Risk Assessment and Evaluation of Analytical Method of Polycyclic Aromatic Hydrocarbons (PAHs) for Deep-Fat Fried Pork Products in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sample Preparation for Evaluation PAH4
2.3. Extraction and Clean-Up for Pretreatment
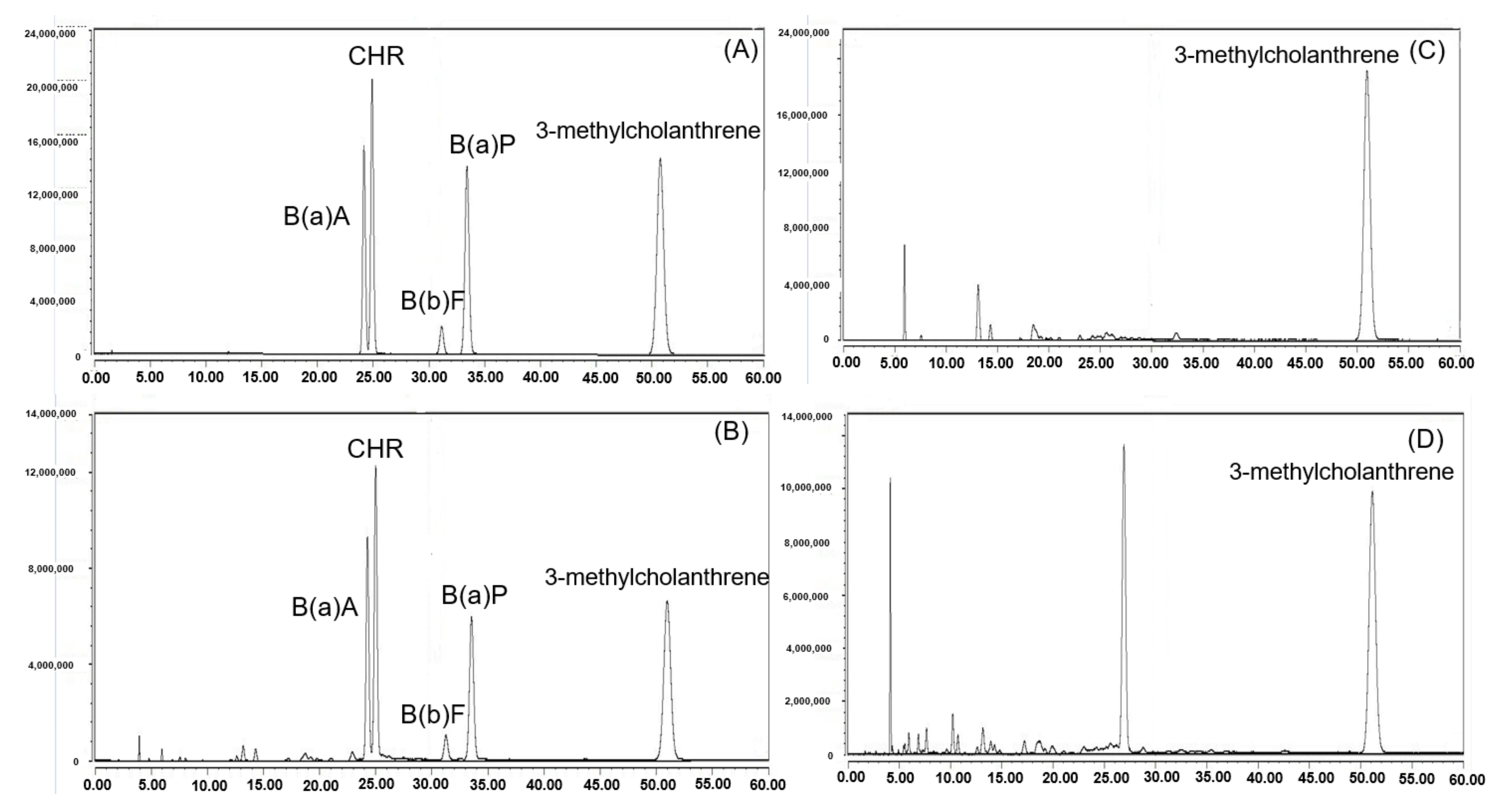
2.4. HPLC-FLD Analysis of PAH4
2.5. Identification and Quantification of PAH4
2.6. Validation of Analytical Method
2.7. Application of TEQ Concentration
2.8. Exposure Assessment
2.9. Risk Characterization
2.10. Statistical Analysis
3. Results and Discussion
3.1. Validation and Analytical Quality Assurance for PAHs Analysis
3.2. Comparison of PAHs Content in Deep-Fat Fried Pork Products according to the Frying Conditions
3.3. Exposure Assessment
3.4. Risk Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramesh, A.; Archibong, A.E.; Hood, D.B.; Guo, Z.; Loganathan, B.G. Global Environmental Distribution and Human Health Effects of Polycyclic Aromatic Hydrocarbons. Glob. Contam. Trends Persistent Org. Chem. 2011, 63, 97–128. [Google Scholar]
- Alexander, C.; Smith, R.; Loganathan, B.; Ertel, J.; Windom, H.L.; Lee, R.F. Pollution History of the Savannah River Estuary and Comparisons with Baltic Sea Pollution History. Limnologica 1999, 29, 267–273. [Google Scholar] [CrossRef] [Green Version]
- Guillen, M.D. Polycyclic aromatic compounds: Extraction and determination in food. Food Addit. Contam. 1994, 11, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Lapviboonsuk; Jutathip; Bommanna Loganathan. Polynuclear Aromatic Hydrocarbons in Sediments and Mussel Tissue from the Lower Tennessee River and Kentucky Lake. J. Ky. Acad. Sci. 2007, 68, 186–197. [Google Scholar] [CrossRef]
- Milton, L. Analytical Chemistry of Polycyclic Aromatic Compounds; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0-12-440840-0. [Google Scholar]
- Purcaro, G.; Navas, J.A.; Guardiola, F.; Conte, L.S.; Moret, S. Polycyclic aromatic hydrocarbons in frying oils and snacks. J. Food Prot. 2006, 69, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals; AOAC International: Gaithersburg, MD, USA, 2002; pp. 12–19. [Google Scholar]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.-P.; Dogliotti, E.; Di Domenico, A.; Fernández-Cruz, M.; Fink-Gremmels, J.; Fürst, P.; Galli, C.; et al. Polycyclic Aromatic Hydrocarbons in Food 1 Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 724, 114. [Google Scholar] [CrossRef]
- Manjinder, S.S.; Ian, R.W.; Klim, M. Systematic Review of the Prospective Cohort Studies on Meat Consumption and Colorectal Cancer Risk: A Meta-Analytical Approach. Cancer Epidemiol. Biomark. Prev. 2001, 10, 439–446. [Google Scholar]
- Sinha, R.; Peters, U.; Cross, A.J.; Kulldorff, M.; Weissfeld, J.L.; Pinsky, P.F.; Rothman, N.; Hayes, R.B. Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Res. 2005, 65, 8034–8041. [Google Scholar] [CrossRef] [Green Version]
- Baan, R.; Steenwinkel, M.-J.; van den Berg, P.; Roggeband, R.; van Delft, J. Molecular dosimetry of DNA damage induced by polycyclic aromatic hydrocarbons; relevance for exposure monitoring and risk assessment. Hum. Exp. Toxicol. 1994, 13, 880–887. [Google Scholar] [CrossRef]
- King, A.; Readman, J.; Zhou, J. Determination of polycyclic aromatic hydrocarbons in water by solid-phase microextraction–gas chromatography–mass spectrometry. Anal. Chim. Acta 2004, 523, 259–267. [Google Scholar] [CrossRef]
- Wise, J.P.; Wise, S.S. Mutation research, genetic toxicology and environmental mutagenesis. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2005, 1, 193–196. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Guillen, M.; Sopelana, P.; Partearroyo, M.A. Food as a source of polycyclic aromatic carcinogens. Rev. Environ. Health 1997, 12, 133–146. [Google Scholar] [CrossRef]
- Wang, L.-F. Mutagenicity and aromatic amine content of fumes from heated cooking oils produced in Taiwan. Food Chem. Toxicol. 1999, 37, 125–134. [Google Scholar] [CrossRef]
- Kafouris, D.; Koukkidou, A.; Christou, E.; Hadjigeorgiou, M.; Yiannopoulos, S. Determination of polycyclic aromatic hydrocarbons in traditionally smoked meat products and charcoal grilled meat in Cyprus. Meat Sci. 2020, 164, 108088. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, N. Effect of household cooking methods and some food additives on polycyclic aromatic hydrocarbons (PAHs) formation in chicken meat. World Applied Sci. J. 2010, 9, 963–974. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, Y.; Kong, Q.; Zheng, F.; Shao, L.; Zhang, T.; Jiang, D.; Gao, X. Occurrence of polycyclic aromatic. Pollut. Res. 2021, 28, 32802–32809. [Google Scholar] [CrossRef]
- Aaslyng, M.D.; Duedahl-Olesen, L.; Jensen, K.; Meinert, L. Content of heterocyclic amines and polycyclic aromatic hydrocarbons in pork, beef and chicken barbecued at home by Danish consumers. Meat Sci. 2013, 93, 85–91. [Google Scholar] [CrossRef]
- U.S.E.P.A.E. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons; Environmental Protection Agency, Office of Research and Development, Office of Health and Environmental Assessment: Washington, DC, USA, 1993; Volume 600. [Google Scholar]
- Tarafdar, A.; Chawda, S.; Sinha, A. Health risk assessment from polycyclic aromatic hydrocarbons (PAHs) present in dietary components: A meta-analysis on a global scale. Polycycl. Aromat. Compd. 2018, 40, 850–861. [Google Scholar] [CrossRef]
- Chen, C.; Chu, M.M. Dose-Response analysis of INGESTED Benzo (a) Pyrene (CAS No. 50-32-8); Environmental Protection Agency: Washington, DC, USA, 1991. [Google Scholar]
- Duedahl-Olesen, L.; Aaslyng, M.; Meinert, L.; Christensen, T.; Jensen, A.; Binderup, M.-L. Polycyclic aromatic hydrocarbons (PAH) in Danish barbecued meat. Food Control. 2015, 57, 169–176. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Patra, J.-K.; Shin, H.-S. Evaluation of analytical method and risk assessment of polycyclic aromatic hydrocarbons for fishery products in Korea. Food Control. 2022, 131, 108421. [Google Scholar] [CrossRef]
| Instrument | Dionex U3000 HPLC | ||
|---|---|---|---|
| Column | ZORBAX Eclipse C18 Plus (4.6 mm × 250 mm, 5 μm) | ||
| Wavelength | Excitation (nm) | Emission (nm) | |
| 0–20 min | 245 | 390 | |
| 20–60 min | 294 | 404 | |
| Mobile phase | Acetonitrile | Water | |
| 0–20 min | 65 | 35 | |
| 20–60 min | 70 | 30 | |
| Flow rate | 1.0 mL/min | ||
| Temperature | 37 °C | ||
| Injection volume | 10.0 μL | ||
| PAHs | LOD (μg/kg) 1 | LOQ (μg/kg) 2 | Linearity (R2) 3 | Recovery (%) 4 |
|---|---|---|---|---|
| Benz[a]anthrancene | 0.14 | 0.42 | y = 0.0251x + 0.0043 R2 = 0.9989 | 99.9 ± 1.1 |
| Chrysene | 0.10 | 0.32 | y = 0.0176x + 0.0217 R2 = 0.9976 | 95.5 ± 1.4 |
| Benzo[b]fluoranthene | 0.16 | 0.50 | y = 0.0192x + 0.0060 R2 = 0.9975 | 88.0 ± 0.2 |
| Benzo[a]pyrene | 0.18 | 0.55 | Y = 0.1340x − 0.0318 R2 = 0.9932 | 91.8 ± 1.4 |
| Oil | Cooking Temperature (°C) | Time | PAHs (μg/kg) 1 | TEQBaP (μg/kg/day) | |||||
|---|---|---|---|---|---|---|---|---|---|
| (min) | BaA | CHR | BbF | BaP | PAH4 | BaP | PAH4 | ||
| 0 | <LOQ 2 | <LOQ | <LOQ | 0.38 ± 0.26 | 0.38 ± 0.26 | 0.38 | 0.38 | ||
| Soybean | 160 | 3 | <LOQ | <LOQ | <LOQ | 0.86 ± 0.14 | 0.86 ± 0.14 | 0.86 | 0.86 |
| 6 | <LOQ | <LOQ | <LOQ | 1.59 ± 0.52 | 1.59 ± 0.52 | 1.59 | 1.59 | ||
| 9 | 0.55 ± 0.07 | <LOQ | <LOQ | 2.66 ± 0.31 | 3.21 ± 0.37 | 2.66 | 2.71 | ||
| 180 | 3 | <LOQ | <LOQ | <LOQ | 1.50 ± 0.15 | 1.50 ± 0.15 | 1.50 | 1.50 | |
| 6 | 0.14 ± 0.24 | <LOQ | <LOQ | 2.42 ± 0.14 | 2.56 ± 0.33 | 2.42 | 2.44 | ||
| 9 | 0.71 ± 0.07 | <LOQ | <LOQ | 3.50 ± 0.56 | 4.21 ± 0.50 | 3.50 | 3.57 | ||
| 200 | 3 | <LOQ | <LOQ | <LOQ | 2.66 ± 0.32 | 2.66 ± 0.32 | 2.66 | 2.66 | |
| 6 | 0.17 ± 0.29 | <LOQ | <LOQ | 3.59 ± 0.12 | 3.76 ± 0.33 | 3.59 | 3.61 | ||
| 9 | 0.86 ± 0.09 | <LOQ | <LOQ | 5.95 ± 2.01 | 6.81 ± 2.00 | 5.95 | 6.03 | ||
| Canola | 160 | 3 | <LOQ | <LOQ | <LOQ | 1.03 ± 0.16 | 1.03 ± 0.16 | 1.03 | 1.03 |
| 6 | <LOQ | <LOQ | <LOQ | 1.63 ± 0.12 | 1.63 ± 0.12 | 1.63 | 1.63 | ||
| 9 | <LOQ | 0.14 ± 0.25 | <LOQ | 3.72 ± 0.58 | 3.86 ± 0.67 | 3.72 | 3.72 | ||
| 180 | 3 | <LOQ | <LOQ | <LOQ | 1.43 ± 0.09 | 1.43 ± 0.09 | 1.43 | 1.43 | |
| 6 | <LOQ | <LOQ | <LOQ | 1.99 ± 0.36 | 1.99 ± 0.36 | 1.99 | 1.99 | ||
| 9 | 0.19 ± 0.32 | 0.18 ± 0.31 | <LOQ | 2.54 ± 0.42 | 2.90 ± 0.14 | 2.54 | 2.56 | ||
| 200 | 3 | <LOQ | <LOQ | <LOQ | 1.71 ± 0.16 | 1.71 ± 0.16 | 1.71 | 1.71 | |
| 6 | <LOQ | 0.15 ± 0.27 | <LOQ | 2.95 ± 0.32 | 3.10 ± 0.59 | 2.95 | 2.95 | ||
| 9 | 0.23 ± 0.39 | 0.22 ± 0.38 | <LOQ | 5.06 ± 1.22 | 5.50 ± 0.88 | 5.06 | 5.08 | ||
| Grape seed | 160 | 3 | <LOQ | <LOQ | <LOQ | 1.06 ± 0.09 | 1.06 ± 0.09 | 1.06 | 1.06 |
| 6 | <LOQ | 0.20 ± 0.34 | <LOQ | 1.49 ± 0.14 | 1.69 ± 0.48 | 1.49 | 1.49 | ||
| 9 | <LOQ | 0.22 ± 0.39 | <LOQ | 2.52 ± 0.03 | 2.74 ± 0.40 | 2.52 | 2.52 | ||
| 180 | 3 | <LOQ | <LOQ | <LOQ | 1.40 ± 0.17 | 1.40 ± 0.17 | 1.40 | 1.40 | |
| 6 | <LOQ | 0.2 ± 0.35 | <LOQ | 2.01 ± 0.07 | 2.21 ± 0.36 | 2.01 | 2.01 | ||
| 9 | <LOQ | 0.24 ± 0.42 | <LOQ | 3.80 ± 1.61 | 4.04 ± 2.03 | 3.80 | 3.80 | ||
| 200 | 3 | <LOQ | 0.16 ± 0.28 | <LOQ | 1.91 ± 0.48 | 2.07 ± 0.55 | 1.91 | 1.91 | |
| 6 | <LOQ | 0.23 ± 0.4 | <LOQ | 2.95 ± 0.32 | 3.18 ± 0.67 | 2.95 | 2.95 | ||
| 9 | 0.27 ± 0.47 | 0.80 ± 0.78 | <LOQ | 6.83 ± 1.33 | 7.90 ± 2.46 | 6.83 | 6.86 | ||
| Sunflower | 160 | 3 | <LOQ | <LOQ | <LOQ | 1.09 ± 0.07 | 1.09 ± 0.07 | 1.09 | 1.09 |
| 6 | <LOQ | <LOQ | <LOQ | 1.33 ± 0.20 | 1.33 ± 0.20 | 1.33 | 1.33 | ||
| 9 | <LOQ | 0.19 ± 0.32 | <LOQ | 2.44 ± 1.21 | 2.63 ± 1.11 | 2.44 | 2.45 | ||
| 180 | 3 | <LOQ | 0.16 ± 0.28 | <LOQ | 1.31 ± 0.07 | 1.47 ± 0.34 | 1.31 | 1.31 | |
| 6 | <LOQ | 0.33 ± 0.28 | <LOQ | 2.63 ± 0.05 | 2.95 ± 0.25 | 2.63 | 2.63 | ||
| 9 | <LOQ | 0.43 ± 0.38 | <LOQ | 4.41 ± 1.56 | 4.84 ± 1.79 | 4.41 | 4.42 | ||
| 200 | 3 | <LOQ | 0.17 ± 0.29 | <LOQ | 2.21 ± 0.59 | 2.37 ± 0.66 | 2.21 | 2.21 | |
| 6 | <LOQ | 0.45 ± 0.41 | <LOQ | 3.42 ± 0.45 | 3.87 ± 0.68 | 3.42 | 3.42 | ||
| 9 | <LOQ | 0.37 ± 0.64 | <LOQ | 6.81 ± 2.48 | 7.17 ± 3.02 | 6.81 | 6.81 | ||
| Age | Soybean Oil | Canola Oil | Grape Seed Oil | Sunflower Oil | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BaP (μg/kg) | PAH4 (μg/kg) | BaP (μg/kg) | PAH4 (μg/kg) | BaP (μg/kg) | PAH4 (μg/kg) | BaP (μg/kg) | PAH4 (μg/kg) | |||||||||
| Mean | 95th | Mean | 95th | Mean | 95th | Mean | 95th | Mean | 95th | Mean | 95th | Mean | 95th | Mean | 95th | |
| >65 | 0.32 | 2.03 | 0.33 | 2.06 | 0.28 | 1.72 | 0.28 | 1.73 | 0.37 | 2.33 | 0.37 | 2.34 | 0.37 | 2.32 | 0.37 | 2.32 |
| 50–64 | 0.38 | 1.90 | 0.39 | 1.93 | 0.33 | 1.62 | 0.33 | 1.62 | 0.44 | 2.18 | 0.44 | 2.19 | 0.44 | 2.18 | 0.44 | 2.18 |
| 30–49 | 0.77 | 3.66 | 0.78 | 3.72 | 0.66 | 3.11 | 0.66 | 3.13 | 0.89 | 4.20 | 0.89 | 4.23 | 0.89 | 4.19 | 0.89 | 4.19 |
| 19–29 | 0.53 | 2.64 | 0.54 | 2.67 | 0.45 | 2.24 | 0.45 | 2.25 | 0.61 | 3.03 | 0.61 | 3.04 | 0.60 | 3.02 | 0.61 | 3.02 |
| 12–18 | 0.36 | 1.61 | 0.37 | 1.63 | 0.31 | 1.37 | 0.31 | 1.37 | 0.42 | 1.85 | 0.42 | 1.86 | 0.42 | 1.84 | 0.42 | 1.84 |
| 6–11 | 0.32 | 1.36 | 0.33 | 1.38 | 0.28 | 1.16 | 0.28 | 1.17 | 0.37 | 1.57 | 0.38 | 1.57 | 0.37 | 1.56 | 0.37 | 1.56 |
| 3–5 | 0.18 | 0.77 | 0.18 | 0.78 | 0.15 | 0.65 | 0.16 | 0.66 | 0.21 | 0.88 | 0.21 | 0.88 | 0.21 | 0.88 | 0.21 | 0.88 |
| 1–2 | 0.09 | 0.52 | 0.09 | 0.53 | 0.08 | 0.44 | 0.08 | 0.44 | 0.11 | 0.60 | 0.11 | 0.60 | 0.11 | 0.60 | 0.11 | 0.60 |
| Age | 3 min | 6 min | 9 min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Dietary Exposure (μg-TEQBaP/kg/day) | 95th Percentile Dietary Exposure (μg-TEQBaP/kg/day) | Average Dietary Exposure (μg-TEQBaP/kg/day) | 95th Percentile Dietary Exposure (μg-TEQBaP/kg/day) | Average Dietary Exposure (μg-TEQBaP/kg/day) | 95th Percentile Dietary Exposure (μg-TEQBaP/kg/day) | |||||||
| BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | |
| >65 | 9.36 ×105 | 4.55 ×106 | 1.50 ×105 | 7.29 ×105 | 6.10 ×105 | 2.96 ×106 | 9.77 ×104 | 4.74 ×105 | 3.50 ×105 | 1.69 ×106 | 5.61 ×104 | 2.71 ×105 |
| 50–64 | 7.90 ×105 | 3.77 ×106 | 1.60 ×105 | 7.63 ×105 | 5.15 ×105 | 2.38 ×106 | 1.04 ×105 | 4.81 ×105 | 2.96 ×105 | 1.29 ×106 | 5.99 ×104 | 2.61 ×105 |
| 30–49 | 3.93 ×105 | 1.91 ×106 | 8.30 ×104 | 3.96 ×105 | 2.56 ×105 | 1.24 ×106 | 5.41 ×104 | 2.50 ×105 | 1.47 ×105 | 7.10 ×105 | 3.11 ×104 | 1.36 ×105 |
| 19–29 | 5.76 ×105 | 2.75 ×106 | 1.15 ×106 | 5.50 ×105 | 3.75 ×105 | 1.73 ×106 | 7.51 ×104 | 3.47 ×105 | 2.15 ×105 | 9.40 ×105 | 4.32 ×104 | 1.88 ×105 |
| 12–18 | 8.38 ×105 | 4.07 ×106 | 1.89 ×105 | 9.19 ×105 | 5.46 ×105 | 2.65 ×106 | 1.23 ×105 | 5.98 ×105 | 3.14 ×105 | 1.51 ×106 | 7.08 ×104 | 3.42 ×105 |
| 6–11 | 9.36 ×105 | 4.54 ×106 | 2.22 ×105 | 1.08 ×105 | 6.10 ×105 | 2.96 ×106 | 1.45 ×105 | 7.04 ×105 | 3.50 ×105 | 1.69 ×106 | 8.34 ×104 | 4.02 ×105 |
| 3–5 | 1.67 ×106 | 8.11 ×106 | 3.97 ×105 | 1.93 ×106 | 1.09 ×107 | 5.28 ×106 | 2.58 ×105 | 1.25 ×106 | 6.25 ×105 | 3.01 ×106 | 1.48 ×105 | 7.16 ×105 |
| 1–2 | 3.28 ×106 | 1.59 ×107 | 5.84 ×105 | 2.84 ×106 | 2.14 ×107 | 1.04 ×107 | 3.80 ×105 | 1.84 ×106 | 1.23 ×106 | 5.92 ×106 | 2.19 ×105 | 1.05 ×106 |
| Age | 160 °C | 180 °C | 200 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Dietary Exposure (μg-TEQBaP/kg/day) | 95th Percentile Dietary Exposure (μg-TEQBaP/kg/day) | Average Dietary Exposure (μg-TEQBaP/kg/day) | 95th Percentile Dietary Exposure (μg-TEQBaP/kg/day) | Average Dietary Exposure (μg-TEQBaP/kg/day) | 95th Percentile Dietary Exposure (μg-TEQBaP/kg/day) | |||||||
| BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | BaP | PAH4 | |
| >65 | 8.66 ×105 | 4.20 ×106 | 1.39 ×105 | 6.73 ×105 | 6.20 ×105 | 3.00 ×106 | 3.00 ×104 | 9.94 ×105 | 4.10 ×105 | 1.99 ×106 | 6.58 ×104 | 3.19 ×105 |
| 50–64 | 7.31 ×105 | 3.46 ×106 | 1.48 ×105 | 7.00 ×105 | 5.23 ×105 | 2.40 ×106 | 1.06 ×105 | 4.86 ×105 | 3.46 ×105 | 1.58 ×106 | 7.01 ×104 | 3.21 ×105 |
| 30–49 | 3.64 ×105 | 1.76 ×106 | 7.68 ×104 | 3.63 ×105 | 2.60 ×105 | 1.26 ×106 | 5.50 ×104 | 2.52 ×105 | 1.72 ×105 | 8.36 ×105 | 3.64 ×104 | 1.66 ×105 |
| 19–29 | 5.32 ×105 | 2.52 ×106 | 1.07 ×105 | 5.05 ×105 | 3.81 ×105 | 1.75 ×106 | 7.64 ×104 | 3.50 ×105 | 2.52 ×105 | 1.15 ×106 | 5.06 ×104 | 2.31 ×105 |
| 12–18 | 7.75 ×105 | 3.76 ×106 | 1.75 ×105 | 8.49 ×105 | 5.55 ×105 | 2.69 ×106 | 1.25 ×105 | 6.07 ×105 | 3.67 ×105 | 1.78 ×106 | 8.29 ×104 | 4.02 ×105 |
| 6–11 | 8.66 ×105 | 4.20 ×106 | 2.06 ×105 | 1.00 ×106 | 6.20 ×105 | 3.00 ×106 | 1.48 ×105 | 7.15 ×105 | 4.10 ×105 | 1.99 ×106 | 9.78 ×104 | 4.74 ×105 |
| 3–5 | 1.54 ×106 | 7.49 ×106 | 3.67 ×105 | 1.78 ×106 | 1.11 ×106 | 5.36 ×106 | 2.63 ×105 | 1.27 ×106 | 7.32 ×105 | 3.55 ×106 | 1.74 ×105 | 8.43 ×105 |
| 1–2 | 3.03 ×106 | 1.47 ×107 | 5.40 ×105 | 2.62 ×106 | 2.17 ×106 | 1.04 ×107 | 3.87 ×105 | 1.88 ×106 | 1.44 ×106 | 6.97 ×106 | 2.56 ×105 | 1.24 ×106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Shin, H.W.; Kim, G.H.; Kim, Y.-Y.; Kang, M.-J.; Shin, H.-S. Risk Assessment and Evaluation of Analytical Method of Polycyclic Aromatic Hydrocarbons (PAHs) for Deep-Fat Fried Pork Products in Korea. Foods 2022, 11, 1618. https://doi.org/10.3390/foods11111618
Kim SY, Shin HW, Kim GH, Kim Y-Y, Kang M-J, Shin H-S. Risk Assessment and Evaluation of Analytical Method of Polycyclic Aromatic Hydrocarbons (PAHs) for Deep-Fat Fried Pork Products in Korea. Foods. 2022; 11(11):1618. https://doi.org/10.3390/foods11111618
Chicago/Turabian StyleKim, Seo Yeon, Hye Won Shin, Geon Hee Kim, Yong-Yeon Kim, Min-Jae Kang, and Han-Seung Shin. 2022. "Risk Assessment and Evaluation of Analytical Method of Polycyclic Aromatic Hydrocarbons (PAHs) for Deep-Fat Fried Pork Products in Korea" Foods 11, no. 11: 1618. https://doi.org/10.3390/foods11111618
APA StyleKim, S. Y., Shin, H. W., Kim, G. H., Kim, Y.-Y., Kang, M.-J., & Shin, H.-S. (2022). Risk Assessment and Evaluation of Analytical Method of Polycyclic Aromatic Hydrocarbons (PAHs) for Deep-Fat Fried Pork Products in Korea. Foods, 11(11), 1618. https://doi.org/10.3390/foods11111618









