Novel Fluorescent Nanocellulose Hydrogel Based on Nanocellulose and Carbon Dots for Detection and Removal of Heavy Metal Ions in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocellulose
2.3. Synthesis of Carboxylated Nanocellulose (CNC)
2.4. Preparation of CDs
2.5. Preparation of Carboxylated Nanocellulose Modified with Carbon Dots (CNC-CDs)
2.6. Preparation of Fluorescent Nanocellulose Hydrogel (FNH)
2.7. Physicochemical Characterization
2.8. Adsorption Studies
3. Results and Discussion
3.1. Physicochemical Characterization
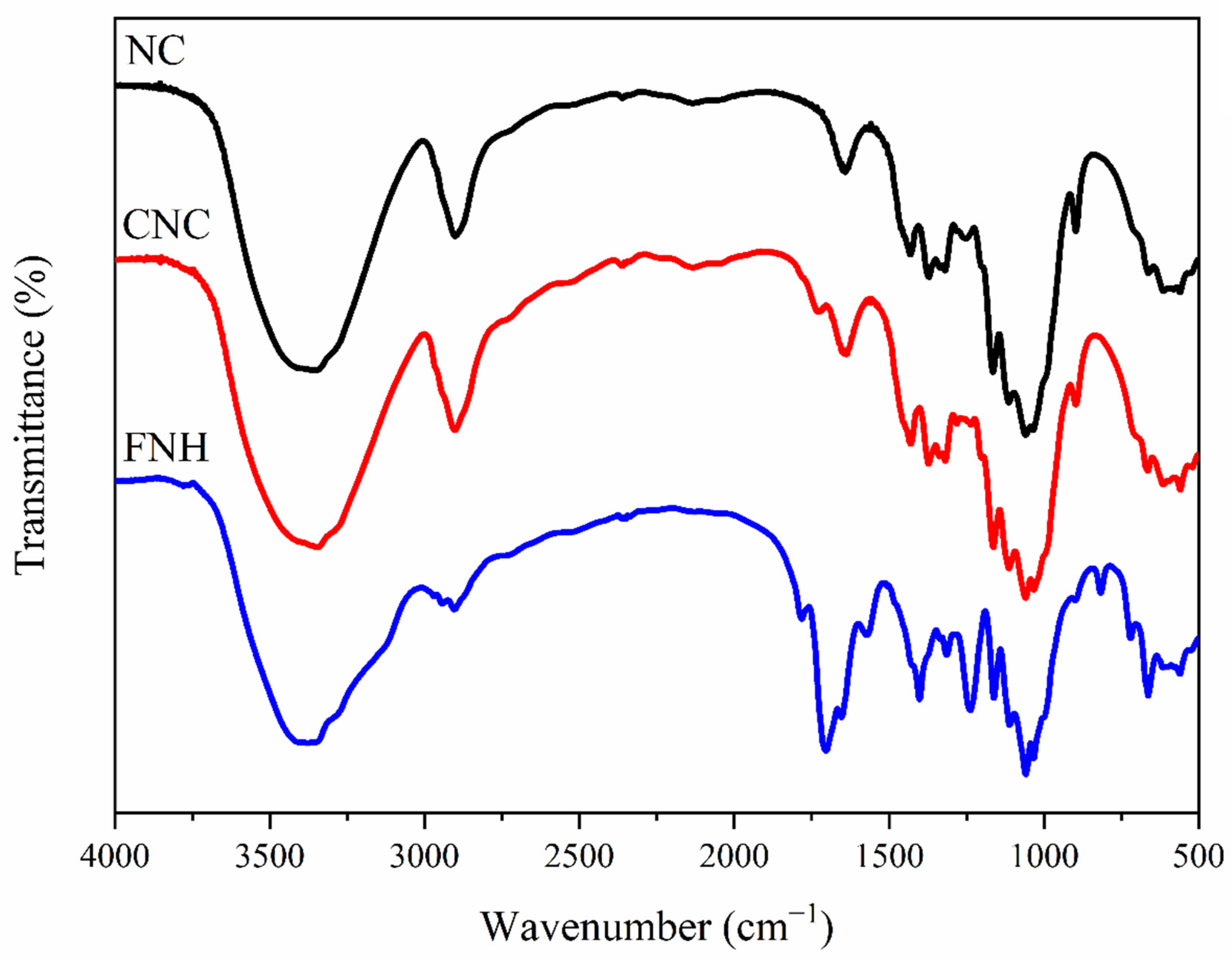
3.1.1. FTIR
3.1.2. SEM
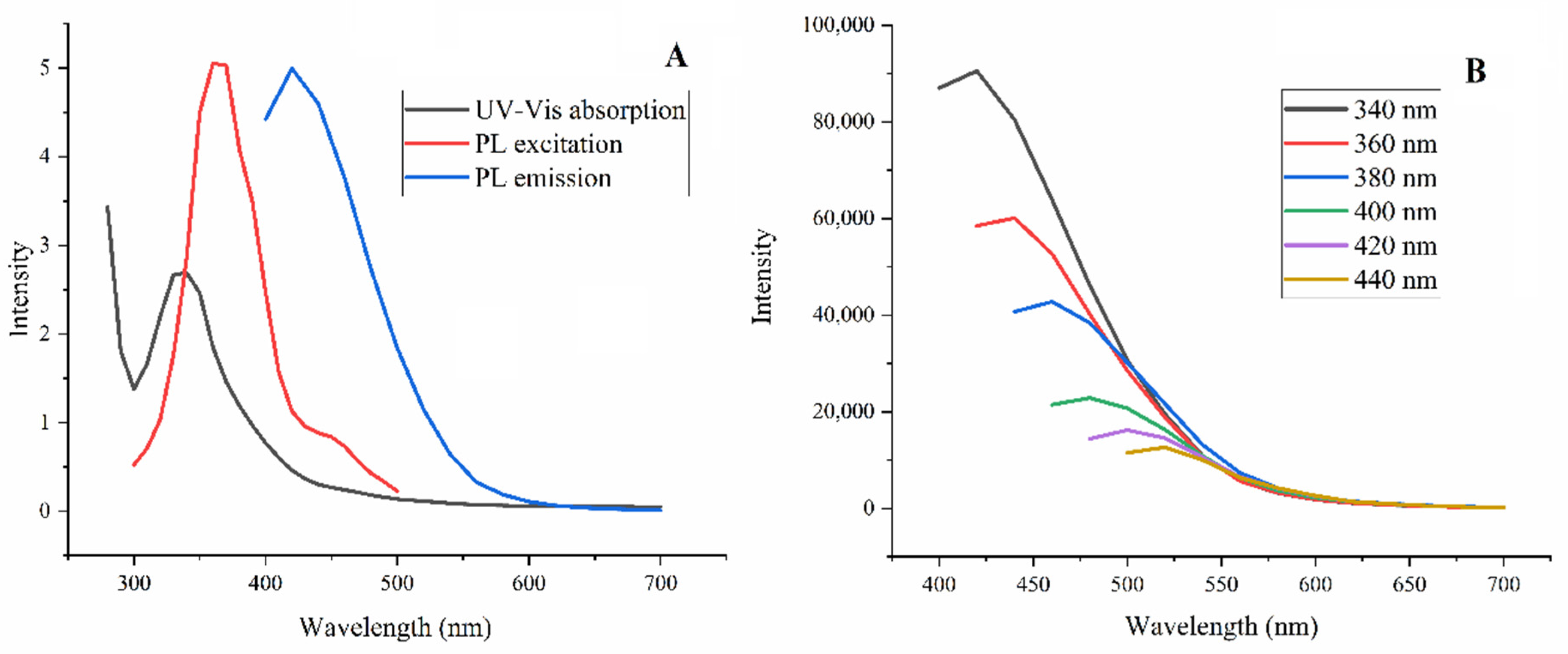
3.1.3. Spectral Features
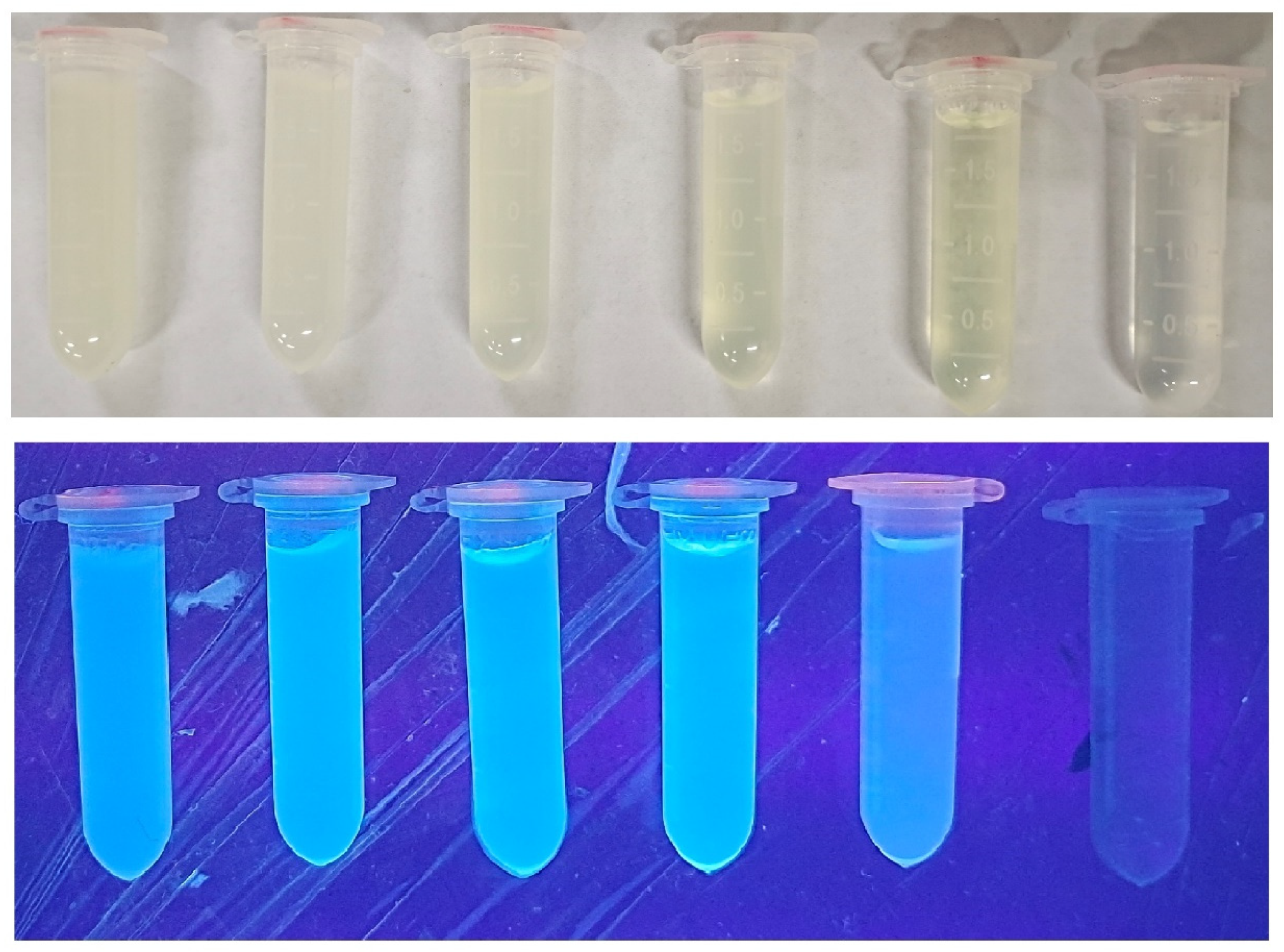
3.2. Appearance and Fluorescence Behavior of FNH
3.3. Effect of Experimental Parameters on Fe3+ and Pb2+ Adsorption Capacity
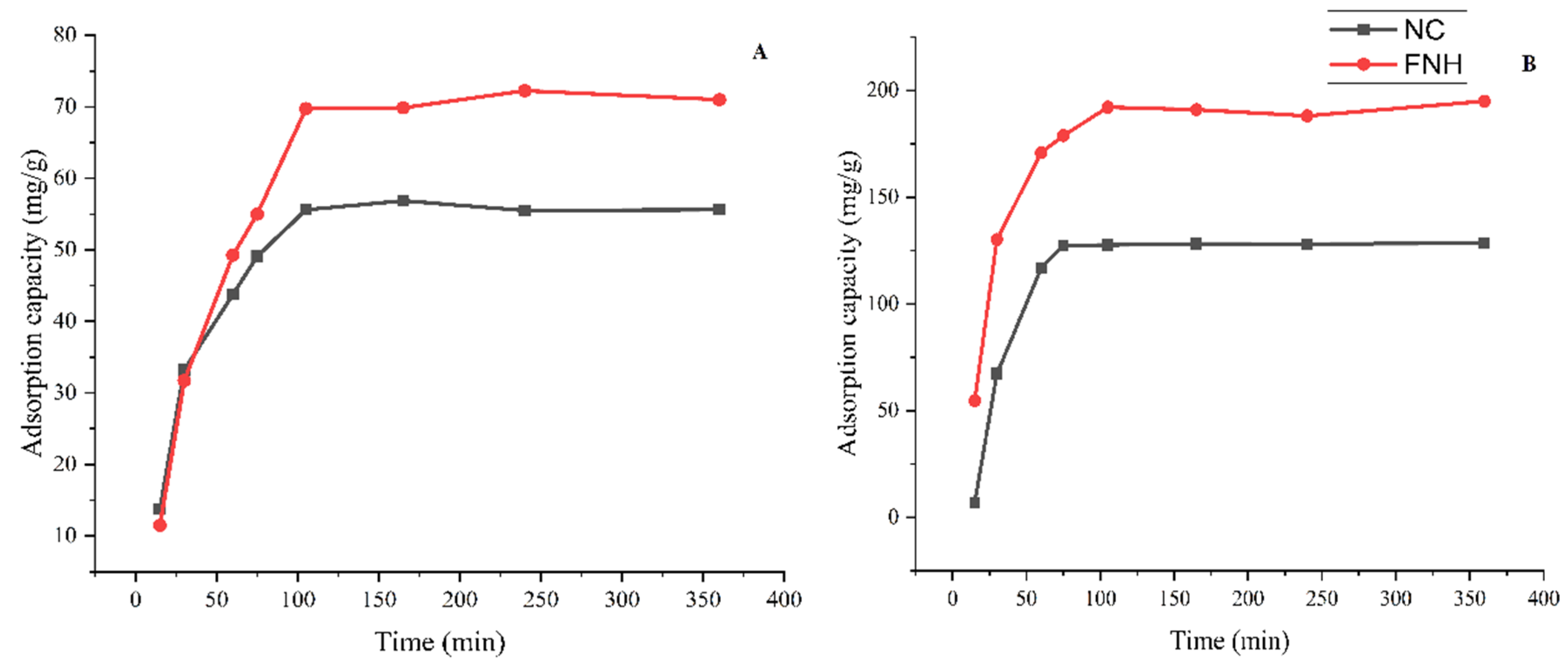
3.3.1. Effect of Contact Time
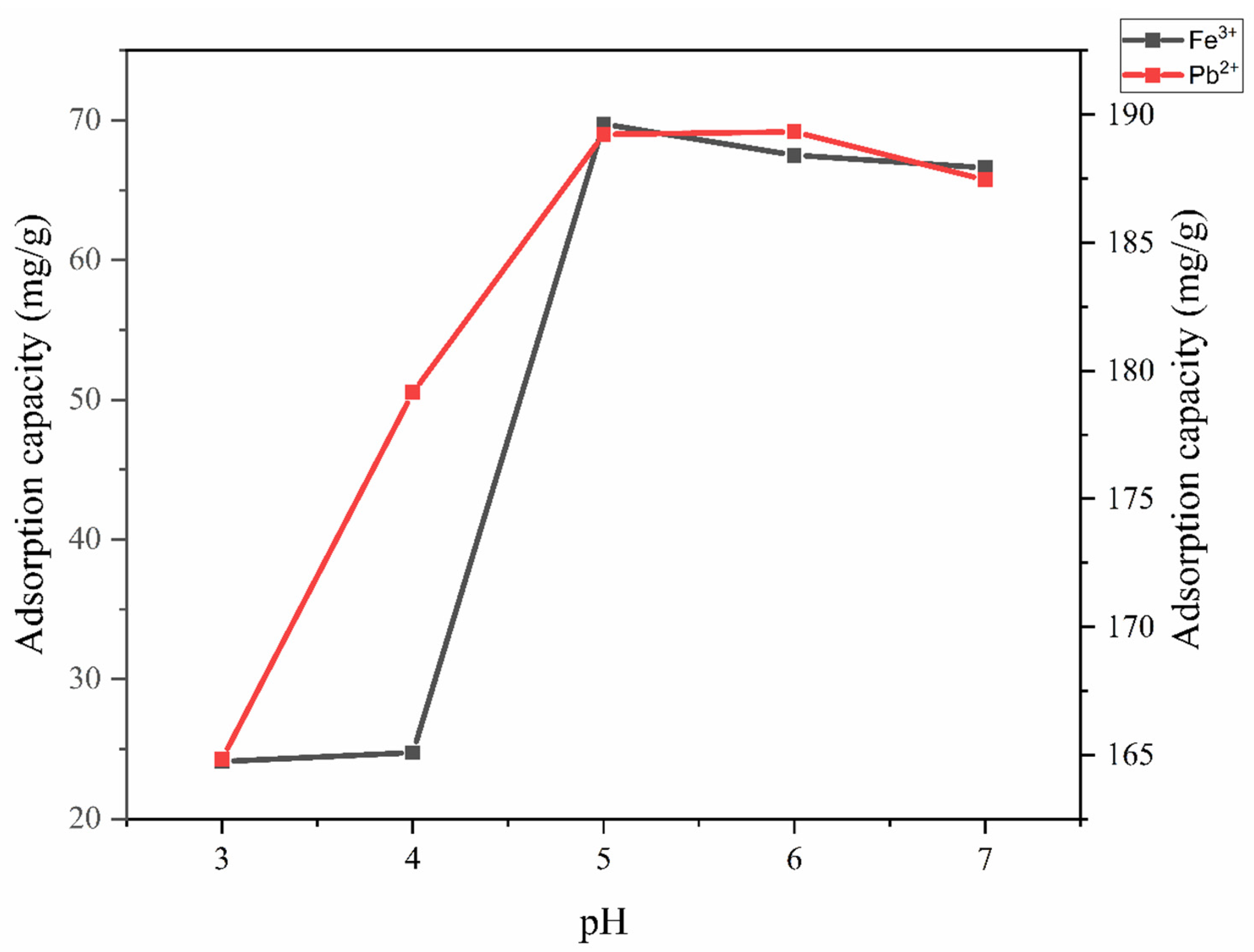
3.3.2. Effect of pH
3.3.3. Effect of Adsorbent Dosage
3.3.4. Adsorption Isotherm
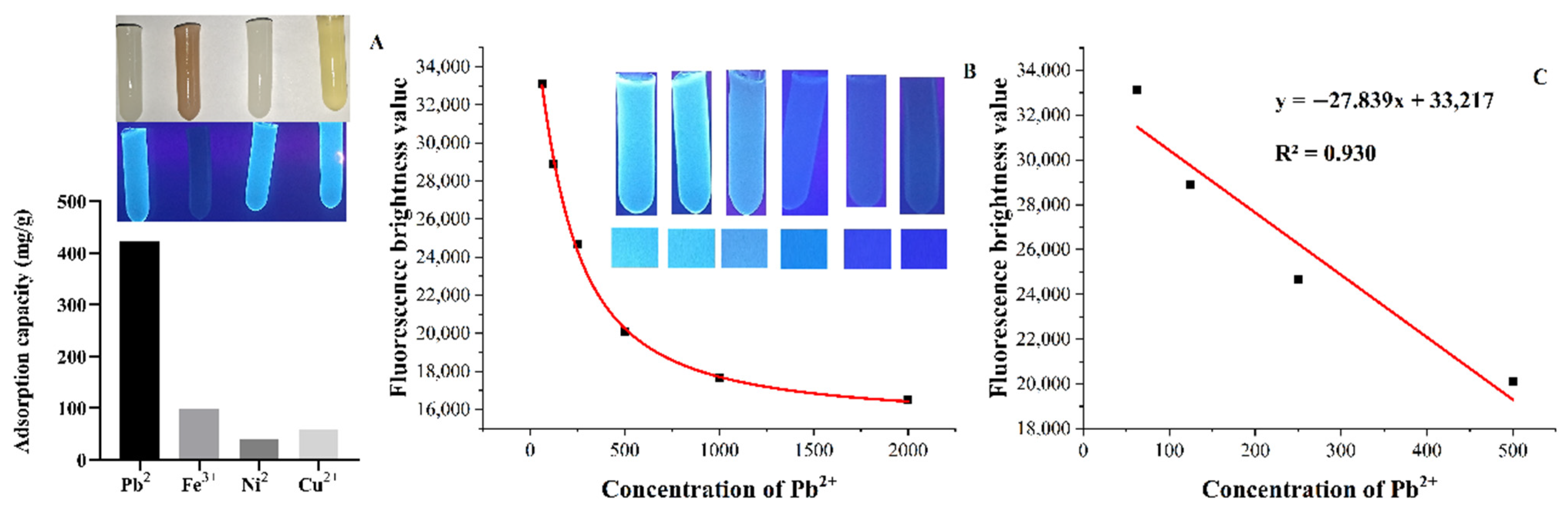
3.4. Fluorescence Sensing of Fe3+
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Q.; Xu, J.; Bu, X.H. Recent advances about metal-organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2018, 378, 17–31. [Google Scholar] [CrossRef]
- Lèbre, E.; Corder, G.; Golev, A. Sustainable practices in the management of mining waste: A focus on the mineral resource. Miner. Eng. 2017, 107, 34–42. [Google Scholar] [CrossRef]
- Liu, J.; Luo, X.; Sun, Y.; Tsang, D.; Qi, J.; Zhang, W.; Li, N.; Yin, M.; Wang, J.; Lippold, H.; et al. Thallium pollution in China and removal technologies for waters: A review. Environ. Int. 2019, 126, 771–790. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Baghbadorani, N.B.; Behzad, T.; Etesami, N.; Heidarian, P. Removal of Cu2+ ions by cellulose nanofibers-assisted starch-g-poly (acrylic acid) superadsorbent hydrogels. Compos. B Eng. 2019, 176, 107084. [Google Scholar] [CrossRef]
- Hong, H.J.; Yu, H.; Park, M.; Jeong, H.S. Recovery of platinum from waste effluent using polyethyleneimine-modified nanocelluloses: Effects of the cellulose source and type. Carbohydr. Polym. 2019, 210, 167–174. [Google Scholar] [CrossRef]
- Vilela, P.B.; Dalalibera, A.; Duminelli, E.C.; Becegato, V.A.; Paulino, A.T. Adsorption and removal of chromium (vi) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 28481–28489. [Google Scholar] [CrossRef]
- Encina, L.; Elgueta, E.; Rivas, B.L.; Pereira, M.; Sanhueza, F. Hydrogels derived from galactoglucomannan hemicellulose with inorganic contaminant removal properties. RSC Adv. 2021, 11, 35960–35972. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Renewable bionanohydrogels based on tragacanth gum for the adsorption of pb2+: Study of isotherm, kinetic models, and phenomenology. Environ. Technol. Innov. 2021, 23, 101723. [Google Scholar] [CrossRef]
- Jsm, A.; Rrf, A.; Agds, A.; Rfsb, A.; Ajdmj, B.; Dsr, A. Biodegradable gelatin composite hydrogels filled with cellulose for chromium (vi) adsorption from contaminated water. Int. J. Biol. Macromol. 2021, 181, 112–124. [Google Scholar]
- Kayra, N.; Aytekin, A.Z. Synthesis of Cellulose-Based Hydrogels: Preparation, Formation, Mixture, and Modification. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 407–434. [Google Scholar]
- Xiong, Y.; Xu, L.; Jin, C.; Sun, Q. Cellulose hydrogel functionalized titanate microspheres with self-cleaning for efficient purification of heavy metals in oily wastewater. Cellulose 2020, 27, 7751–7763. [Google Scholar] [CrossRef]
- Jing, Y.; Na, S.; Zhang, Y.K.; Zhong, S.X.; Wang, A.J.; Chen, J. Green preparation of carbon dots by jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sens. Actuators B Chem. 2015, 214, 29–35. [Google Scholar]
- Li, S.; Li, Y.; Fu, Z.; Lu, L.; Cheng, J.; Fei, Y. A ‘top modification’ strategy for enhancing the ability of a chitosan aerogel to efficiently capture heavy metal ions. J. Colloid Interface Sci. 2021, 594, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Huo, P.; Gu, J. Exploration of zwitterionic cellulose acetate antifouling ultrafiltration membrane for bovine serum albumin (bsa) separation. Carbohydr. Polym. 2017, 165, 266–275. [Google Scholar] [CrossRef]
- Sheikhi, A.; Kakkar, A.; Van, D. Biomimetic scale-resistant polymer nanocomposites: Towards universal additive-free scale inhibition. J. Mater. Chem. A 2018, 6, 10189–10195. [Google Scholar] [CrossRef]
- Van, D.; Sheikhi, A. Hairy cellulose nanocrystalloids: A novel class of nanocellulose. Nanoscale 2016, 8, 15101–15114. [Google Scholar]
- Veenhuyzen, B.; Tichapondwa, S.; Hörstmann, C.; Chirwa, E.; Brink, H.G. High capacity Pb(II) adsorption characteristics onto raw- and chemically activated waste activated sludge. J. Hazard. Mater. 2021, 416, 125943. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.L.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Dan, W. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr. Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Yuan, H.; Luo, Q.; Wu, Y. A novel fluorescent nanocellulosic hydrogel based on carbon dots for efficient adsorption and sensitive sensing in heavy metals. J. Mater. Chem. A 2019, 7, 27081–27088. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Y.; Wang, H.; Liu, S.; Song, L.; Li, S.; Tan, M. Carbon quantum dots from roasted Atlantic salmon (Salmo salar L.): Formation, biodistribution and cytotoxicity. Food Chem. 2019, 293, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Jang, H.J.; Park, S.W.; Jie, W.; Yun, K.J. Folic acid functionalized carbon dot/polypyrrole nanoparticles for specific bioimaging and photothermal therapy. ACS Appl. Bio. Mater. 2021, 4, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.L.; Soliman, A.; Ali, A.B.; Abou-Zeid, N.Y.; Nada, A.A. Hydrogel bioink based on clickable cellulose derivatives: Synthesis, characterization and in vitro assessment. Int. J. Biol. Macromol. 2020, 163, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Yan, Q.; Ning, Y.; Tian, C.; Huang, Y. A green route to prepare fluorescent and absorbent nano-hybrid hydrogel for water detection. Sci. Rep. 2017, 7, 4380. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Li, M.; Zhang, H.; Tang, X.; Chen, K. Fabrication of regenerated cellulose films by dmac dissolution using parenchyma cells via low-temperature pulping from yunnan-endemic bamboos. Ind. Crops Prod. 2020, 160, 113116. [Google Scholar] [CrossRef]
- Barsbay, M.; Kavaklı, P.A.; Tilki, S.; Kavaklı, C.; Güven, O. Porous cellulosic adsorbent for the removal of Cd (II), Pb(II) and Cu(II) ions from aqueous media. Radiat. Phys. Chem. 2018, 142, 70–76. [Google Scholar] [CrossRef]
- Mo, L.; Pang, H.; Lu, Y.; Li, Z.; Kang, H.; Wang, M.; Zhang, S.; Li, J. Wood-inspired nanocellulose aerogel adsorbents with excellent selective pollutants capture, superfast adsorption, and easy regeneration. J. Hazard. Mater. 2021, 415, 125612. [Google Scholar] [CrossRef]
- Kriechbaum, K.; Bergström, L. Antioxidant and UV-Blocking Leather-Inspired Nanocellulose-Based Films with High Wet Strength. Biomacromolecules 2020, 21, 1720–1728. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Zhang, Y.; Zhang, Z.; Li, Y.; Li, W. Nanocellulose aerogel for highly efficient adsorption of uranium (VI) from aqueous solution. Carbohydr. Polym. 2021, 267, 118233. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Du, S.; Huo, Y.; He, J.; Cui, S. Removal of copper ions from aqueous solution by adsorption onto novel polyelectrolyte film-coated nanofibrous silk fibroin non-wovens. Appl. Surf. Sci. 2015, 345, 169–174. [Google Scholar] [CrossRef]
- Li, J.; Zuo, K.; Wu, W.; Xu, Z.; Yi, Y.; Jing, Y.; Dai, H.; Fang, G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jin, R.N.; Liu, C.; Wang, Y.F.; Ouyang, X.K. Magnetic carboxylated cellulose nanocrystals as adsorbent for the removal of pb(ii) from aqueous solution. Int. J. Biol. Macromol. 2016, 93, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal—Based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]


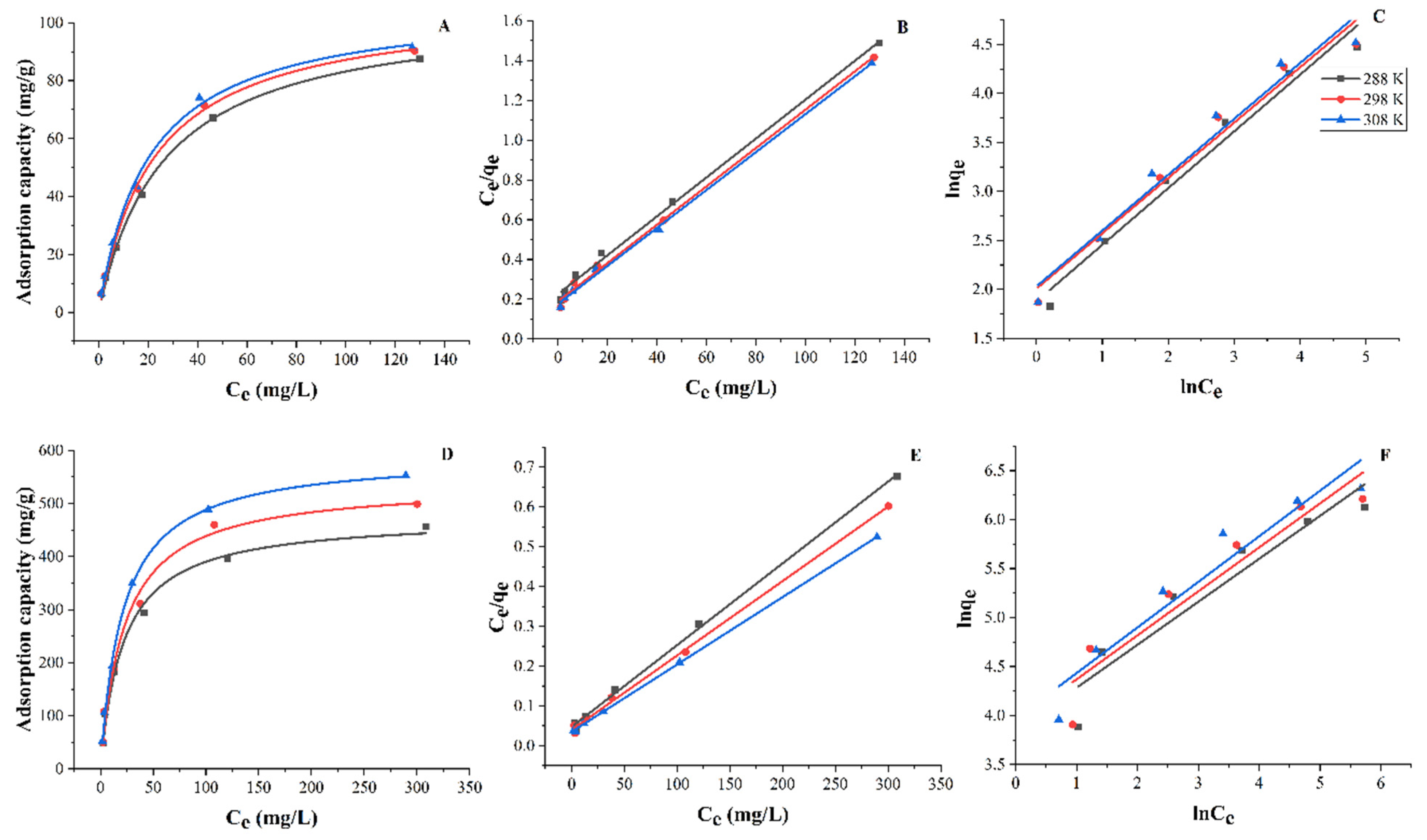
| Temperature | R2 | qcal,mas (mg/g) | qexp,max (mg/g) | KL (L/mg) | |
|---|---|---|---|---|---|
| 288 K a | Fe3+ | 0.9981 | 86.66 | 87.50 | 0.0377 |
| 298 K | 0.9975 | 90.24 | 90.25 | 0.0446 | |
| 308 K | 0.9967 | 91.76 | 91.50 | 0.0491 | |
| 288 K | Pb2+ | 0.9872 | 443.8 | 456.3 | 0.0460 |
| 298 K | 0.9830 | 492.0 | 498.8 | 0.0439 | |
| 308 K | 0.9975 | 549.3 | 552.5 | 0.0473 |
| Temperature | R2 | n | KL | |
|---|---|---|---|---|
| 288 K | Fe3+ | 0.9594 | 2.232 | 10.42 |
| 298 K | 0.9443 | 2.341 | 12.09 | |
| 308 K | 0.9324 | 2.393 | 12.94 | |
| 288 K | Pb2+ | 0.9287 | 3.068 | 75.57 |
| 298 K | 0.9071 | 3.046 | 84.06 | |
| 308 K | 0.9051 | 3.031 | 92.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Luo, Z.; Wang, M. Novel Fluorescent Nanocellulose Hydrogel Based on Nanocellulose and Carbon Dots for Detection and Removal of Heavy Metal Ions in Water. Foods 2022, 11, 1619. https://doi.org/10.3390/foods11111619
Yang J, Luo Z, Wang M. Novel Fluorescent Nanocellulose Hydrogel Based on Nanocellulose and Carbon Dots for Detection and Removal of Heavy Metal Ions in Water. Foods. 2022; 11(11):1619. https://doi.org/10.3390/foods11111619
Chicago/Turabian StyleYang, Jiachuan, Zhixin Luo, and Min Wang. 2022. "Novel Fluorescent Nanocellulose Hydrogel Based on Nanocellulose and Carbon Dots for Detection and Removal of Heavy Metal Ions in Water" Foods 11, no. 11: 1619. https://doi.org/10.3390/foods11111619





