1. Introduction
Emulsified meat products, such as sausages, meatballs and burgers, are the most popular ready-to-eat foods for consumers because of their convenience and high sensory quality [
1]. In general, emulsified meat products are produced with a high level of fat, ranging from 20% to 30% [
2]. Excessive consumption can easily lead to obesity and certain chronic diseases, threatening human health. As consumers become increasingly aware of the relationship between red meat consumption and health, the decrease in the fat content of emulsified meat products has been one of the developing directions of the meat processing industry. Animal fat plays a key role in determining the quality of emulsified meat products, such as improved texture, cooking yield, color and unique flavor [
3]. Previous studies have reported that the direct fat-reduction method by the addition of more water or meat leads to greater cooking loss and inferior emulsion stability of final products [
4]. It remains a challenge to reduce the content of animal fat and improve or maintain the quality of lower-fat meat products. Meat scientists and producers introduce bioactive components during processing and design novel formulations to increase product value of processed meat, thus achieving a balance between fat replacement and desirable meat quality [
5,
6]. In recent years, edible fungi have been added into meat emulsions systems to obtain healthier meat products with lower fat, reduced salt and fewer calories, as well as the introduction of bioactive components, such as dietary fibers and natural antioxidants.
Edible fungi are rich in unsaturated fatty acids, amino acids, minerals and other nutrients, as well as bioactive substances such as polysaccharides and phenols, which represent a much healthier alternative for a balanced diet [
7,
8]. These nutrients have antioxidant, bacteriostatic and anticancer functions. Edible fungi are widely used in meat product processing and are advantageous in improving the nutritional attributes, textural properties and cooking yield of finished meat products [
9,
10]. Recent studies concerned the addition of various edible mushroom flours into meat products for decreasing salt and fat content and improving the quality of the final products, such as water retention, textural characteristics and flavor [
11,
12,
13]. Cerón-Guevara et al. found that the addition of
Agaricus bisporus and
Pleurotus ostreatus flours as a partial substitute of fat and salt into cold-stored beef patties effectively improved its cooked yield, but did not improve the springiness and cohesiveness compared to the high-fat beef patties [
14]. Patinho et al. reported that the fat could be partially replaced by the addition of 10% and 20%
Agaricus bisporus in beef burgers to produce low-fat beef burgers, while retaining the instrumental characteristics and a good overall acceptability [
15].
Oudemansiella raphanipies is a rare edible fungus with fresh and tender meat, unique taste and high nutritional value, which has been welcomed by consumers [
7]. In China, it is mainly distributed in Yunnan, Sichuan, Fujian, Xizang, Jiangsu and other places.
Oudemansiella raphanipies contains high protein, low fat and a high content of umami amino acids and antioxidant components such as polyphenols, polysaccharides and flavonoids. At present, more research has focused on the activity characterization of functional components in the
Oudemansiella raphanipies. Thus, it has potential as a functional food used in analgesic, anti-inflammatory contexts, improving immunity, repairing damaged organs and regulating body functions, which may be applied in meat products to improve nutritional values and functional properties. However, the application of
Oudemansiella raphanipies powder (ORP) in the development of meat products is scarce. It is essential to further understand whether ORP has the ability to promote the formation of meat proteins matrix formation and a better water-holding capacity (WHC) of lower-fat meat batter.
Therefore, the aim of this work was to investigate the effect of ORP on the physicochemical, textural and rheological properties, water distribution and movement and protein secondary structure of lower-fat pork batters. Our findings will provide a promising method to achieve nutritious meat products, while maintaining texture and water retention.
2. Materials and Methods
2.1. Materials
Oudemansiella raphanipies was obtained from Yichun Minghe Kangtai Agricultural Development Co., Ltd. (Nanchang, Jiangxi, China). Pork leg lean meat (M. semitendinosus, 24–48 h postmortem, pH 5.6–5.8), pork back fat, salt, sugar and white pepper powder were purchased from Zhengzhou Dennis Department Store Co., Ltd. (Zhengzhou, Henan, China). All other chemicals and reagents used in this study were of analytical grade.
2.2. Preparation of Oudemansiella raphanipies Powder
The Oudemansiella raphanipies fungi were washed in warm water at 60 °C to remove sediment, and dispersed into triple distilled water (w:w) with an ultrasound cleaner (Ningbo Xinzhi Biotechnology Co., Ltd., Ningbo, China) at 300 W for 30 min. The samples were dried at 80 °C for 16 h (electric constant humidity air drying box, DHG-9076A Shanghai Jinghong Experimental Equipment Co., Ltd., Shanghai, China). Next, they were grounded to a powder by high-speed mill (QE-100, Zhejiang Yili Industry & Trade Co., Ltd., Quzhou, China). Afterward, they were sieved by 60 mesh sieve (aperture 0.28 mm) to obtain Oudemansiella raphanipies powder (ORP). The ORP was repacked in a vacuum bag and stored at room temperature for subsequent experiments.
2.3. Determination of Chemical Composition and Hydrolytic Amino Acid Content of OPR
The moisture, protein, crude fat, carbohydrate, crude fiber and ash contents of
Oudemansiella raphanipies powder were measured according to the method of Yu et al. (2020) [
16]. Amino acid composition of ORP was determined according to the guideline of GB 5009.124-2016 [
17].
2.4. Preparation of Lower-Fat Meat Batters
Five groups of lower-fat pork meat batters were formulated, as presented in
Table 1. Specifically, the C group was set as the control. The other four treatment groups, labeled T1, T2, T3 and T4, were formulated with 1%, 2%, 3% and 4% (
w/
w) ORP of the meat (M
meat = M
Pork lean meat + M
Pork back-fat; M stands for mass), respectively. Details for meat batter preparation were described in the previous study [
18]. Briefly, all external fat and connective tissue were removed from the meat. The pork lean meat and pork back-fat were crushed by a 6 mm meat grinder (HM 740, Qingdao Hanshang Electric Appliance Co., Ltd., Qingdao, Shandong, China) and then frozen for later use.
The abovementioned minced meat was thawed at 4 °C overnight. A total of 160 g pork leg lean meat was ground with the required amount of Oudemansiella raphanipies powder and 10 g ice water in a bowl chopper (Retsch GM 300, Haan, Germany) at 3000 rpm for 20 s. Next, 3 g salt, 0.48 g tripolyphosphate and 10 g ice water were added into meat batter and chopped for another 20 s, followed by a 2 min break. Afterward, 40 g pork fat, 7.2 g sugar water, 0.32 g white pepper and 10 g ice water were added to the mixtures and chopped for 20 s. The prepared raw meat batter was stored at 4 °C for later use.
Approximately 20 g of the prepared meat batter was stuffed into 50 mL polypropylene centrifuge tube and sealed hermetically. To remove the residual air bubbles, the plastic containers were centrifuged at 500× g for 5 min at 4 °C. Some of the centrifuged meat batters were heated in a water bath at 80 °C for 20 min until the core temperature reached 72 °C, then chilled immediately in an ice bath for 30 min and kept overnight at 4 °C for the recording pH measurement, color, texture profile analysis (TPA) and Raman spectroscopy. Each group was made in six parallel samples. A total of thirty meat batter samples were obtained (6 × 5 groups). The remaining meat batters were stored at 4 °C to evaluate the dynamic rheology, cooking yield, water retention measurement and low-field nuclear magnetic resonance (LF-NMR) spectroscopy.
2.5. pH
After cooling at 4 °C overnight, the cooked lower-fat pork batters were placed at room temperature (20 °C) for 2 h. Approximately 10 g of the cooked meat batter was blended with 40 mL distilled ice water using a homogenizer at 15,000 rpm for 10 s (T25 digital, IKA Ltd., Staufen, Germany), and the pH was measured by a digital pH meter (Hanna, Italy). Each treatment group was performed in three replicates.
2.6. Color Evaluation
The color of cooked lower-fat pork batter was determined according to the method of Li et al. [
18] with some modifications. After cooling at 4 °C overnight, the cooked lower-fat pork batters were placed at room temperature (20 °C) for 2 h. Then, the boiled pellets were cut into 2 cm-thick cylinders, and then the color of the samples was measured with a portable high-quality spectrophotometer (NS 800, 3 NH Technology Co., Ltd., Shenzhen, China) calibrated with an 8 mm aperture and D
65 illuminant.
Before testing, the instrument was immediately calibrated against a standard white plate (L* = 96.28, a* = 0.26, b* = 1.26). The lightness (L*), redness (a*) and yellowness (b*) values were recorded individually. Six samples taken from each formulation group were evaluated for the internal color.
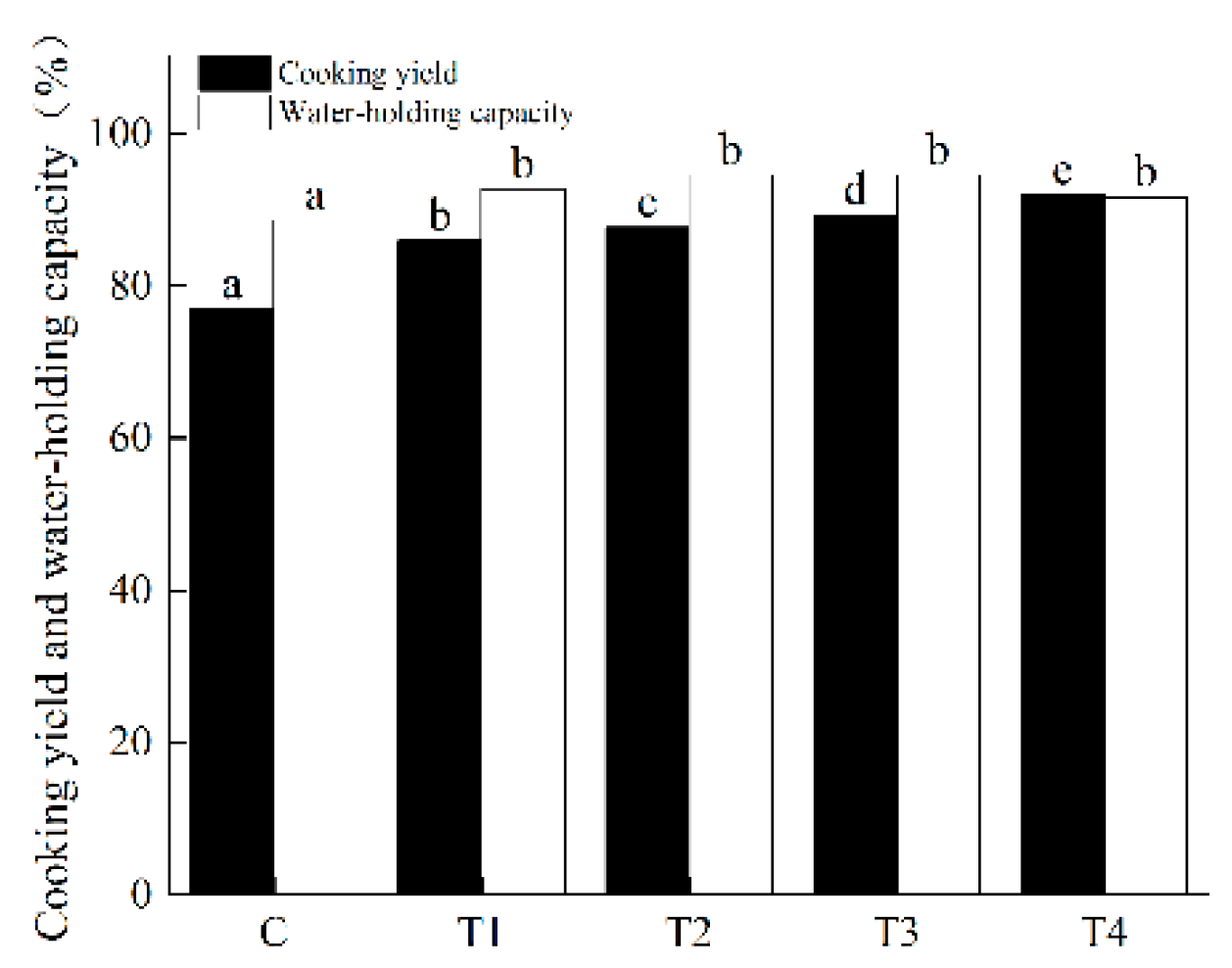
2.7. Cooking Yield
The cooking yield (%) of the samples was measured and evaluated based on the procedure described by Sompugdee et al. [
5]. Briefly, after refrigerated storage at 4 °C overnight, the meat batters prepared in
Section 2.4 were kept at room temperature for 30 min to achieve an equilibrium. We poured the sample out of the centrifugal tube and removed the excess water with absorbable paper and weighed the mass meter (m
3) of each pellet sample.
The cooking loss (%) was calculated as a percentage from the known weights of meat batter before cooking.
where m
1 is the mass of an empty 50 mL centrifugal tube (g); m
2 is the total mass of sample and centrifugal tube before cooking (g); and m
3 is the sample mass (g) of dried water after cooking.
2.8. WHC
Approximately 1.00 g (exact sample weights were recorded) of meat batter was wrapped in a double filter paper and filled into 50 mL plastic tubes, centrifuged at 3500 rpm for 5 min at 4 °C. After centrifugation, we removed and dried the surface moisture and measured its mass immediately. The pre-centrifugation weight (m
4) and the post- centrifugation weight (m
5) of the sample were accurately weighed to be used for the following calculation formula:
2.9. Texture Profile Analysis
The cooked meat batters prepared in
Section 2.4 were equilibrated at 25 °C for at least 2 h to keep the internal and external temperature of the pellet consistent, and then cut into a 20 mm-high meat cylinder. Textural profile analysis (TPA) was conducted using a texture analyzer (Model TA-XT Icon, Stable Micro Systems Co., Ltd. Godalming, Surrey, UK) equipped with a P/36R cylindrical probe. The setting parameters were as follows: pre-test speed, 2.0 mm/s; test speed, 2.0 mm/s; post-test speed, 5.0 mm/s; strain, 50%; time, 5 s; trigger type, automatic. The obtained parameters comprise hardness, springiness, cohesiveness and chewiness. Hardness is the peak force for the first compression (g). Springiness is the ratio of the sample recovered after the first compression (mm). Cohesiveness is the ratio of the area of the second compression to the first compression (dimensionless). Chewiness is the product of hardness × springiness × cohesiveness (g.mm). Each parallel treatment group was tested six times.
2.10. Dynamic Rheological Measurement
To measure the dynamic rheological behaviors of the uncooked lower-fat meat batter during thermal processing, a temperature ramp sweep test of samples was performed using a hybrid rheometer (Discovery HR-1, TA Instruments Inc., New Castle, DE, USA) following the method of Li et al. [
19]. Approximately 5 g of uncooked low-fat meat batter was placed between the 40 mm diameter smart-swap, the gap between upper and lower of the parallel plates was 0.5 mm in diameter, and then the edge of sample was sealed with a thin layer of silicone oil to avoid moisture evaporation during the thermal processing. The dynamic viscoelastic measurements were determined at a 0.5% strain and a fixed frequency of 0.1 Hz in an oscillating mode. The heating procedure was programmed to hold each sample for 10 min at 20 °C, followed by a heat-induced process from 20 °C to 80 °C at a heating rate of 1 °C/min via a programmable circulating water bath. The rheological storage modulus (G′) and loss modulus (G″) were recorded continuously during the gel-forming process. Each sample was performed in triplicate.
2.11. Low-Field NMR Measurement
Approximately 1.0 g of raw meat batter was put into a 1.5 mL centrifuge tube at 80 °C for 20 min, then further cooled at room temperature for 2 h. The meat sample was taken out and the surface moisture was dried. The meat sample was put into a new 1.5 mL centrifuge tube and then put into a nuclear magnetic tube with a diameter of 15 mm. Low-field NMR was determined in the analyzer, which was operated at a proton resonance frequency of 18 MHz at 32 °C. Spin–spin relaxation time T2 was measured with a Carr-Purcell-Meiboom-Gill (CPMG) sequence, resulting in an exponential decay pattern. The operation conditions were as follows: the sampling frequency was 200 kHz and the half-echo time τ- (the time between pulse of 90° and 180°) was 200 µs. Data from 12,000 echoes were acquired as 16 scan repetitions with the interval of 110 ms. Data of six measurements per samples were recorded.
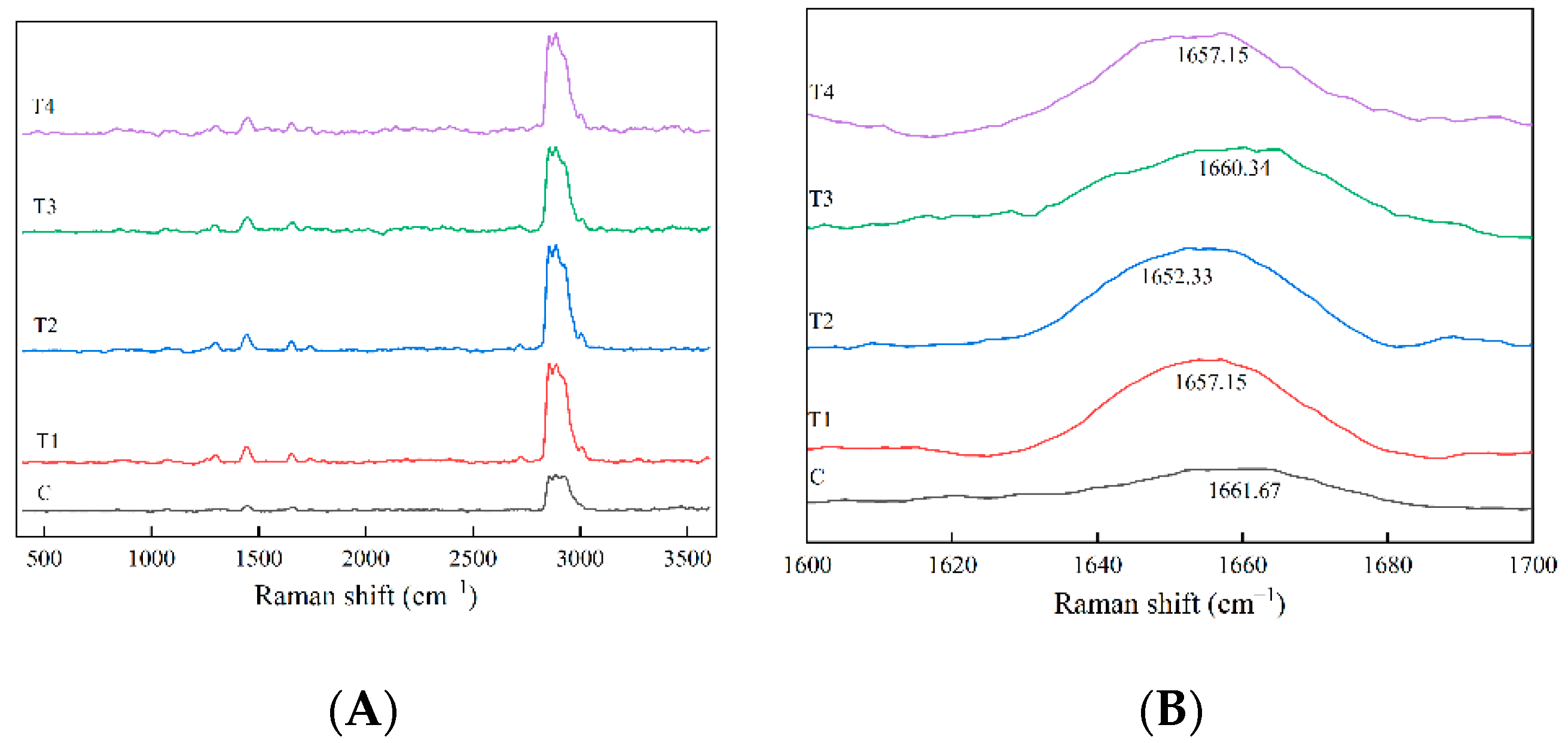
2.12. Raman Spectrometry Analysis
The Raman spectra of cooked pork batter were measured using a Laser Confocal Raman Microscope (inVia Qontor, Renishaw, UK) equipped with a 532 nm argon ion laser. Approximately 0.5 g of sample was put into the center of a slide, and a Raman spectrum in the range of 400~3600 cm
−1 was collected. The test parameters of each spectrum were acquired as follows: 3 scans, 30 s of exposure time, 1 cm
−1 resolution and laser power was 10% of the total power. The obtained spectra for baseline and smooth were analyzed using Peakfit 4.12 software. The secondary structures including the α-helix, β-sheet, β-turn and random coil content of samples were determined based on the method of Alix et al. [
20].
2.13. Statistical Analysis
Statistical analysis was performed by one-way ANOVA using SPSS V.21.0 (SPSS Inc., Chicago, IL, USA). A significant difference among lower-fat pork batter samples with or without ORP was determined at the p < 0.05 level and verified by Duncan’s multiple range test. The results are displayed as the mean ± standard deviation (Means ± SD). The software Origin 8.5 was used to make the figures.