Synergistic Action of Mild Heat and Essential Oil Treatments on Culturability and Viability of Escherichia coli ATCC 25922 Tested In Vitro and in Fruit Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Inoculum Preparation
2.2. Bacterial Culturability and Viability Assays
2.2.1. Standard Plate Count Method
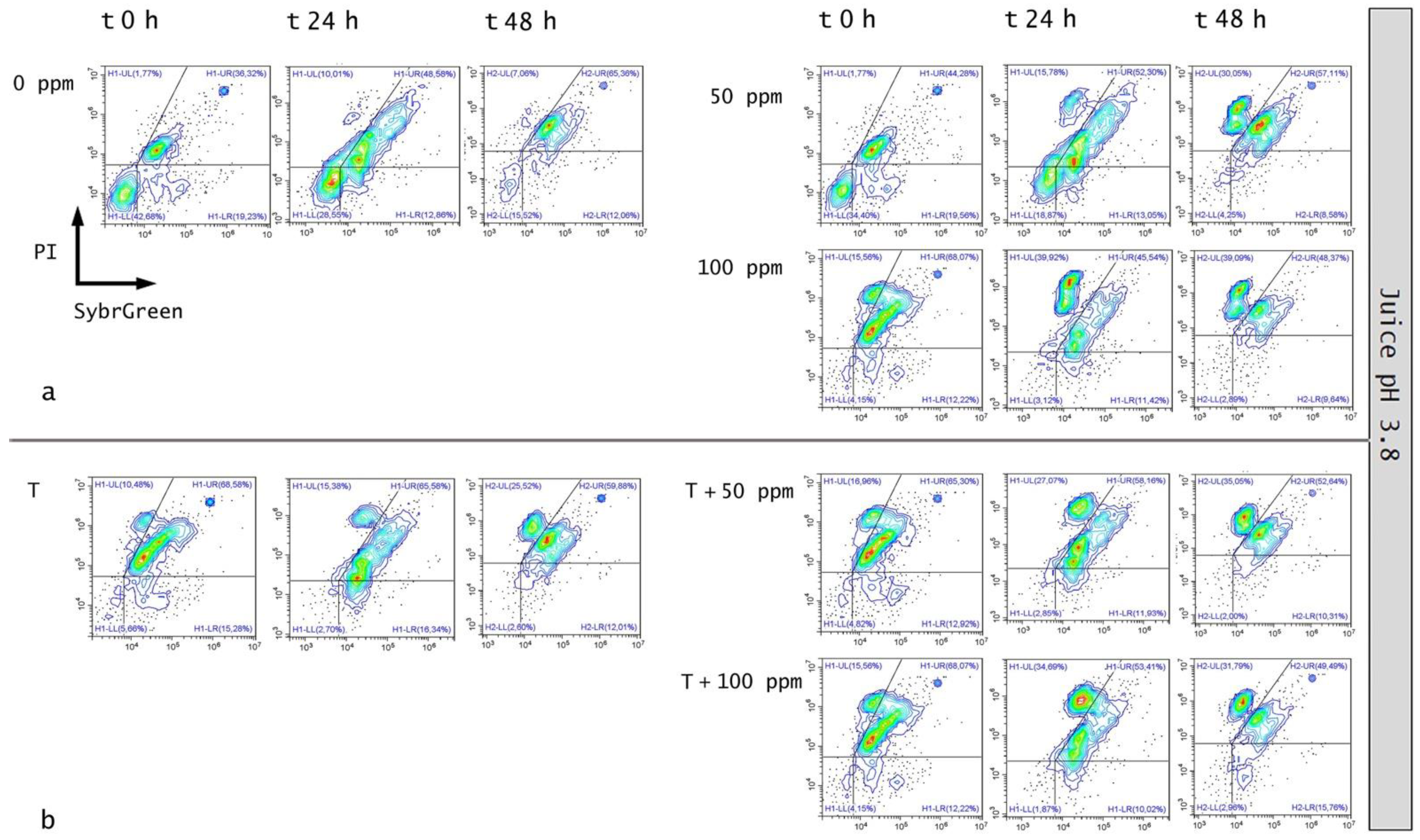
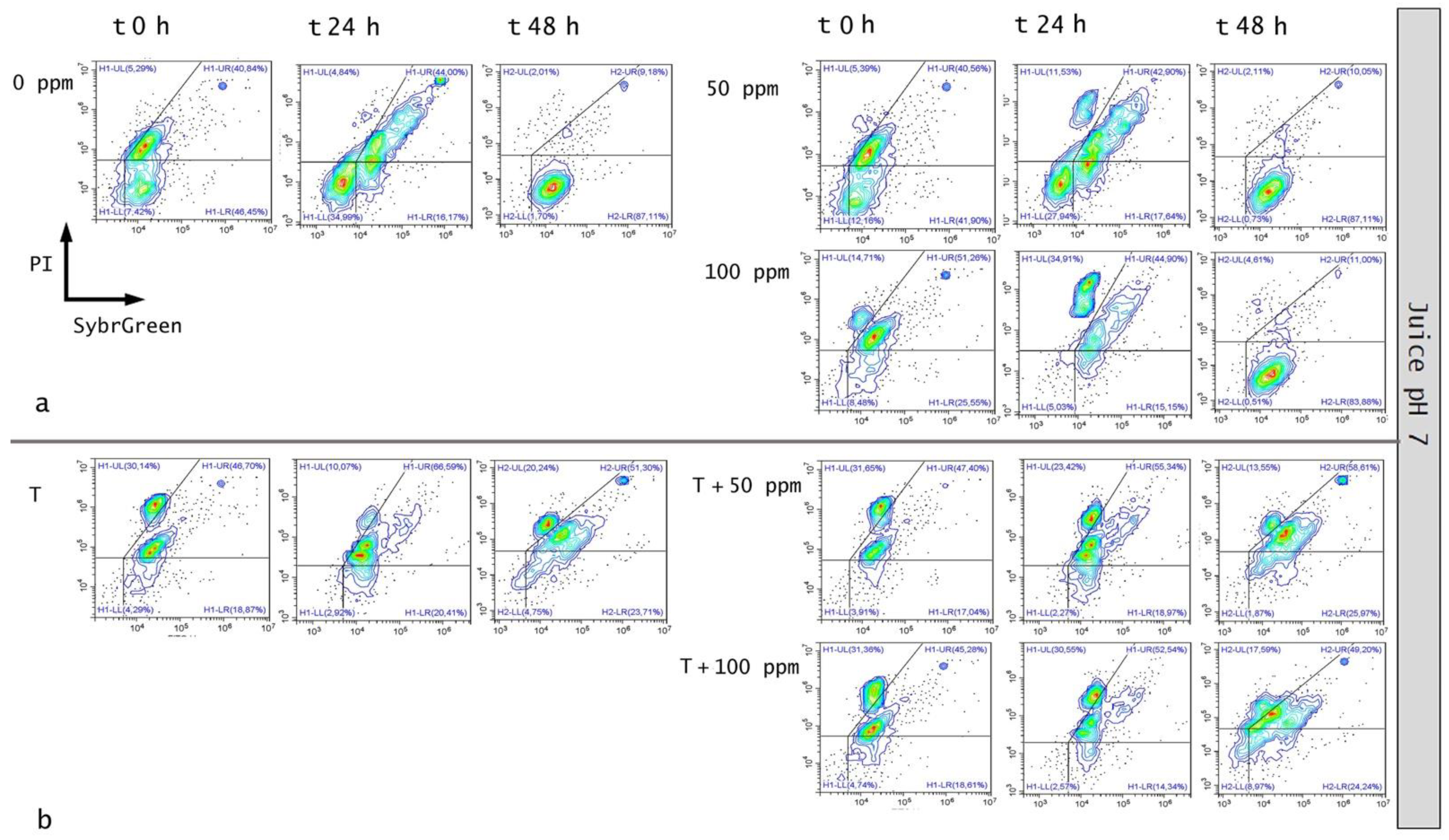
2.2.2. Flow Cytometry Analysis (FCM)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Effect of Thermal Treatments on E. coli ATCC 25922
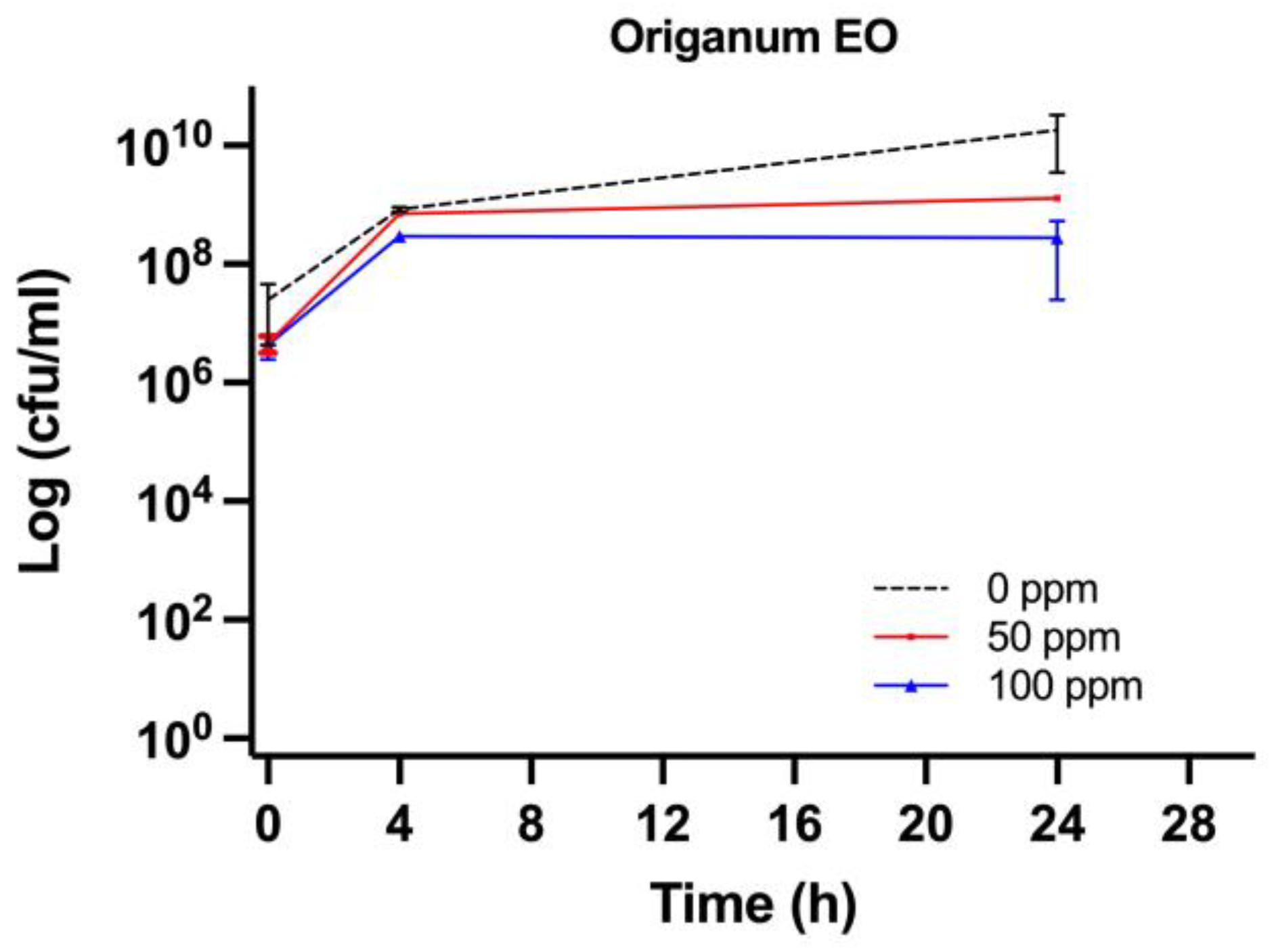
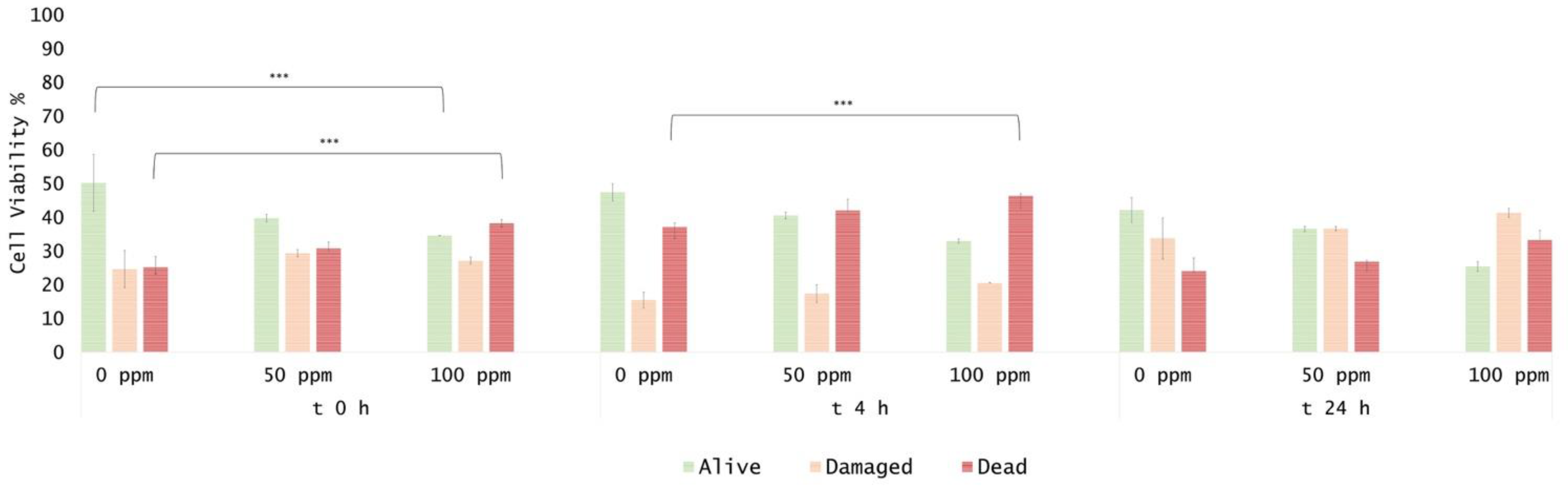
3.2. Effect of OEO Treatment
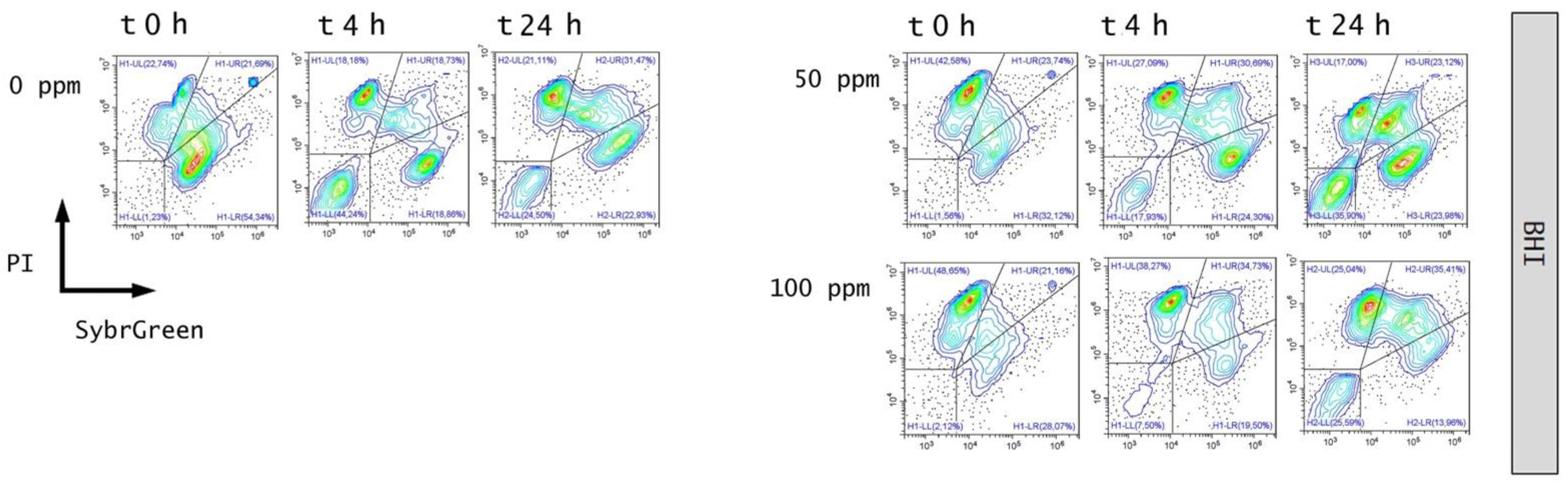
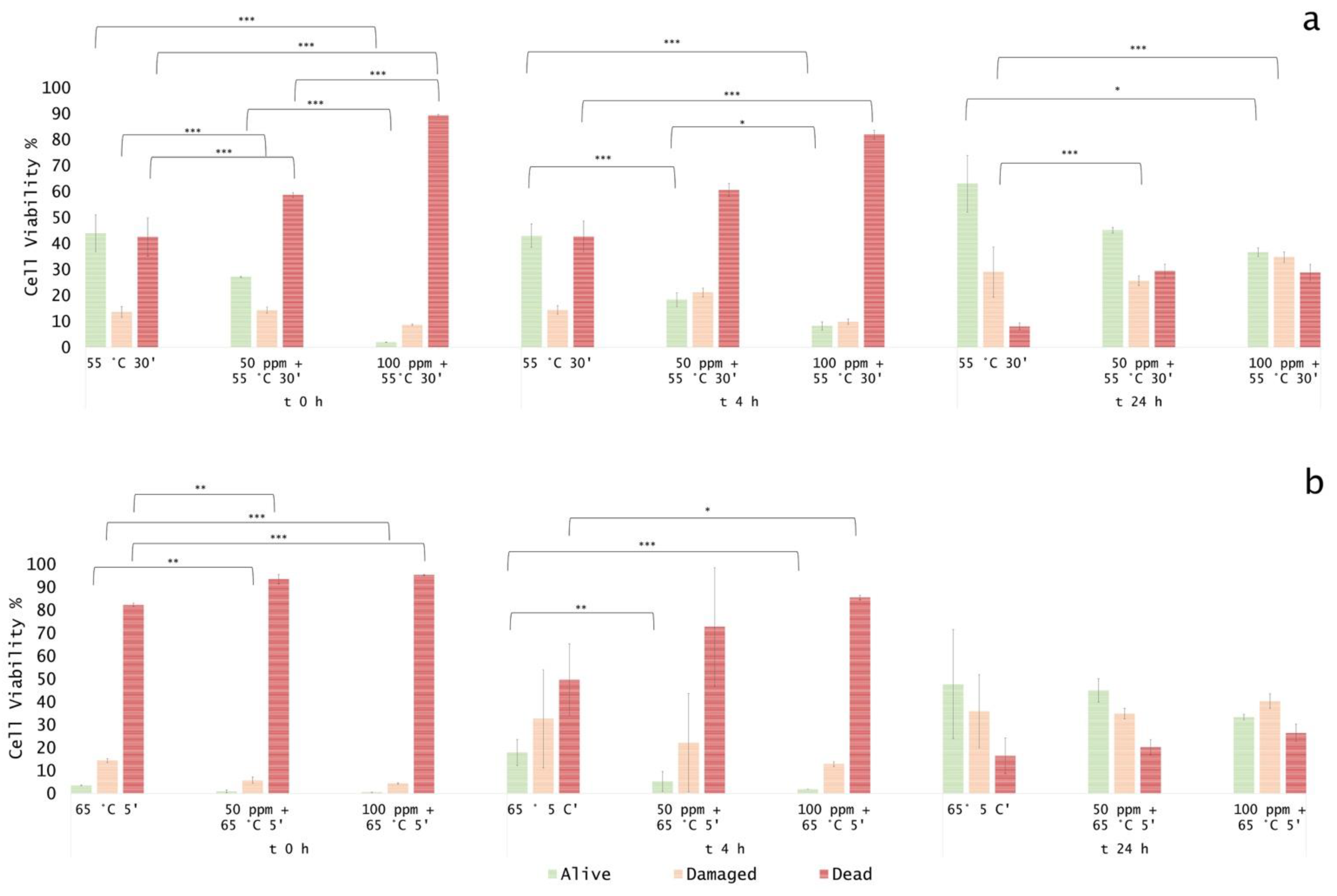
3.3. Effect of Antimicrobial Treatment in Synthetic Medium and Recovery Ability: Mild Heat-OEO
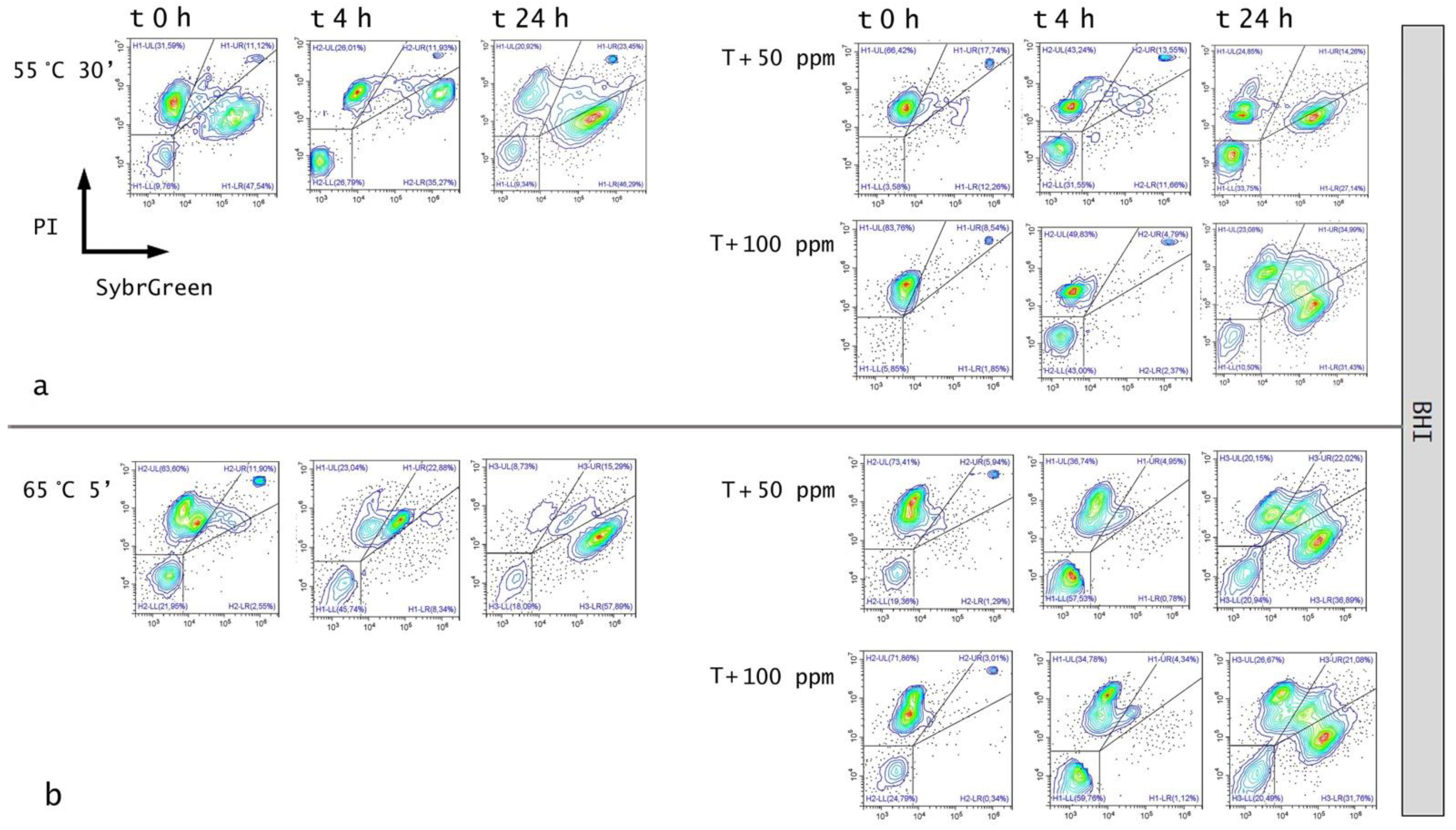
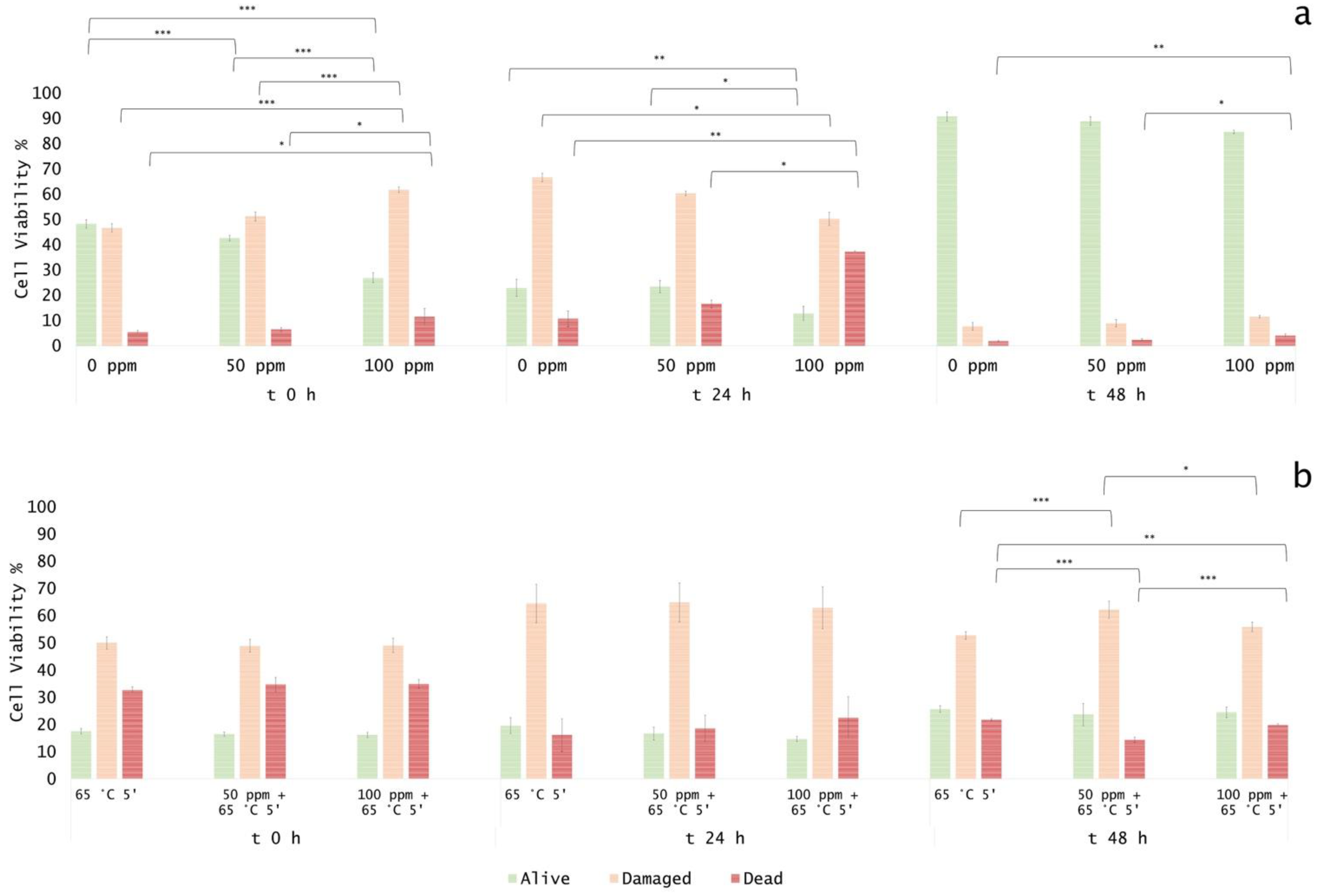
3.4. In Vitro Treatments Efficacy Assessment: Subpopulations Culturability
3.5. Effect of Antimicrobial Treatment in Fruit Juice and Recovery Ability: Mild Heat-OEO
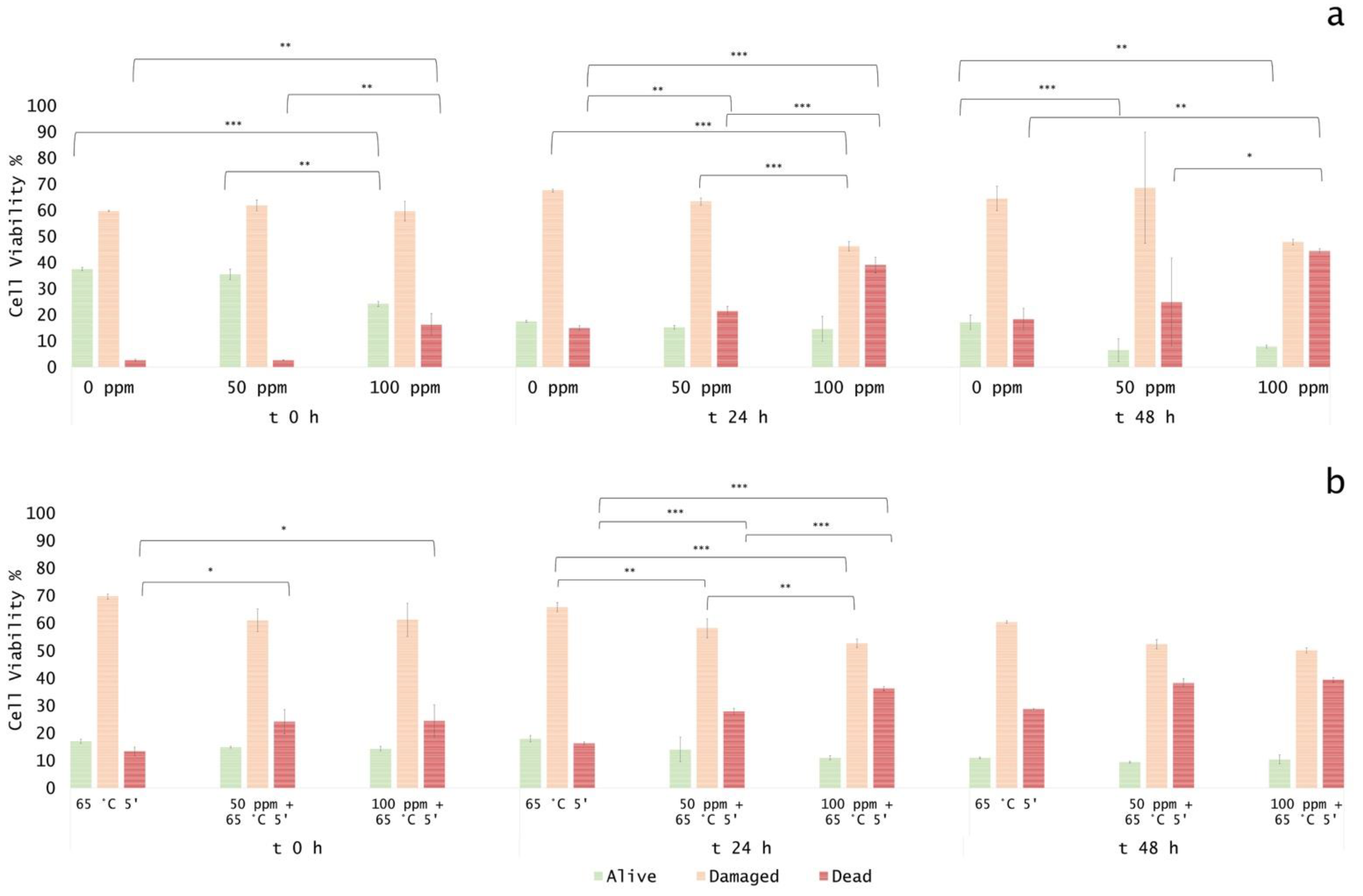
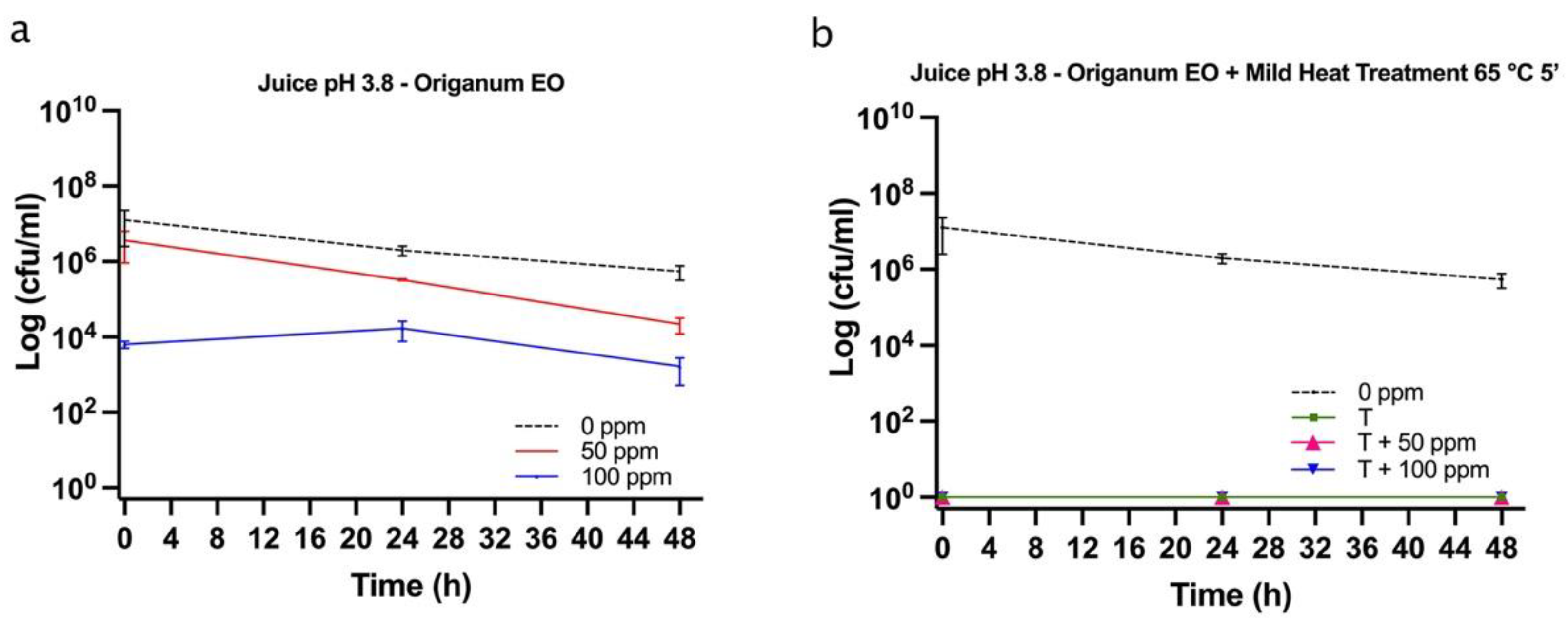
3.5.1. Microbial Challenge Test in Fruit Juice at pH 3.8
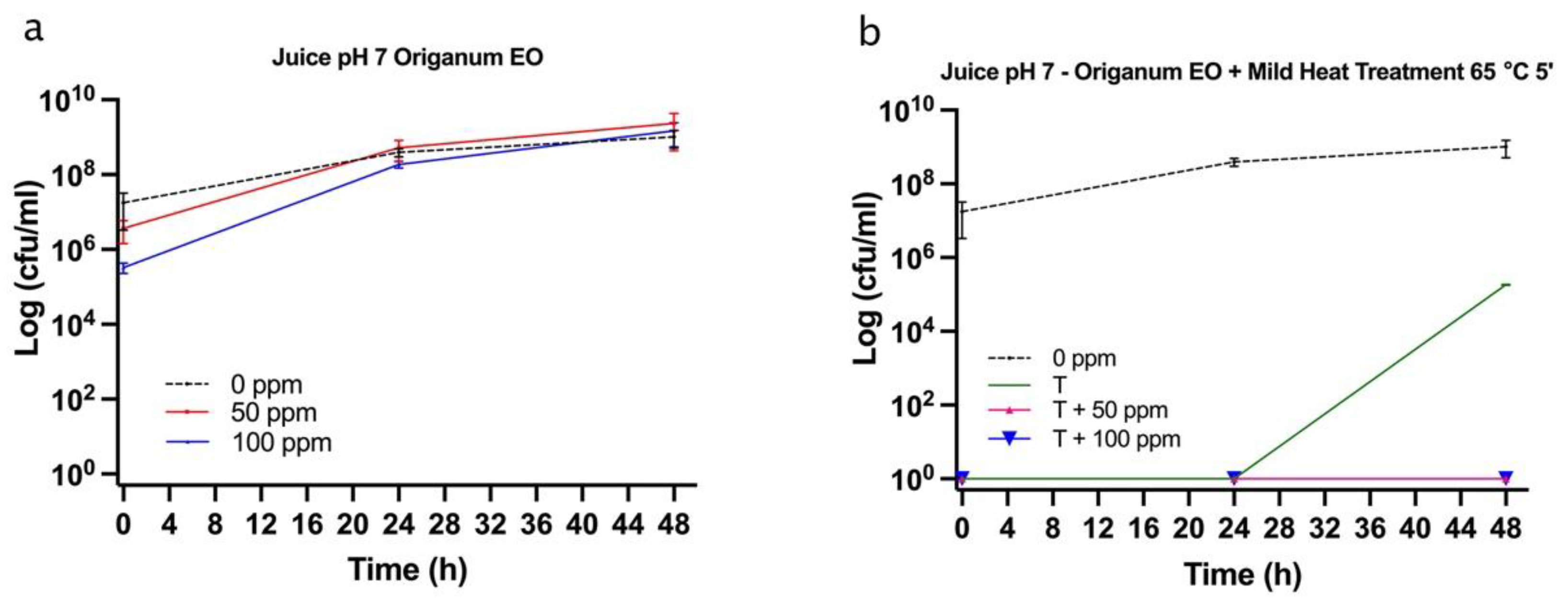
3.5.2. Microbial Challenge Test in Fruit Juice at pH 7.0
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal treatments for fruit and vegetable juices and beverages: A literature overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, R.; Ahn, J.; Balasubramaniam, V.M.; Saona, L.R.; Yousef, A.E. Food Safety Engineering; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128148037. [Google Scholar]
- Hui, Y.H.; Nip, W.K.; Nollet, L.M.L.; Paliyath, G.; Simpson, B.K. Food Biochemistry and Food Processing; Wiley: Hoboken, NJ, USA, 2007; ISBN 0813803780. [Google Scholar]
- Pasha, I.; Saeed, F.; Sultan, M.T.; Khan, M.R.; Rohi, M. Recent developments in minimal processing: A tool to retain nutritional quality of food. Crit. Rev. Food Sci. Nutr. 2014, 54, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, P.; Verbeke, W.; Devlieghere, F.; Debevere, J. Consumer perception and choice of minimally processed vegetables and packaged fruits. Food Qual. Prefer. 2004, 15, 259–270. [Google Scholar] [CrossRef]
- Tchuenchieu, A.; Ngang, J.-J.; Juengue, M.; Kamdem, S.; Etoa, F.-X. Effect of acid adaptation of Listeria monocytogenes on its mild thermal inactivation in a simulated fruit juice supplemented with carvacrol. Br. Microbiol. Res. J. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Tchuenchieu, A.; Kamdem, S.S.; Bevivino, A.; Etoa, F.X.; Ngang, J.J.E. Development of a predictive model of the microbial inactivation of L. monocytogenes during low thermal treatment of fruit juices in combination with carvacrol as aroma compound. Curr. Res. Food Sci. 2022, 5, 374–381. [Google Scholar] [CrossRef]
- Dhar, R.; Basak, S.; Chakraborty, S. Pasteurization of fruit juices by pulsed light treatment: A review on the microbial safety, enzymatic stability, and kinetic approach to process design. Compr. Rev. Food Sci. Food Saf. 2022, 21, 499–540. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Quentin, B.; Assemat, S.; Remize, F. Maintaining physicochemical, microbiological, and sensory quality of pineapple juice (Ananas comosus, Var. ’Queen Victoria’) through mild heat treatment. Processes 2020, 8, 1186. [Google Scholar] [CrossRef]
- Jan, A.; Sood, M.; Sofi, S.A.; Norzom, T. Non-thermal processing in food applications: A review. Int. J. Food Sci. Nutr. 2017, 2, 171–180. [Google Scholar]
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.P.; Marchesseau, S. Potentialities and limits of some non-thermal technologies to improve sustainability of food processing. Front. Nutr. 2019, 5, 130. [Google Scholar] [CrossRef]
- Chiozzi, V.; Agriopoulou, S.; Varzakas, T. Advances, Applications, and comparison of thermal (pasteurization, sterilization, and aseptic packaging) against non-thermal (ultrasounds, UV radiation, ozonation, high hydrostatic pressure) technologies in food processing. Appl. Sci. 2022, 12, 2202. [Google Scholar] [CrossRef]
- Carvalho, R.J.D.; Souza, G.T.D.; Pagán, E.; García-Gonzalo, D.; Magnani, M.; Pagán, R. Nanoemulsions of Mentha piperita L. essential oil in combination with mild heat, pulsed electric fields (PEF) and high hydrostatic pressure (HHP) as an alternative to inactivate Escherichia coli O157: H7 in fruit juices. Innov. Food Sci. Emerg. Technol. 2018, 48, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Novel thermal and non-thermal processing of watermelon juice. Trends Food Sci. Technol. 2019, 93, 234–243. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchuenchieu, A.; Ngang, J.-J.; Pop, C.; Kamdem, S.; Rotar, A.; Etoa, F.-X.; Mudura, E. Antimicrobial potential of carvacrol and its effect at sub-lethal concentration during low thermal pasteurization of fruit uices. J. Adv. Microbiol. 2018, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tchuenchieu, A.; Sylvain, S.K.; Pop, C.; Jean-Justin, E.N.; Mudura, E.; Etoa, F.X.; Rotar, A. Low thermal inactivation of Escherichia coli ATCC 25922 in pineapple, orange and watermelon juices: Effect of a prior acid-adaptation and of carvacrol supplementation. J. Food Saf. 2018, 38, e12415. [Google Scholar] [CrossRef]
- Belletti, N.; Kamdem, S.S.; Tabanelli, G.; Lanciotti, R.; Gardini, F. Modeling of combined effects of citral, linalool and β-pinene used against Saccharomyces cerevisiae in citrus-based beverages subjected to a mild heat treatment. Int. J. Food Microbiol. 2010, 136, 283–289. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Pagán, R.; García-Gonzalo, D. Effect of citral on the thermal inactivation of Escherichia coli O157:H7 in citrate phosphate buffer and apple juice. J. Food Prot. 2010, 73, 2189–2196. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Ouazzou, A.A.; Condón, S.; García-Gonzalo, D.; Pagán, R. Inactivation of Escherichia coli O157:H7 in fruit juices by combined treatments of citrus fruit essential oils and heat. Int. J. Food Microbiol. 2012, 159, 9–16. [Google Scholar] [CrossRef]
- Arioli, S.; Montanari, C.; Magnani, M.; Tabanelli, G.; Patrignani, F.; Lanciotti, R.; Mora, D.; Gardini, F. Modelling of Listeria monocytogenes Scott A after a mild heat treatment in the presence of thymol and carvacrol: Effects on culturability and viability. J. Food Eng. 2019, 240, 73–82. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Fikry, S.; Khalil, N.; Salama, O. Chemical profiling, biostatic and biocidal dynamics of Origanum vulgare L. essential oil. AMB Express 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Tchuenchieu, A.; Ngang, J.J.E.; Servais, M.; Dermience, M.; Kamdem, S.S.; Etoa, F.X.; Sindic, M. Effect of low thermal pasteurization in combination with carvacrol on color, antioxidant capacity, phenolic and vitamin C contents of fruit juices. Food Sci. Nutr. 2018, 6, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Ngang, J.J.E.; Nyegue, M.A.; Ndoye, F.C.; Kamgain, A.D.T.; Kamdem, S.L.S.; Lanciotti, R.; Gardini, F.; Etoa, F.X. Characterization of Mexican coriander (Eryngium foetidum) essential oil and its inactivation of Listeria monocytogenes in vitro and during mild thermal pasteurization of pineapple juice. J. Food Prot. 2014, 77, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Guevara, L.; Antolinos, V.; Palop, A.; Periago, P.M. Impact of moderate heat, carvacrol, and thymol treatments on the viability, injury, and stress eesponse of Listeria monocytogenes. Biomed Res. Int. 2015, 2015, 548930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chueca, B.; Ramírez, N.; Arvizu-Medrano, S.M.; García-Gonzalo, D.; Pagán, R. Inactivation of spoiling microorganisms in apple juice by a combination of essential oils’ constituents and physical treatments. Food Sci. Technol. Int. 2016, 22, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, G.T.D.S.; Carvalho, R.J.D.; Berdejo, D.; Souza, E.L.D.; Pagán, R.; Magnani, M. Control of autochthonous spoilage lactic acid bacteria in apple and orange juices by sensorially accepted doses of Citrus Spp. essential oils combined with mild heat treatments. J. Food Sci. 2019, 84, 848–858. [Google Scholar] [CrossRef]
- Gayán, E.; Geens, E.; Berdejo, D.; García-Gonzalo, D.; Pagán, R.; Aertsen, A.; Michiels, C.W. Combination of mild heat and plant essential oil constituents to inactivate resistant variants of Escherichia coli in buffer and in coconut water. Food Microbiol. 2020, 87, 103388. [Google Scholar] [CrossRef]
- Pedrosa, G.T.D.S.; Souza, E.L.D.; Melo, A.N.F.D.; Almeida, E.T.D.C.; Guedes, J.P.D.S.; Carvalho, R.J.D.; Pagán, R.; Magnani, M. Physiological alterations involved in inactivation of autochthonous spoilage bacteria in orange juice caused by Citrus essential oils and mild heat. Int. J. Food Microbiol. 2020, 334, 108837. [Google Scholar] [CrossRef]
- Montanari, C.; Tabanelli, G.; Barbieri, F.; Mora, D.; Duncan, R.; Gardini, F.; Arioli, S. Listeria monocytogenes sensitivity to antimicrobial treatments depends on cell origin. Sci. Rep. 2021, 11, 21263. [Google Scholar] [CrossRef]
- Fleischmann, S.; Robben, C.; Alter, T.; Rossmanith, P.; Mester, P. How to evaluate non-growing cells—Current strategies for determining antimicrobial resistance of vbnc bacteria. Antibiotics 2021, 10, 115. [Google Scholar] [CrossRef]
- Wideman, N.E.; Oliver, J.D.; Crandall, P.G.; Jarvis, N.A. Detection and potential virulence of viable but non-culturable (VBNC) Listeria monocytogenes: A review. Microorganisms 2021, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.W.; Ding, T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayrapetyan, M.; Oliver, J.D. The viable but non-culturable state and its relevance in food safety. Curr. Opin. Food Sci. 2016, 8, 127–133. [Google Scholar] [CrossRef]
- Sourri, P.; Tassou, C.C.; Nychas, G.E. Fruit juice spoilage by Alicyclobacillus: Detection and control methods—A comprehensive review. Foods 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Suo, Y.; Xiang, Q.; Zhao, X.; Chen, S.; Ye, X.; Liu, D. Significance of viable but nonculturable Escherichia coli: Induction, detection, and control. J. Microbiol. Biotechnol. 2017, 27, 417–428. [Google Scholar] [CrossRef]
- Asakura, H.; Kawamoto, K.; Haishima, Y.; Igimi, S.; Yamamoto, S.; Makino, S.I. Differential expression of the outer membrane protein W (OmpW) stress response in enterohemorrhagic Escherichia coli O157: H7 corresponds to the viable but non-culturable state. Res. Microbiol. 2008, 159, 709–717. [Google Scholar] [CrossRef]
- Fu, Y.; Jia, Y.; Fan, J.; Yu, C.; Yu, C.; Shen, C. Induction of Escherichia coli O157: H7 into a viable but non-culturable state by high temperature and its resuscitation. Environ. Microbiol. Rep. 2020, 12, 568–577. [Google Scholar] [CrossRef]
- Zand, E.; Froehling, A.; Schoenher, C.; Zunabovic-Pichler, M.; Schlueter, O.; Jaeger, H. Potential of flow cytometric approaches for rapid microbial detection and chracterization in the food Industry—A review. Foods 2021, 10, 3112. [Google Scholar] [CrossRef]
- Bridier, A.; Hammes, F.; Canette, A.; Bouchez, T.; Briandet, R. Fluorescence-based tools for single-cell approaches in food microbiology. Int. J. Food Microbiol. 2015, 213, 2–16. [Google Scholar] [CrossRef]
- Léonard, L.; Chibane, L.B.; Bouhedda, B.O.; Degraeve, P.; Oulahal, N. Recent advances on multi-parameter flow cytometry to characterize antimicrobial treatments. Front. Microbiol. 2016, 7, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.; Beltran, A.; Valdes, A.; Peltzer, M.A.; Jimenez, A.; Garrigos, M.C.; Zaikov, G.E. Carvacrol and thymol for fresh food packaging. J. Bioequivalence Bioavailab. 2013, 5, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Nostro, A.; Papalia, T. Antimicrobial activity of carvacrol: Current progress and future prospectives. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Wang, S.; Chen, C.; Zhang, W.; Zhang, Y. Ultra-rapid drug susceptibility testing for Klebsiella pneumoniae clinical isolates in 60 min by SYBR Green I/Propidium iodide viability assay. Front. Microbiol. 2021, 12, 694522. [Google Scholar] [CrossRef]
- Foglia, C.; Allesina, S.; Amoruso, A.; Prisco, A.D.; Pane, M. New insights in enumeration methodologies of probiotic cells in finished products. J. Microbiol. Methods 2020, 175, 105993. [Google Scholar] [CrossRef]
- Russell, A.D. Lethal effects of heat on bacterial physiology and structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Teixeira, P.; Fernandes, B.; Silva, A.M.; Dias, N.; Azeredo, J. Evaluation by flow cytometry of Escherichia coli viability in lettuce after disinfection. Antibiotics 2020, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Afari, G.K.; Hung, Y.C. Detection and verification of the viable but nonculturable (VBNC) state of Escherichia coli O157:H7 and Listeria monocytogenes using flow cytometry and standard plating. J. Food Sci. 2018, 83, 1913–1920. [Google Scholar] [CrossRef]
- Massicotte, R.; Mafu, A.A.; Ahmad, D.; Deshaies, F.; Pichette, G.; Belhumeur, P. Comparison between flow cytometry and traditional culture methods for efficacy assessment of six disinfectant agents against nosocomial bacterial species. Front. Microbiol. 2017, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Zhao, X. Induction of viable but nonculturable Escherichia coli O157: H7 by low temperature and its resuscitation. Front. Microbiol. 2018, 9, 2728. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Jiang, L.; Zhang, R. Induction of a viable but non-culturable state in Salmonella Typhimurium by thermosonication and factors affecting resuscitation. FEMS Microbiol. Lett. 2018, 365, fnx249. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Ponce, A.G.; Valle, C.E.D.; Roura, S.I. Inhibitory parameters of essential oils to reduce a foodborne pathogen. LWT Food Sci. Technol. 2005, 38, 565–570. [Google Scholar] [CrossRef]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar] [CrossRef]
- Kennedy, D.; Cronin, U.P.; Wilkinson, M.G. Responses of Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus to simulated food processing treatments, determined using fluorescence-activated cell sorting and plate counting. Appl. Environ. Microbiol. 2011, 77, 4657–4668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevel, S.V.; Koetzsch, S.; Weilenmann, H.U.; Boon, N.; Hammes, F. Routine bacterial analysis with automated flow cytometry. J. Microbiol. Methods 2013, 94, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.M. Flow cytometry of bacterial membrane potential and permeability. Methods Mol. Med. 2008, 142, 175–186. [Google Scholar] [CrossRef]
- Wilkinson, M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: A review. Trends Food Sci. Technol. 2018, 78, 1–10. [Google Scholar] [CrossRef]
- Wilkinson, M.G. Flow cytometry in food microbiology: Challenges, opportunities and progress to date. Téc. Lab. 2016, 417, 722–728. [Google Scholar]
- Müller, S.; Nebe-Von-Caron, G. Functional single-cell analyses: Flow cytometry and cell sorting of microbial populations and communities. FEMS Microbiol. Rev. 2010, 34, 554–587. [Google Scholar] [CrossRef] [Green Version]
- Gurtler, J.B.; Fan, X.; Jin, T.; Niemira, B.A. Influence of antimicrobial agents on the thermal sensitivity of foodborne pathogens: A review. J. Food Prot. 2019, 82, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Tenreiro, A.; Cunha, M.V. When FLOW-FISH met FACS: Combining multiparametric, dynamic approaches for microbial single-cell research in the total environment. Sci. Total Environ. 2022, 806, 150682. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, D.; Loureiro, J.; Antoniadi, I.; Bainard, J.; Bureš, P.; Cápal, P.; Castro, M.; Castro, S.; Čertner, M.; Čertnerová, D.; et al. Best practices in plant cytometry. Cytom. Part A 2021, 99, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Schraivogel, D.; Kuhn, T.M.; Rauscher, B.; Rodríguez-Martínez, M.; Paulsen, M.; Owsley, K.; Middlebrook, A.; Tischer, C.; Ramasz, B.; Ordoñez-Rueda, D.; et al. High-speed fluorescence image—Enabled cell sorting. Science 2022, 320, 315–320. [Google Scholar] [CrossRef]
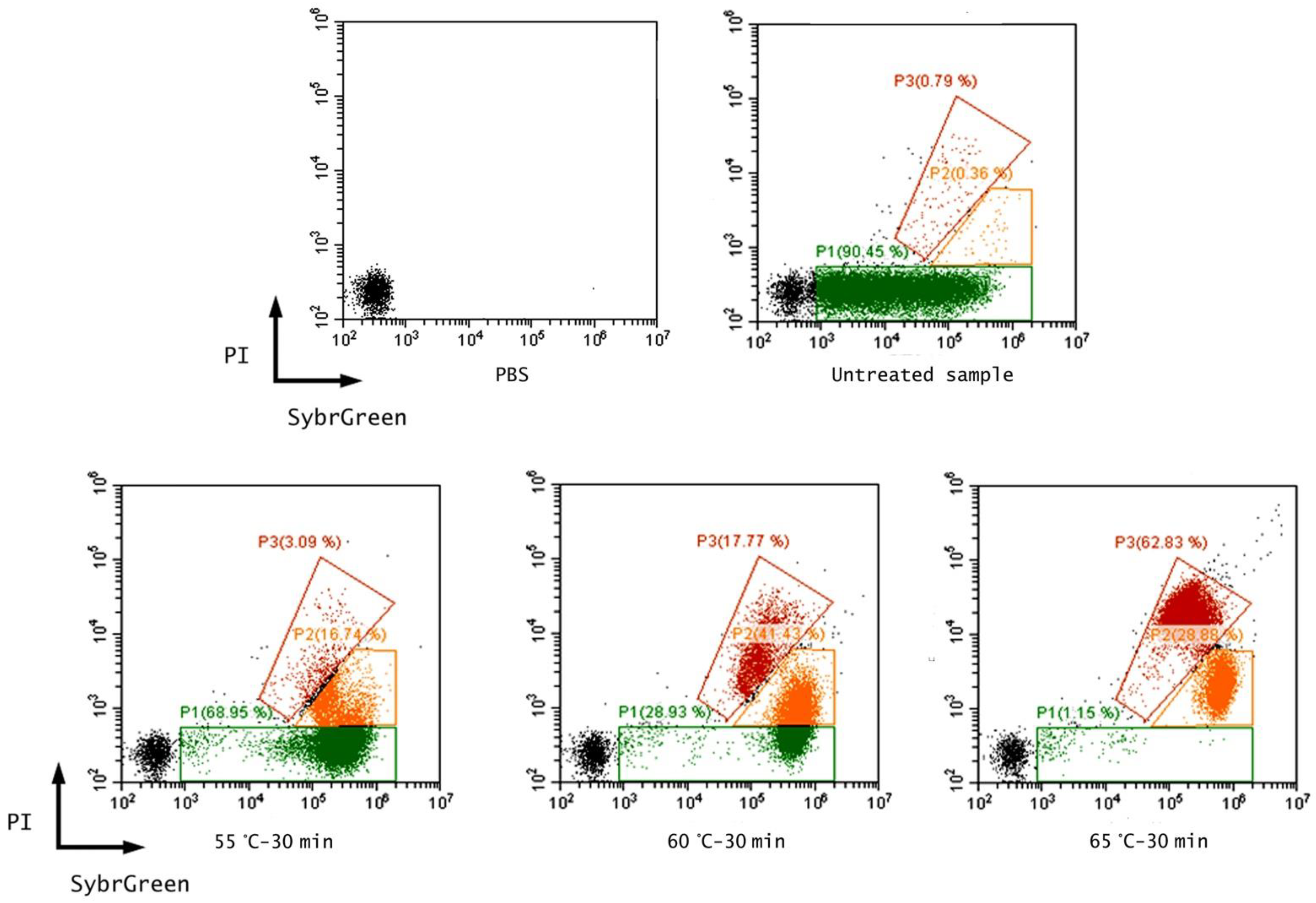

| Treatment | Culture-Based Method | Flow Cytometry (% of Events) * | Equivalent Alive Cell Content (Log AFU/mL) ** | ||||
|---|---|---|---|---|---|---|---|
| Log CFU/mL | Eq Log Reduction | Eq % of Dead Ells | Alive | Damaged | Dead | ||
| Untreated sample | 7.44 ± 0.23 a | 0 | 0 | 98.90 ± 0.13 | 0.30 ± 0.08 | 0.80 ± 0.06 | 7.23 ± 0.02 a |
| 55 °C-30 min | 4.99 ± 0.90 a | −2.45 ± 0.90 | 98.68 ± 2.07 | 78.06 ± 0.88 | 18.37 ± 0.88 | 3.57 ± 0.09 | 7.05 ± 0.01 b |
| 60 °C-30 min | 3.96 ± 0.82 a | −3.48 ± 0.82 | 99.60 ± 0.16 | 33.14 ± 0.27 | 46.42 ± 0.60 | 20.44 ± 0.40 | 6.72 ± 0.01 b |
| 65 °C-30 min | 1.33 ± 1.24 a | −6.11 ± 1.24 | 99.99 ± 0.001 | 1.15 ± 0.18 | 34.33 ± 3.51 | 64.59 ± 3.43 | 5.25 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gregorio, L.; Tchuenchieu, A.; Poscente, V.; Arioli, S.; Del Fiore, A.; Costanzo, M.; Giorgi, D.; Lucretti, S.; Bevivino, A. Synergistic Action of Mild Heat and Essential Oil Treatments on Culturability and Viability of Escherichia coli ATCC 25922 Tested In Vitro and in Fruit Juice. Foods 2022, 11, 1615. https://doi.org/10.3390/foods11111615
Di Gregorio L, Tchuenchieu A, Poscente V, Arioli S, Del Fiore A, Costanzo M, Giorgi D, Lucretti S, Bevivino A. Synergistic Action of Mild Heat and Essential Oil Treatments on Culturability and Viability of Escherichia coli ATCC 25922 Tested In Vitro and in Fruit Juice. Foods. 2022; 11(11):1615. https://doi.org/10.3390/foods11111615
Chicago/Turabian StyleDi Gregorio, Luciana, Alex Tchuenchieu, Valeria Poscente, Stefania Arioli, Antonella Del Fiore, Manuela Costanzo, Debora Giorgi, Sergio Lucretti, and Annamaria Bevivino. 2022. "Synergistic Action of Mild Heat and Essential Oil Treatments on Culturability and Viability of Escherichia coli ATCC 25922 Tested In Vitro and in Fruit Juice" Foods 11, no. 11: 1615. https://doi.org/10.3390/foods11111615
APA StyleDi Gregorio, L., Tchuenchieu, A., Poscente, V., Arioli, S., Del Fiore, A., Costanzo, M., Giorgi, D., Lucretti, S., & Bevivino, A. (2022). Synergistic Action of Mild Heat and Essential Oil Treatments on Culturability and Viability of Escherichia coli ATCC 25922 Tested In Vitro and in Fruit Juice. Foods, 11(11), 1615. https://doi.org/10.3390/foods11111615