.1 | 2337.7 g ± 61.8 | 887.7 i ± 10.1 | 77.5 b ± 0.0 | 92.4 ± 0.3 a | 197.0 ± 33.4 b |
| 100C-4h | 1369.0 d ± 6.1 | 1144.0 e ± 14.7 | 1764.3 e ± 21.1 | 620.3 e ± 6.5 | 78.6 c ± 0.0 | 91.8 ± 0.5 a | 225.0 ± 9.0 b |
| 100C-24h | 139.0 ab ± 10.0 | 131.0 b ± 10.0 | 229.0 b ± 15.5 | 98.0 b ± 5.6 | - | 95.1 ± 0.0 c | 8.0 ± 0.0 a |
| 100C-48h | 67.0 ab ± 1.7 | 62.3 ab ± 1.2 | 91.7 a ± 0.6 | 29.3 a ± 0.6 | - | 95.0 ± 0.0 c | 4.7 ± 0.6 a |
| 120C-2h | 504.7 c ± 19.1 | 493.3 d ± 16.3 | 806.0 d ± 26.6 | 312.7 d ± 10.5 | 84.0 d ± 0.8 | 95.0 ± 0.0 c | 15.3 ± 6.5 a |
| 120C-4h | 218.0 b ± 7.0 | 204.0 c ± 4.6 | 370.3 c ± 10.7 | 166.3 c ± 6.7 | - | 95.0 ± 0.0 c | 16.7 ± 6.0 a |
| 120C-24h | 73.3 ab ± 5.0 | 70.0 ab ± 5.6 | 101.0 a ± 7.5 | 31.0 a ± 2.0 | - | 95.0 ± 0.0 c | 4.3 ± 2.1 a |
| 120C-48h | 36.7 a ± 2.5 | 34.0 a ± 2.6 | 50.0 a ± 3.0 | 16.0 a ± 1.0 | - | 95.0 ± 0.0 c | 3.0 ± 1.0 a |
| Native | 80C-24h | 80C-48 | 100C-2h | 100C-4h | 120C-2h | |
|---|---|---|---|---|---|---|
| SV (mL/g) | 1.66 b ± 0.06 | 1.51 a ± 0.07 | 1.62 ab ± 0.04 | 1.56 ab ± 0.10 | 1.57 ab ± 0.08 | 1.56 ab ± 0.03 |
| Crumb properties | ||||||
| Moisture content (%) | 49.2 a ± 0.6 | 49.7 a ± 0.4 | 49.1 a ± 0.7 | 49.9 a ± 0.6 | 49.2 a ± 0.7 | 49.6 a ± 0.6 |
| Hardness (N) | 17.1 d ± 1.8 | 15.8 cd ± 1.7 | 14.3 bc ± 1.3 | 11.6 a ± 1.3 | 12.4 ab ± 1.4 | 12.1 a ± 2.1 |
| Cohesiveness | 0.63 ab ± 0.02 | 0.63 ab ± 0.02 | 0.60 a ± 0.05 | 0.68 c ± 0.05 | 0.66 bc ± 0.02 | 0.65 bc ± 0.02 |
| Springiness | 0.90 ab ± 0.01 | 0.90 ab ± 0.01 | 0.88 a ± 0.03 | 0.91 bc ± 0.01 | 0.91 bc ± 0.01 | 0.92 c ± 0.03 |
| Resilience | 0.31 ab ± 0.01 | 0.31 ab ± 0.02 | 0.29 a ± 0.04 | 0.35 c ± 0.03 | 0.33 bc± 0.01 | 0.31 bc ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzetti, S.; Heetesonne, I.; Ngadze, R.T.; Linnemann, A.R. Dry Heating of Cowpea Flour below Biopolymer Melting Temperatures Improves the Physical Properties of Bread Made from Climate-Resilient Crops. Foods 2022, 11, 1554. https://doi.org/10.3390/foods11111554
Renzetti S, Heetesonne I, Ngadze RT, Linnemann AR. Dry Heating of Cowpea Flour below Biopolymer Melting Temperatures Improves the Physical Properties of Bread Made from Climate-Resilient Crops. Foods. 2022; 11(11):1554. https://doi.org/10.3390/foods11111554
Chicago/Turabian StyleRenzetti, Stefano, Ine Heetesonne, Ruth T. Ngadze, and Anita R. Linnemann. 2022. "Dry Heating of Cowpea Flour below Biopolymer Melting Temperatures Improves the Physical Properties of Bread Made from Climate-Resilient Crops" Foods 11, no. 11: 1554. https://doi.org/10.3390/foods11111554
APA StyleRenzetti, S., Heetesonne, I., Ngadze, R. T., & Linnemann, A. R. (2022). Dry Heating of Cowpea Flour below Biopolymer Melting Temperatures Improves the Physical Properties of Bread Made from Climate-Resilient Crops. Foods, 11(11), 1554. https://doi.org/10.3390/foods11111554