Effect of Fibril Entanglement on Pickering Emulsions Stabilized by Whey Protein Fibrils for Nobiletin Delivery
Abstract
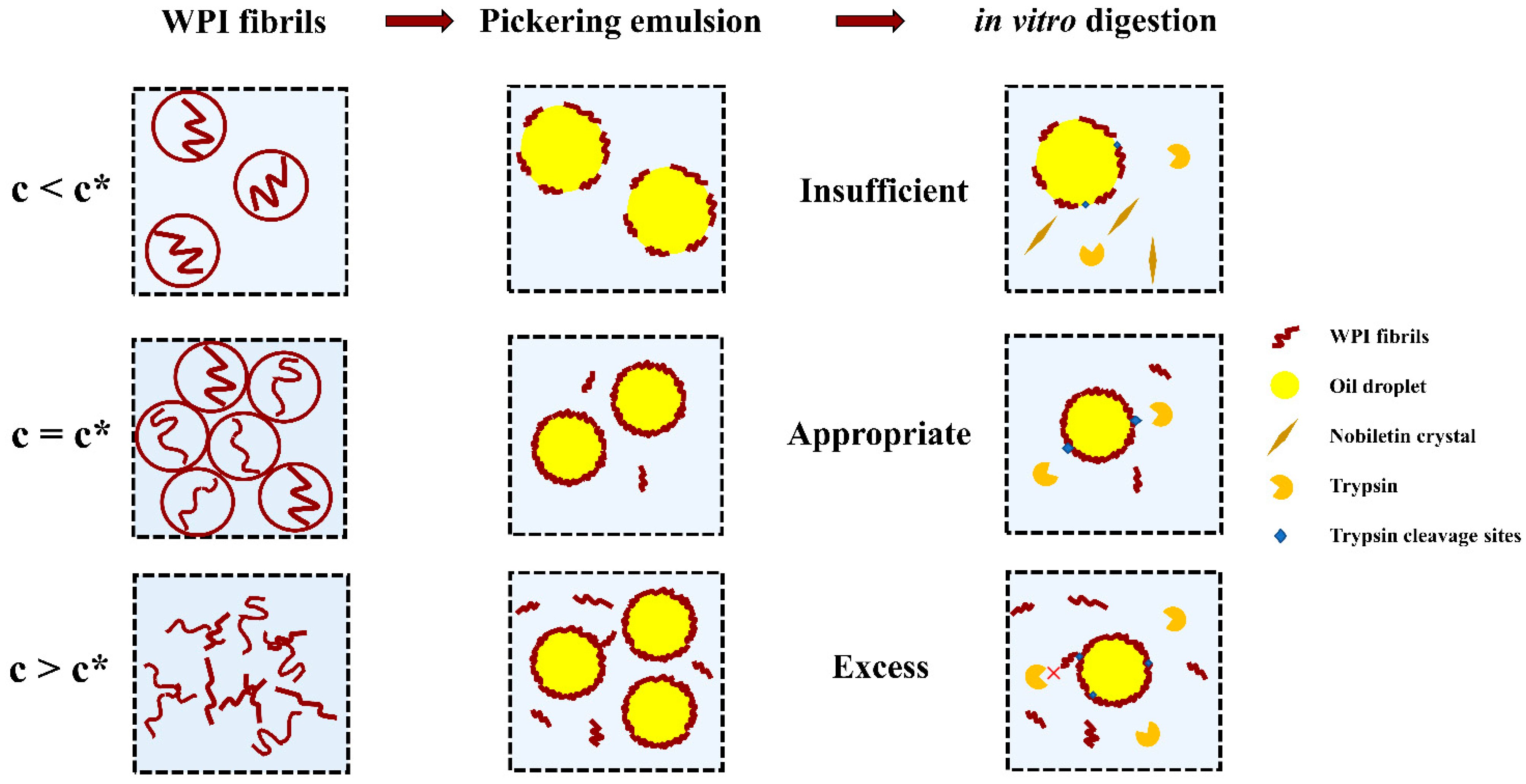
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPI Fibrils
2.3. Rheology Analysis
2.4. SAXS Analysis
2.5. Dilatational Interfacial Rheology
2.6. Preparation of WPI Fibril-Stabilized Pickering Emulsion with and without Nobiletin
2.6.1. Preparation of Pickering Emulsions Stabilized by WPI Fibrils
2.6.2. Preparation of Nobiletin-Loaded Pickering Emulsions Stabilized by WPI Fibrils
2.7. Characterization of WPI Fibril-Stabilized Pickering Emulsion
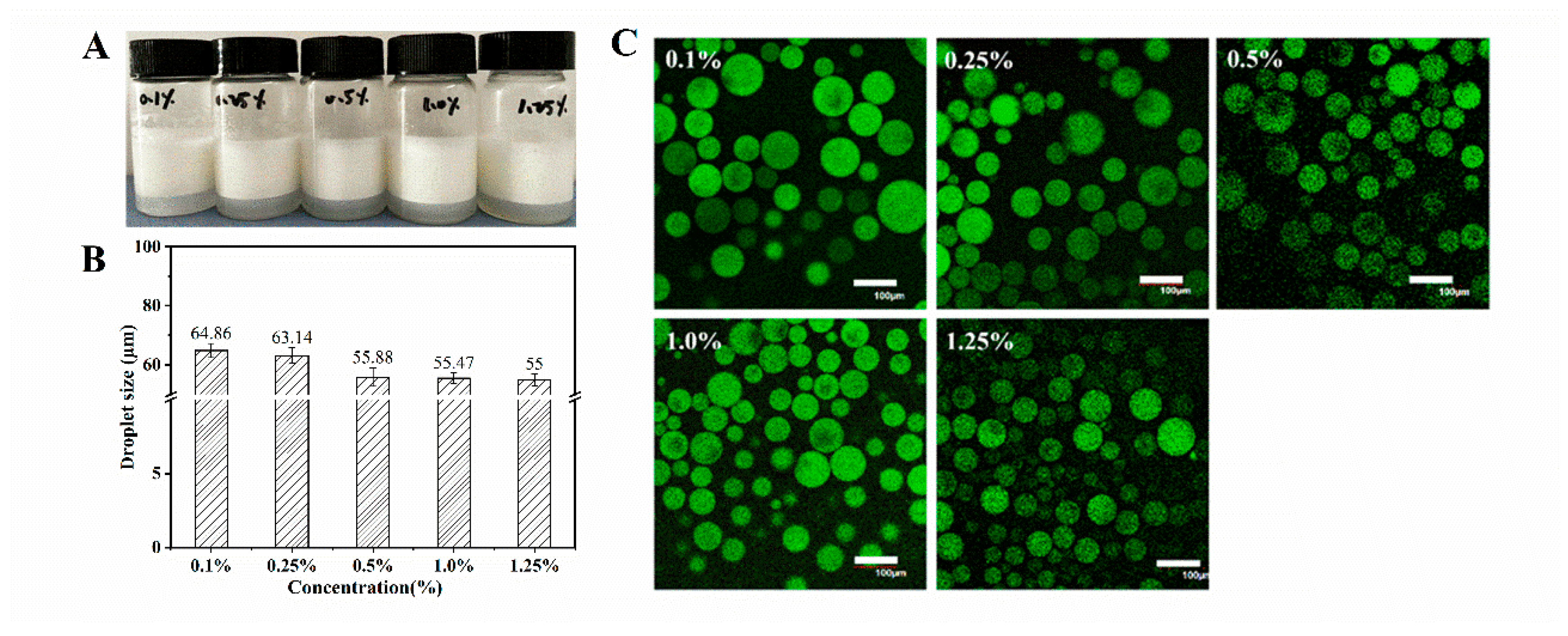
Confocal Laser Scanning Microscopy
2.8. Optical Microscopy
2.9. Physicochemical Stability of WPI Fibril- Stabilized Pickering Emulsions
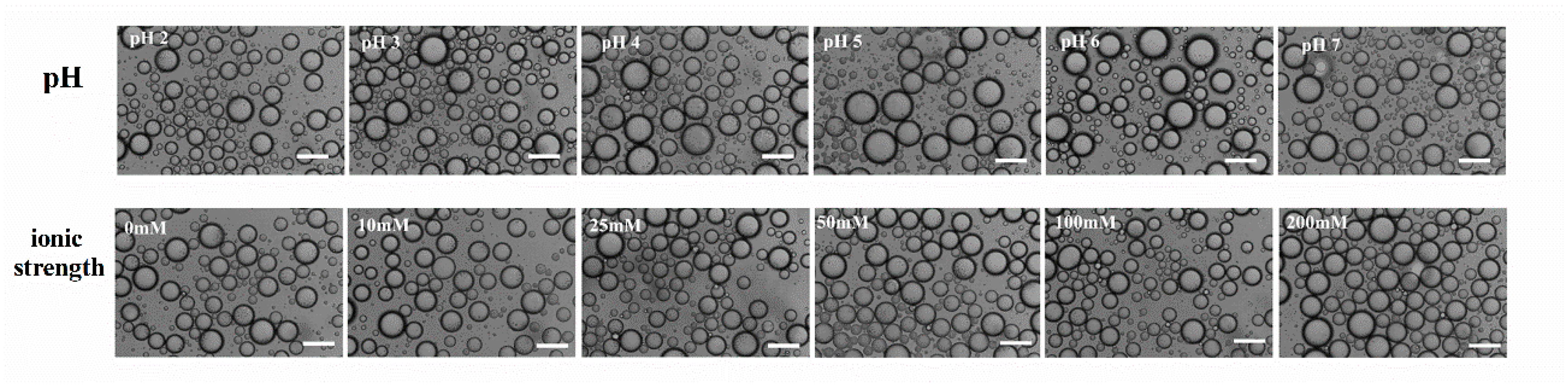
2.9.1. Effect of pH
2.9.2. Effect of Ionic Strength
2.9.3. Effect of Storage Time
2.10. In Vitro Digestion Analysis, Free Fatty Acid Release, and Bioaccessibility of Nobiletin
2.10.1. Digestion of Pickering Emulsions Stabilized by WPI Fibrils
2.10.2. High-Performance Liquid Chromatography (HPLC) Analysis of Nobiletin
2.10.3. Determination of Nobiletin Bioaccessibility
2.11. Statistical Analysis
3. Results and Discussion
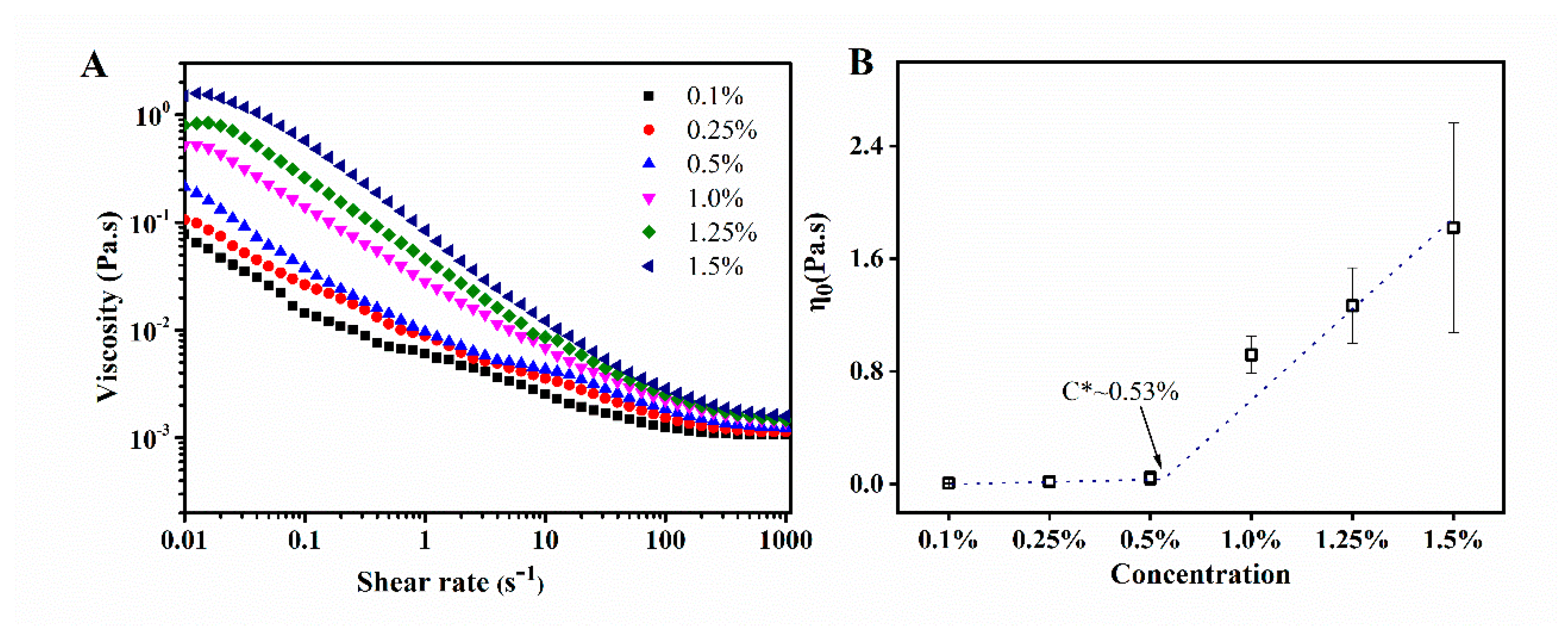
3.1. Rheology
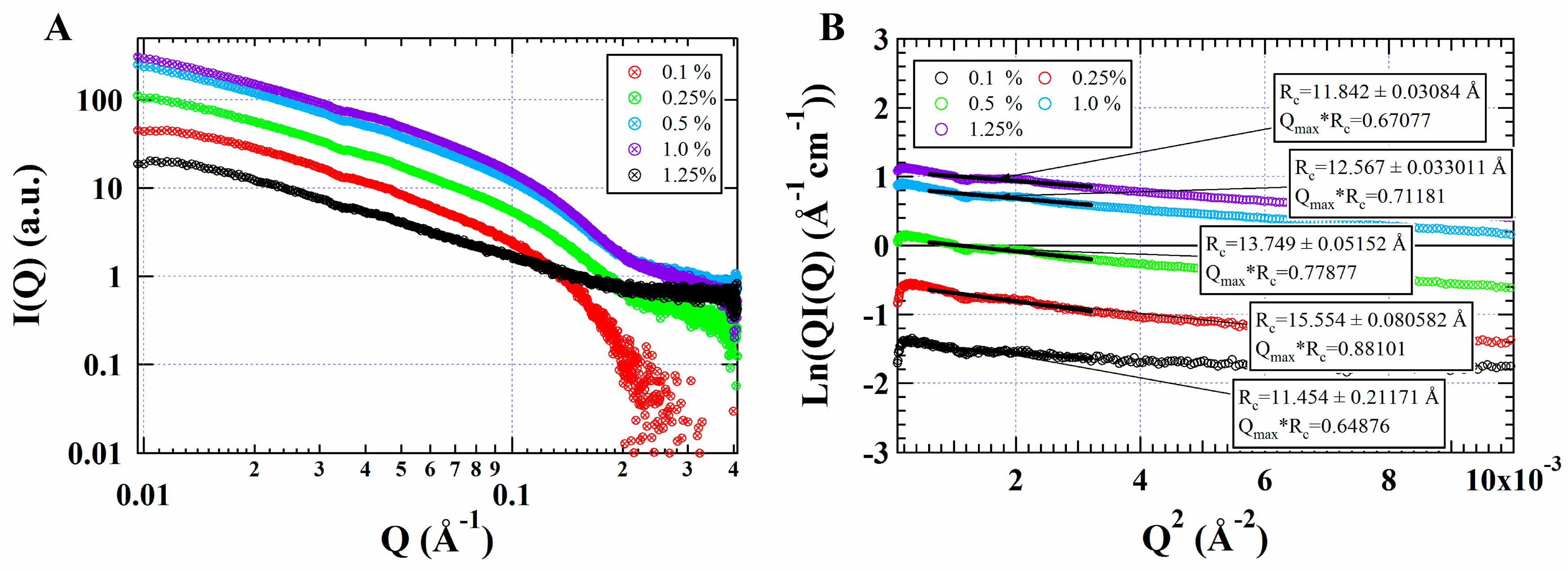
3.2. SAXS
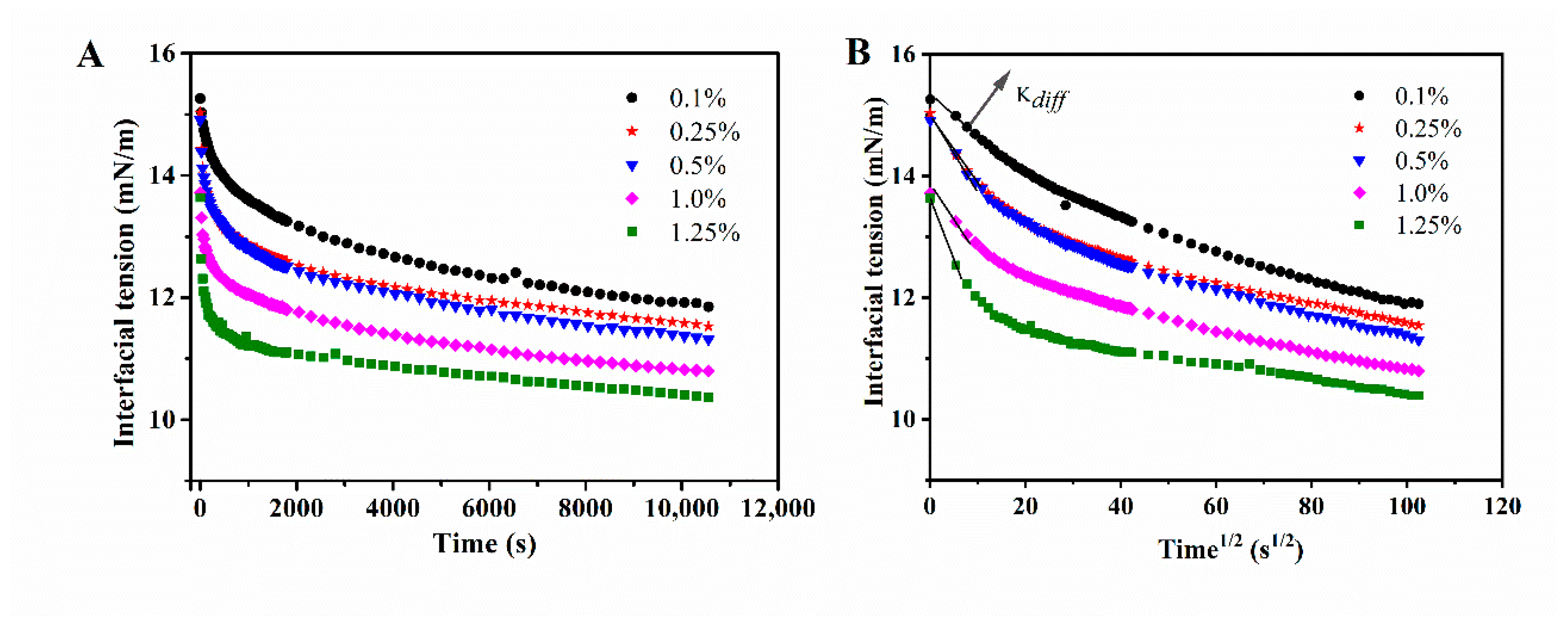
3.3. Interfacial Adsorption Behavior
3.4. Characterization of WPI Fibril-Stabilized Emulsions
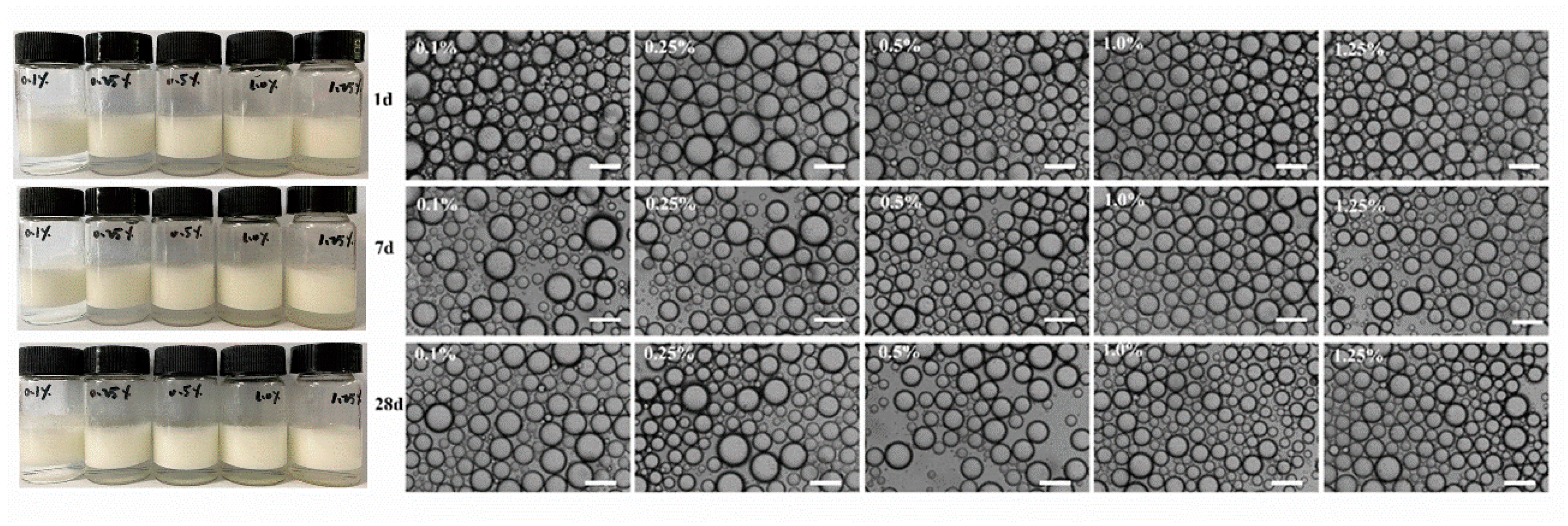
3.5. Physicochemical Stability of WPI Fibril-Stabilized Pickering Emulsions
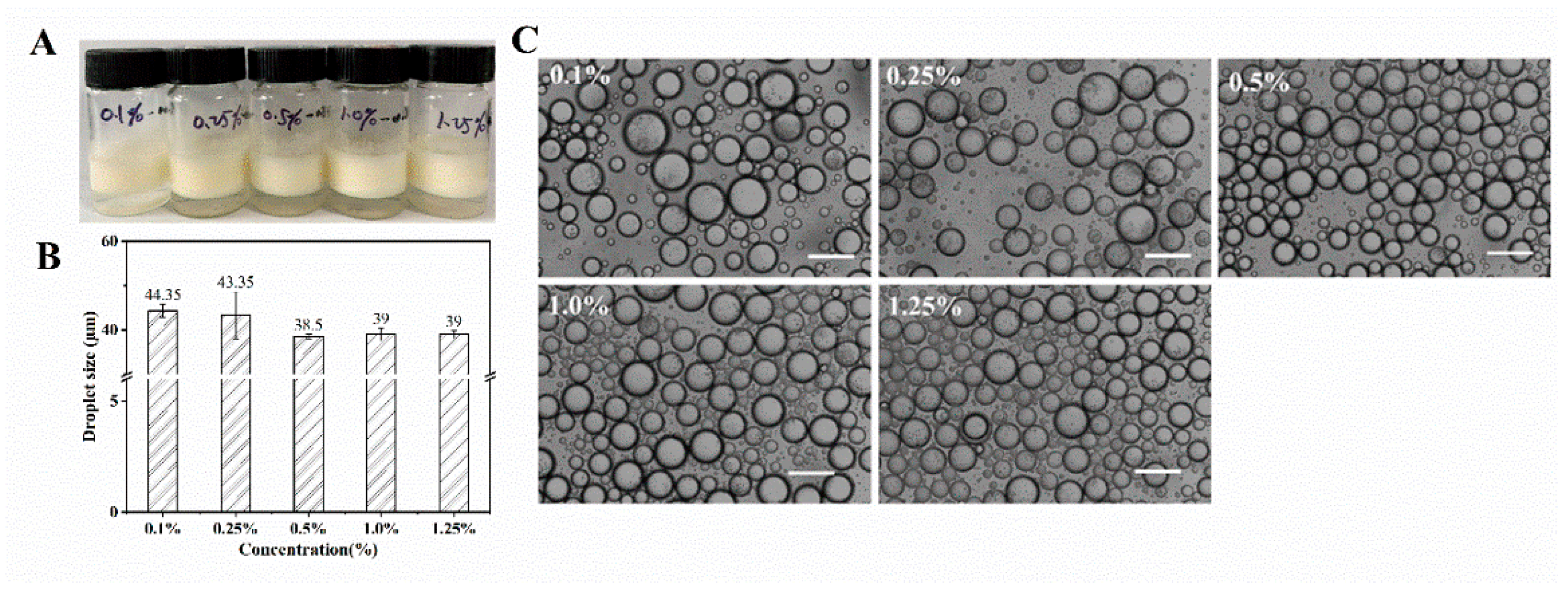
3.6. Characterization of Nobiletin-Loaded WPI Fibrils Stabilized Pickering Emulsion
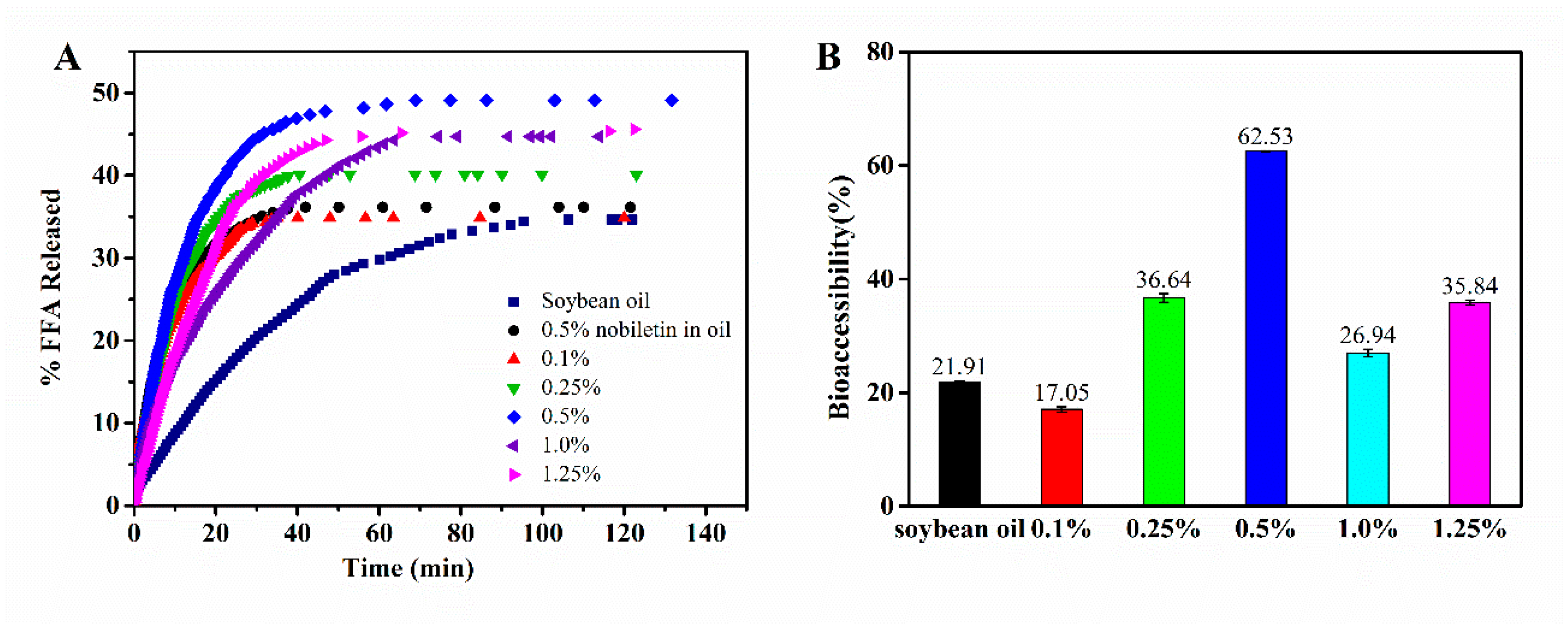
3.7. Lipolysis and Bioaccessibility of Nobiletin in WPI Fibril-Stabilized Pickering Emulsions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, Z.; Cheng, Y.; Zhu, J.; Huang, Q. Genipin-crosslinked ovotransferrin particle-stabilized Pickering emulsions as delivery vehicles for hesperidin. Food Hydrocoll. 2019, 94, 561–573. [Google Scholar] [CrossRef]
- Su, J.; Guo, Q.; Chen, Y.; Dong, W.; Mao, L.; Gao, Y.; Yuan, F. Characterization, and formation mechanism of lutein Pickering emulsion gels stabilized by β-lactoglobulin-gum arabic composite colloidal nanoparticles. Food Hydrocoll. 2020, 98, 105276. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocoll. 2021, 123, 107180. [Google Scholar] [CrossRef]
- Dai, L.; Zhan, X.; Wei, Y.; Sun, C.; Mao, L.; Mcclements, D.J.; Gao, Y. Composite zein-propylene glycol alginate particles prepared using solvent evaporation: Characterization and application as Pickering emulsion stabilizers. Food Hydrocoll. 2018, 85, 281–290. [Google Scholar] [CrossRef]
- Jiang, F.; Pan, Y.; Peng, D.; Huang, W.; Shen, W.; Jin, W.; Huang, Q. Tunable self-assemblies of whey protein isolate fibrils for Pickering emulsions structure regulation. Food Hydrocoll. 2022, 124, 107264. [Google Scholar] [CrossRef]
- Feng, X.; Dai, H.; Ma, L.; Fu, Y. Properties of Pickering emulsion stabilized by food-grade gelatin nanoparticles: Influence of the nanoparticles concentration. Colloids Surf. B Biointerfaces 2020, 196, 111294. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.; Khan, N.M.; Ali, F.; Khan, Z.U.; Muhammad, N. Effect of protein and oil volume concentrations on emulsifying properties of acorn protein isolate. Food Chem. 2020, 324, 126894. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, J.; Huang, Y.; Yao, X.; Zhang, K.; Fang, Y.; Nishinari, K.; Phillips, G.O.; Jiang, F.; Yang, H. Edible Pickering emulsion stabilized by protein fibrils. Part 1: Effects of pH and fibrils concentration. LWT-Food Sci. Technol. 2017, 76, 1–8. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. Pea protein exhibits a novel Pickering stabilization for oil-in-water emulsions at pH 3.0. LWT-Food Sci. Technol. 2014, 58, 463–469. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C.-H. Gel-like pea protein Pickering emulsions at pH 3.0 as a potential intestine-targeted and sustained-release delivery system for beta-carotene. Food Res. Int. 2016, 79, 64–72. [Google Scholar] [CrossRef]
- Bttcher, S.; Keppler, J.K.; Drusch, S.J.C.; Physicochemical, S.A. Mixtures of Quillaja saponin and beta-lactoglobulin at the oil/water-interface: Adsorption, interfacial rheology and emulsion properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 518, 46–56. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C. Soy glycinin as food-grade Pickering stabilizers: Part. I. Structural characteristics, emulsifying properties and adsorption/arrangement at interface. Food Hydrocoll. 2016, 60, 606–619. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Lu, W.; Sun, C.; Fang, Y. Evolution of physicochemical and antioxidant properties of whey protein isolate during fibrillization process. Food Chem. 2021, 357, 129751. [Google Scholar] [CrossRef] [PubMed]
- Bolder, S.G.; Vasbinder, A.J.; Sagis, L.M.C.; Linden, E.V.D. Heat-induced whey protein isolate fibrils: Conversion, hydrolysis, and disulphide bond formation. Int. Dairy J. 2007, 17, 846–853. [Google Scholar] [CrossRef]
- Loveday, S.M.; Su, J.; Rao, M.A.; Anema, S.G.; Singh, H.J.B. Effect of calcium on the morphology and functionality of whey protein nanofibrils. Biomacromolecules 2011, 12, 3780–3788. [Google Scholar] [CrossRef]
- Oboroceanu, D.; Wang, L.; Magner, E.; Auty, M.A.E. Fibrillization of whey proteins improves foaming capacity and foam stability at low protein concentrations. J. Food Eng. 2014, 121, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, T.; Galindo-Rosales, F.J.; Campo-Deaño, L. Critical overlap concentration and intrinsic viscosity data of xanthan gum aqueous solutions in dimethyl sulfoxide. Data Brief 2020, 33, 106431. [Google Scholar] [CrossRef]
- Serfert, Y.; Lamprecht, C.; Tan, C.P.; Keppler, J.K.; Appel, E.; Rossier-Miranda, F.J.; Schroen, K.; Boom, R.M.; Gorb, S.; Selhuber-Unkel, C.J.J. Characterisation and use of β-lactoglobulin fibrils for microencapsulation of lipophilic ingredients and oxidative stability thereof. J. Food Eng. 2014, 143, 53–61. [Google Scholar] [CrossRef]
- Zhang, B.; Lei, M.; Huang, W.; Liu, G.; Jin, W. Improved Storage Properties and Cellular Uptake of Casticin-Loaded Nanoemulsions Stabilized by Whey Protein-Lactose Conjugate. Foods 2021, 10, 1640. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Jin, W.; Sagis, L.M.C.; Li, B. Adsorption of microgel aggregates formed by assembly of gliadin nanoparticles and a β-lactoglobulin fibril-peptide mixture at the air/water interface: Surface morphology and foaming behavior. Food Hydrocoll. 2022, 122, 1070399. [Google Scholar] [CrossRef]
- Lai, C.S.; Li, S.; Chai, C.Y.; Wang, Y.J. Anti-inflammatory and antitumor promotional effects of a novel urinary metabolite, 3’,4’-didemethylnobiletin, derived from nobiletin. Carcinogenesis 2008, 29, 2415–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Zheng, J.; Hang, X.; Mcclements, D. Nanoemulsion-based delivery systems for poorly water-soluble bioactive compounds: Influence of formulation parameters on polymethoxyflavone crystallization. Food Hydrocoll. 2012, 27, 517–528. [Google Scholar]
- Li, S.; Wang, H.; Guo, L.; Zhao, H.; Ho, C.T. Chemistry and bioactivity of nobiletin and its metabolites. J. Funct. Foods 2014, 6, 2–10. [Google Scholar] [CrossRef]
- Wagoner, T.B.; Cakir-Fuller, E.; Drake, M.A.; Foegeding, E.A. Sweetness perception in protein-polysaccharide beverages is not explained by viscosity or critical overlap concentration. Food Hydrocoll. 2019, 94, 229–237. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Lan, Y.; Xia, C.; Lin, Z.; Rogers, M.A.; Huang, Q. Viscoelastic Emulsion Improved the Bioaccessibility and Oral Bioavailability of Crystalline Compound: A Mechanistic Study Using in Vitro and in Vivo Models. Mol. Pharm. 2015, 12, 2229. [Google Scholar] [CrossRef]
- Heller, C. Capillary electrophoresis of proteins and nucleic acids in gels and entangled polymer solutions. J. Chromatogr. A 1995, 698, 19–31. [Google Scholar] [CrossRef]
- Blanchet, C.E.; Svergun, D.I. Small-angle X-ray scattering on biological macromolecules and nanocomposites in solution. Annu. Rev. Phys. Chem. 2013, 64, 37–54. [Google Scholar] [CrossRef]
- Shi, C.; Li, Y. Progress on the Application of Small-angle X-ray Scattering in the Study of Protein and Protein Complexes. Acta Polym. Sin. 2015, 8, 871–883. [Google Scholar]
- Li, Y.; Xia, Q.; Shi, K.; Huang, Q. Scaling behaviors of α-zein in acetic acid solutions. J. Phys. Chem. B 2011, 115, 9695–9702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tobin, J.T.; Drusch, S.; Hogan, S.A. Dynamic adsorption and interfacial rheology of whey protein isolate at oil-water interfaces: Effects of protein concentration, pH and heat treatment. Food Hydrocoll. 2021, 116, 106640. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, Y.; Kong, X.; Zhang, C.; Hua, Y. Emulsifying Properties and Oil/Water (O/W) Interface Adsorption Behavior of Heated Soy Proteins: Effects of Heating Concentration, Homogenizer Rotating Speed, and Salt Addition Level. J. Agric. Food Chem. 2014, 62, 1634–1642. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; Li, J.; Tang, C.; Li, B. Foams Stabilized by β-Lactoglobulin Amyloid Fibrils: Effect of pH. J. Agric. Food Chem. 2017, 65, 10658–10665. [Google Scholar] [CrossRef]
- Silva, A.; Almeida, F.S.; Sato, A. Functional characterization of commercial plant proteins and their application on stabilization of emulsions. J. Food Eng. 2020, 292, 110277. [Google Scholar] [CrossRef]
- Peng, J.; Simon, J.R.; Venema, P.; van der Linden, E. Protein Fibrils Induce Emulsion Stabilization. Langmuir 2016, 32, 2164–2174. [Google Scholar] [CrossRef]
- Ebert, S.; Grossmann, L.; Hinrichs, J.; Weiss, J. Emulsifying properties of water-soluble proteins extracted from the microalgae Chlorella sorokiniana and Phaeodactylum tricornutum. Food Funct. 2019, 10, 754–764. [Google Scholar] [CrossRef]
- Wu, J.; Shi, M.; Li, W.; Zhao, L.; Wang, Z.; Yan, X.; Norde, W.; Li, Y. Pickering emulsions stabilized by whey protein nanoparticles prepared by thermal cross-linking. Colloids Surf. B Biointerfaces 2015, 127, 96–104. [Google Scholar] [CrossRef]
- Liu, G.; Li, W.; Qin, X.; Zhong, Q. Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocoll. 2019, 101, 105503. [Google Scholar] [CrossRef]
- Lv, P.; Wang, D.; Chen, Y.; Yuan, F. Pickering emulsion gels stabilized by novel complex particles of high-pressure-induced WPI gel and chitosan: Fabrication, characterization and encapsulation. Food Hydrocoll. 2020, 108, 105992. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Wang, Y.; Huang, Q. Oleogel-based Pickering emulsions stabilized by ovotransferrin–carboxymethyl chitosan nanoparticles for delivery of curcumin. LWT-Food Sci. Technol. 2022, 157, 113121. [Google Scholar] [CrossRef]
- Lesmes, U.; Baudot, P.; Mcclements, D.J. Impact of Interfacial Composition on Physical Stability and In Vitro Lipase Digestibility of Triacylglycerol Oil Droplets Coated with Lactoferrin and/or Caseinate. J. Agric. Food Chem. 2010, 58, 7962–7969. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, C.; Huang, Q. Kafirin Nanoparticle-Stabilized Pickering Emulsions as Oral Delivery Vehicles: Physicochemical Stability and in Vitro Digestion Profile. J. Agric. Food Chem. 2015, 63, 10263–10270. [Google Scholar] [CrossRef]
- Infantes-Garcia, M.R.; Verkempinck, S.H.E.; Gonzalez-Fuentes, P.G.; Hendrickx, M.E.; Grauwet, T. Lipolysis products formation during in vitro gastric digestion is affected by the emulsion interfacial composition. Food Hydrocoll. 2021, 110, 106163. [Google Scholar] [CrossRef]
- Li, Y.; Mcclements, D.J. New Mathematical Model for Interpreting pH-Stat Digestion Profiles: Impact of Lipid Droplet Characteristics on in Vitro Digestibility. J. Agric. Food Chem. 2010, 58, 8085–8092. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Chen, C.; Wang, X.; Huang, W.; Jin, W.; Huang, Q. Effect of Fibril Entanglement on Pickering Emulsions Stabilized by Whey Protein Fibrils for Nobiletin Delivery. Foods 2022, 11, 1626. https://doi.org/10.3390/foods11111626
Jiang F, Chen C, Wang X, Huang W, Jin W, Huang Q. Effect of Fibril Entanglement on Pickering Emulsions Stabilized by Whey Protein Fibrils for Nobiletin Delivery. Foods. 2022; 11(11):1626. https://doi.org/10.3390/foods11111626
Chicago/Turabian StyleJiang, Fangcheng, Chunling Chen, Xinlan Wang, Wenjing Huang, Weiping Jin, and Qingrong Huang. 2022. "Effect of Fibril Entanglement on Pickering Emulsions Stabilized by Whey Protein Fibrils for Nobiletin Delivery" Foods 11, no. 11: 1626. https://doi.org/10.3390/foods11111626
APA StyleJiang, F., Chen, C., Wang, X., Huang, W., Jin, W., & Huang, Q. (2022). Effect of Fibril Entanglement on Pickering Emulsions Stabilized by Whey Protein Fibrils for Nobiletin Delivery. Foods, 11(11), 1626. https://doi.org/10.3390/foods11111626





