Variations in Fatty Acids Affected Their Derivative Volatiles during Tieguanyin Tea Processing
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Tea Samples
2.3. Extraction and Analysis of Fatty Acids
2.4. Extraction and Analysis of the Odorant Compounds
2.5. Data Processing
3. Results and Discussion
3.1. Change Properties of Fatty Acids during Tieguanyin Tea Processing
3.1.1. Fatty Acid Composition and Content in Tieguanyin Tea Samples
3.1.2. Change in TFA, USFA, and SFA during Tieguanyin Tea Manufacturing
3.1.3. Change in Fatty Acids Composition during Tieguanyin Tea Manufacturing
3.2. Change in FADV Content during Tieguanyin Tea Processing
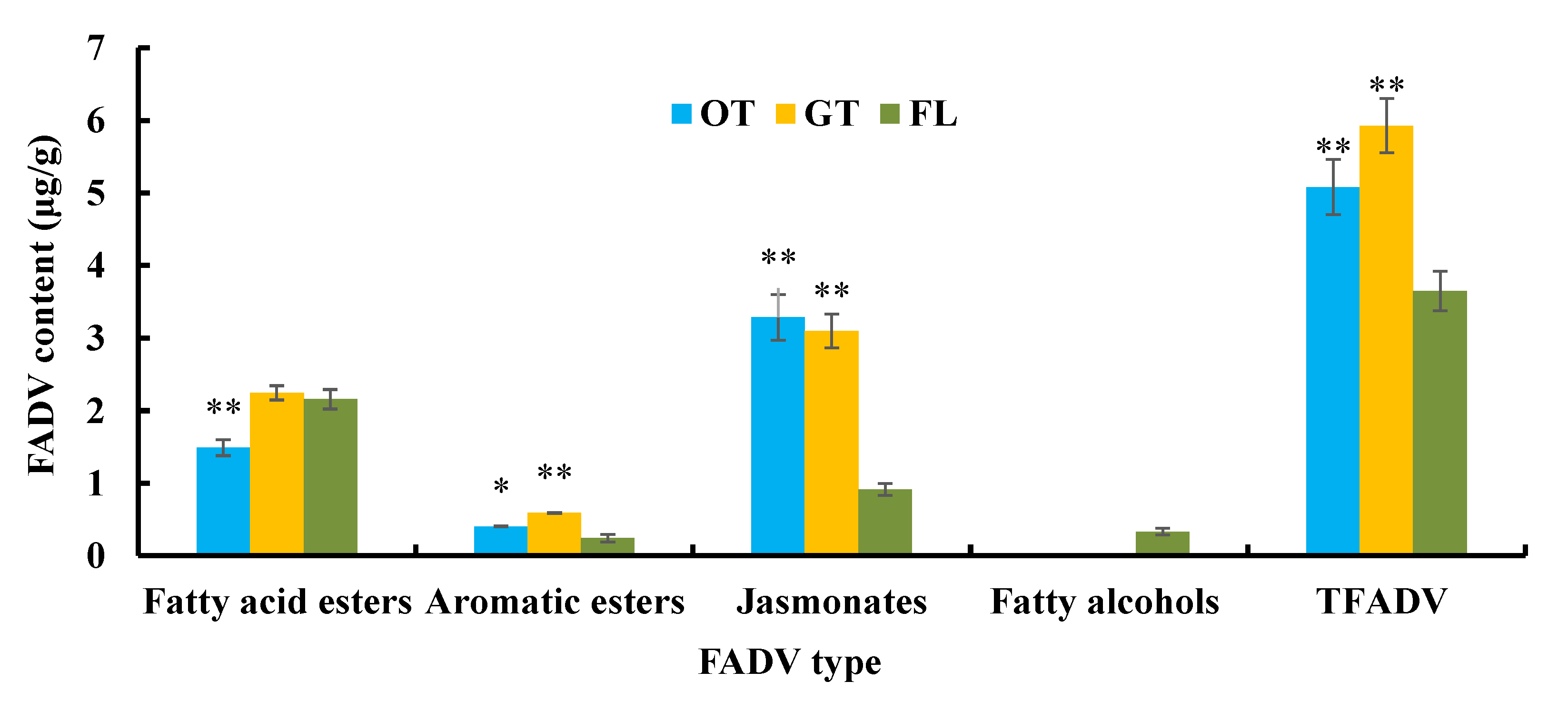
3.2.1. FADV Profiles and Contents in Tieguanyin Tea Samples
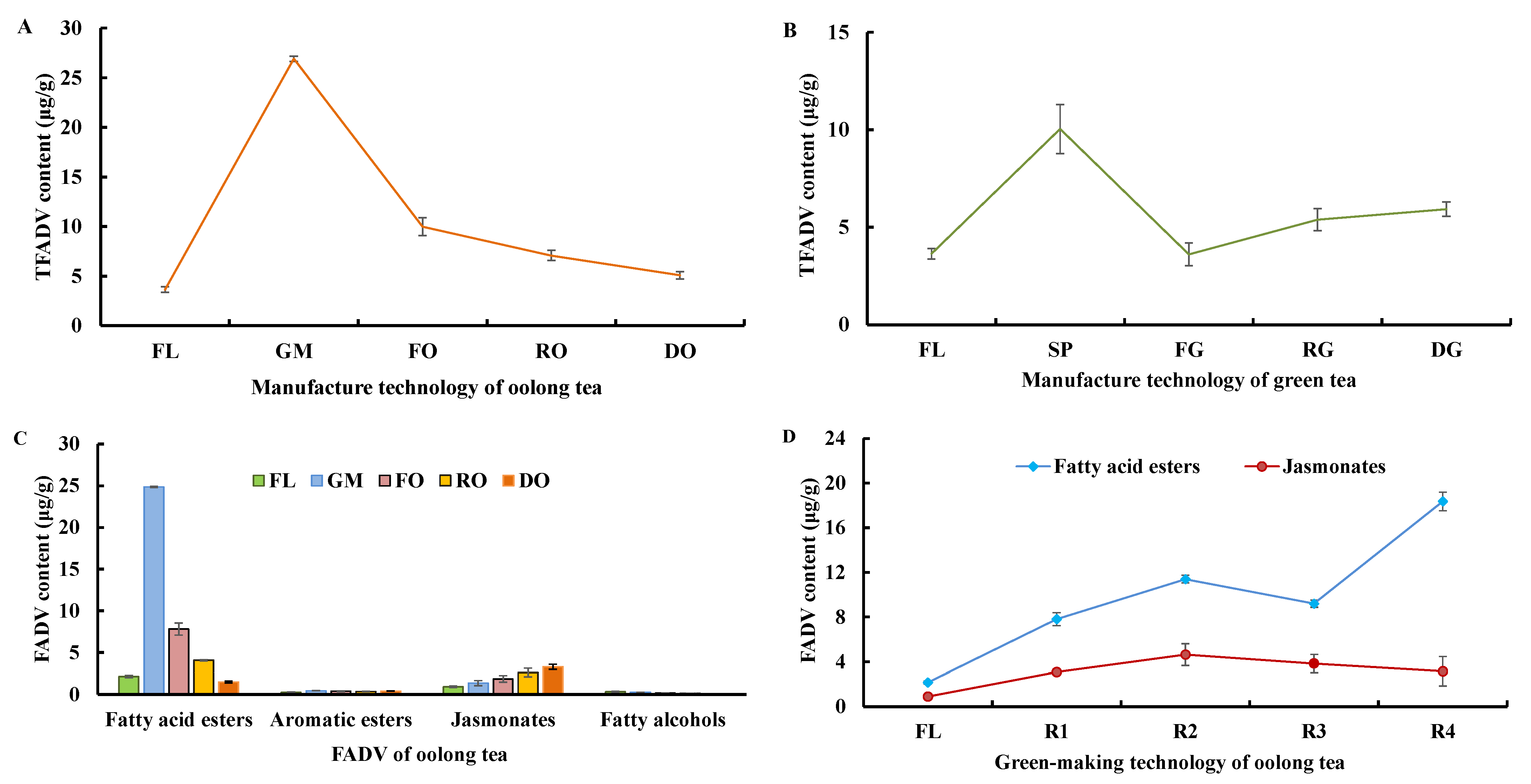
3.2.2. Changes in FADV during Tieguanyin Tea Manufacturing
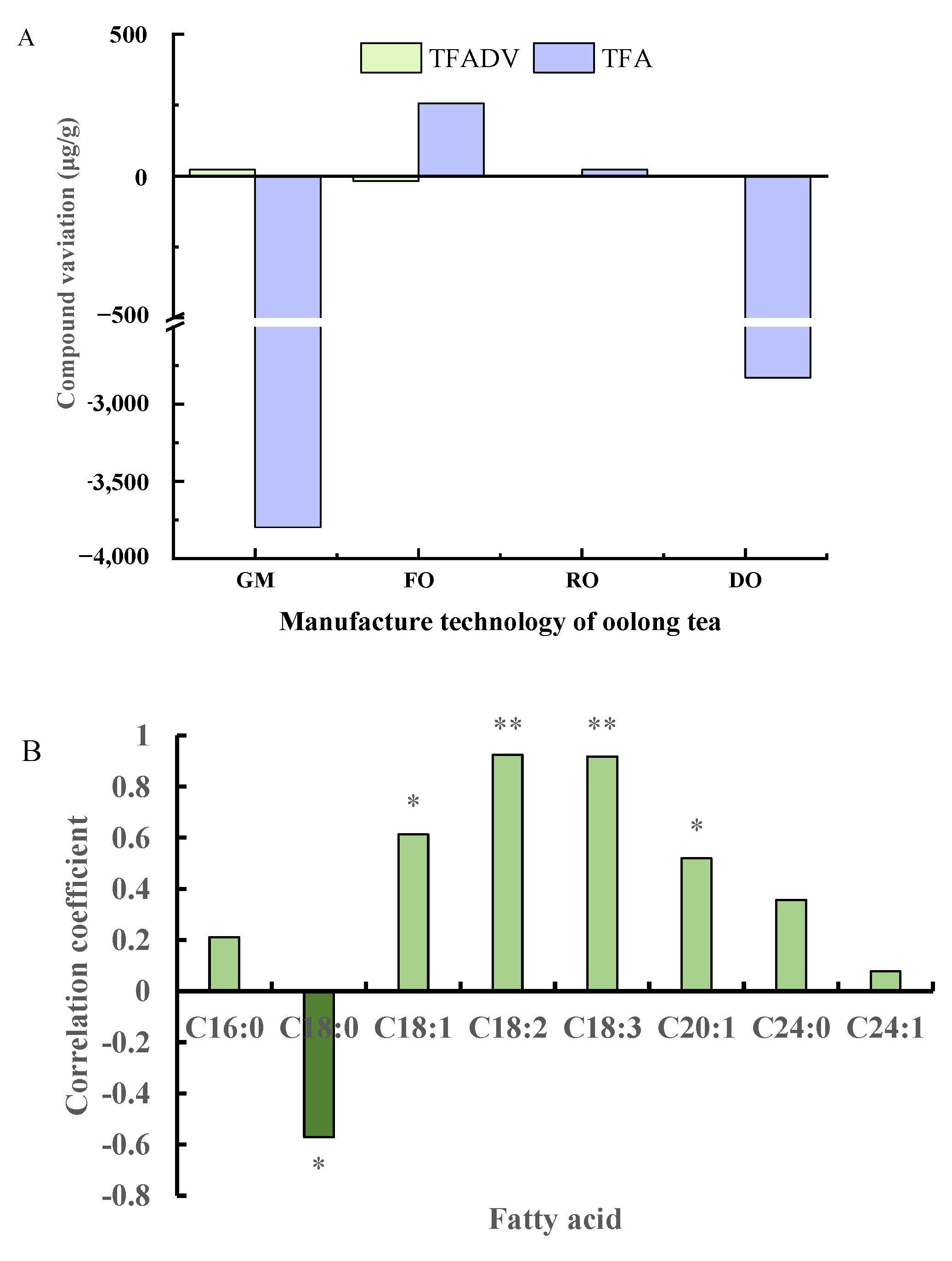
3.3. Correlations of Fatty Acids and Their Derivatives during Oolong Tea Processing
3.4. Relationship of the Key FADV with Fatty Acids during Oolong Tea Green-Making Stage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Lv, S.; Wu, Y.; Gao, X.; Li, J.; Zhang, W.; Meng, Q. Oolong tea made from tea plants from different locations in Yunnan and Fujian, China showed similar aroma but different taste characteristics. Springerplus 2016, 5, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Jin, S.; Guo, Y. Exploration of a Method of Distinguishing Different Nongxiang Tieguanyin Tea Grades Based on Aroma Determined by GC-MS Combined with Chemometrics. Molecules 2019, 24, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Lin, Q.X.; Zhang, Y.G.; Chen, J.; Wang, L.L.; Yu, W.Q.; You, Z.M. Aroma Profiling of Tieguanyin oolong tea with different flavor characteristics based on chemical pattern recognition. J. Tea Sci. 2018, 38, 253–262. [Google Scholar]
- Lin, Z.-k.; Hua, Y.-f.; Gu, Y.-h.; Pan, Y.-s.; Zheng, D.-g. Studies on the aroma constituents of Fujien Ti Kuan Yin tea, Se Chung tea, Wu-I Shui Hsien tea and Wu-I Chi Chung tea in China. J. Integr. Plant Biol. 1984, 26, 513–522. [Google Scholar]
- Ji, W.; Liu, P.; Xu, Y.; Jiang, Y.; Chen, J.; Yin, J. Comparative study of the aroma components of several oolong teas. J. Tea Sci. 2016, 36, 523–530. [Google Scholar]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-Olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, Y.; Fu, X.; Liao, Y.; Yuan, Y.; Jia, Y.; Dong, F.; Yang, Z. Biosynthesis of Jasmine Lactone in Tea (Camellia sinensis) Leaves and Its Formation in Response to Multiple Stresses. J. Agric. Food Chem. 2018, 66, 3899–3909. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Zhao, Y.; Zhang, M.; Zhou, Z.; Li, Y.; Hu, Y.; Wu, Y.; Feng, Z.; Schwab, W.; et al. Biosynthesis of orchid-like volatile methyl jasmonate in tea (Camellia sinensis) leaves in response to multiple stresses during the shaking process of oolong tea. LWT 2021, 143, 111184. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, X.; Su, X.; Yang, Z. Chinese oolong tea: An aromatic beverage produced under multiple stresses. Trends Food Sci. Technol. 2020, 106, 242–253. [Google Scholar] [CrossRef]
- Ma, C.; Li, J.; Chen, W.; Wang, W.; Qi, D.; Pang, S.; Miao, A. Study of the aroma formation and transformation during the manufacturing process of oolong tea by solid-phase micro-extraction and gas chromatography–mass spectrometry combined with chemometrics. Food Res. Int. 2018, 108, 413–422. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhao, X.; Li, X.; Shan, W.; Wang, X.; Wang, S.; Yu, W.; Yang, Z.; Yu, X. Non-targeted metabolomics analysis reveals dynamic changes of volatile and non-volatile metabolites during oolong tea manufacture. Food Res. Int. 2019, 128, 108778. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 2, 585–599. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, L.P.; Tamuly, P.; Mahanta, P.K. Lipid content and fatty acid composition of tea shoot and manufactured tea. J. Agric. Food Chem. 1991, 39, 1159–1162. [Google Scholar] [CrossRef]
- Okal, A.W.; Owuor, P.O.; Kamau, D.M.; Mang’uro, L.O. Effects of production locations and plucking intervals on clonal tea fatty acids levels in the Kenya Higlands. Food Sci. Technol. Res. 2012, 18, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.J.; Tong, H.R. Studies on the fatty acid and glycoside aroma precursors of different tea varieties. J. Southwest Univ. (Nat. Sci. Ed.) 2008, 30, 62–66. [Google Scholar]
- Guo, L.; Du, Z.H.; Wang, Z.; Lin, Z.; Guo, Y.L.; Chen, M.J. Location affects fatty acid composition in Camellia sinensis cv Tieguanyin fresh leaves. J. Food Sci. Technol. 2020, 57, 96–101. [Google Scholar] [CrossRef]
- Guo, L.; Lyu, H.P.; Chen, M.J.; Zhang, Y.; Ba, Y.C.; Guo, Y.L.; Lin, Z. Analysis of fatty acids contents and differences of Fujian oolong tea. J. Tea Sci. 2019, 39, 611–618. [Google Scholar]
- Zhou, J.S.; Lv, S.D.; Jiang, D.H.; Wu, X.D.; Lian, M.; Wang, C.; Meng, Q.X. Analysis of volatile components of Tieguanyin and Dongding oolong teas by simultaneous distillation extraction coupled with gas chromatography-mass spectrometry. Asian J. Chem. 2015, 27, 1899–1902. [Google Scholar] [CrossRef]
- Zhan, Q.; Ren, H.T.; Yang, X.M.; Li, Y.L.; Li, J.H. Analysis on the aroma components in Wuyi rock tea with baking of electric and charcoal and conventional baking Tieguanyin oolong tea. J. Yunnan Agric. Univ. (Nat. Sci.) 2018, 33, 113–119. [Google Scholar]
- Chen, L.; Yu, W.; Zhang, Y.; Xiang, L.; Wang, L.; You, Z. Aroma profiling of oolong tea by SDE and HS-SPME in combination with GC-MS. J. Tea Sci. 2019, 39, 692–704. [Google Scholar]
- Kumazawa, K.; Wada, Y.; Masuda, H. Characterization of epoxydecenal isomers as potent odorants in black tea (Dimbula) infusion. J. Agric. Food Chem. 2006, 54, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Amano, Y.; Wada, Y.; Kumazawa, K. Characterization of the potent odorants contributing to the characteristic aroma of matcha by gas chromatography-olfactometry techniques. J. Agric. Food Chem. 2017, 65, 2984–2989. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Du, Z.; Liu, R.; Guo, L.; Chen, C.; Wu, H.; Chen, M. Establishing a quantitative volatile measurement method in tea by integrating sample extraction method optimizations and data calibration. Flavour Fragr. J. 2020, 36, 64–74. [Google Scholar] [CrossRef]
- Chen, M.; Guo, L.; Zhou, H.; Guo, Y.; Zhang, Y.; Lin, Z.; Sun, M.; Zeng, W.; Wu, H. Absolute quantitative volatile measurement from fresh tea leaves and the derived teas revealed contributions of postharvest synthesis of endogenous volatiles for the aroma quality of made teas. Appl. Sci. 2021, 11, 613. [Google Scholar] [CrossRef]
- Ai, F.F.; Bin, J.; Zhang, Z.M.; Huang, J.H.; Wang, J.B.; Liang, Y.Z.; Yu, L.; Yang, Z.Y. Application of random forests to select premium quality vegetable oils by their fatty acid composition. Food Chem. 2014, 143, 472–478. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Verardo, V.; del Mar Contreras, M. Fatty acid and sterol composition of tea seed oils: Their comparison by the "FancyTiles" approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef]
- He, M.; Ding, N.Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carvalho, I.S.; Brodelius, M. Omega-3 fatty acid desaturase genes isolated from purslane (Portulaca oleracea L.): Expression in different tissues and response to cold and wound stress. J. Agric. Food Chem. 2010, 58, 1870–1877. [Google Scholar] [CrossRef]
- Wang, P.J.; Cao, H.L.; Chen, D.; Chen, D.; Chen, G.X.; Yang, J.F.; Ye, N.X. Cloning and expression analysis of fatty acid desaturase family genes in Camellia sinensis. Acta Hortic. Sin. 2020, 47, 1141–1152. [Google Scholar]
- Qiao, D.; Mi, X.; An, Y.; Xie, H.; Cao, K.; Chen, H.; Chen, M.; Liu, S.; Chen, J.; Wei, C. Integrated metabolic phenotypes and gene expression profiles revealed the effect of spreading on aroma volatiles formation in postharvest leaves of green tea. Food Res. Int. 2021, 149, 110680. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kubota, K.; Kobayashi, A. Optical Isomers of methyl jasmonate in tea aroma. J. Agric. Chem. Soc. Jpn. 1996, 60, 508–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, B.; Zhu, Y.; Lv, H.P.; Yan, H.; Peng, Q.H.; Lin, Z. The enantiomeric distributions of volatile constituents in different tea cultivars. Food Chem. 2018, 265, 329–336. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Li, Y.; Wan, X.; Yang, X. Characterization of the orchid-like aroma contributors in selected premium tea leaves. Food Res. Int. 2020, 129, 108841. [Google Scholar] [CrossRef]
- Gonzalez, J.G.; Coggon, P.; Sanoerson, G.W. A research note biochemistry of tea fermentation: Formation of t-2-hexenal from linolenic acid. J. Food Sci. 2010, 37, 797–798. [Google Scholar] [CrossRef]
- Sang, S. Tea: Chemistry and Processing. Encycl. Food Health 2016, 268–272. [Google Scholar] [CrossRef]
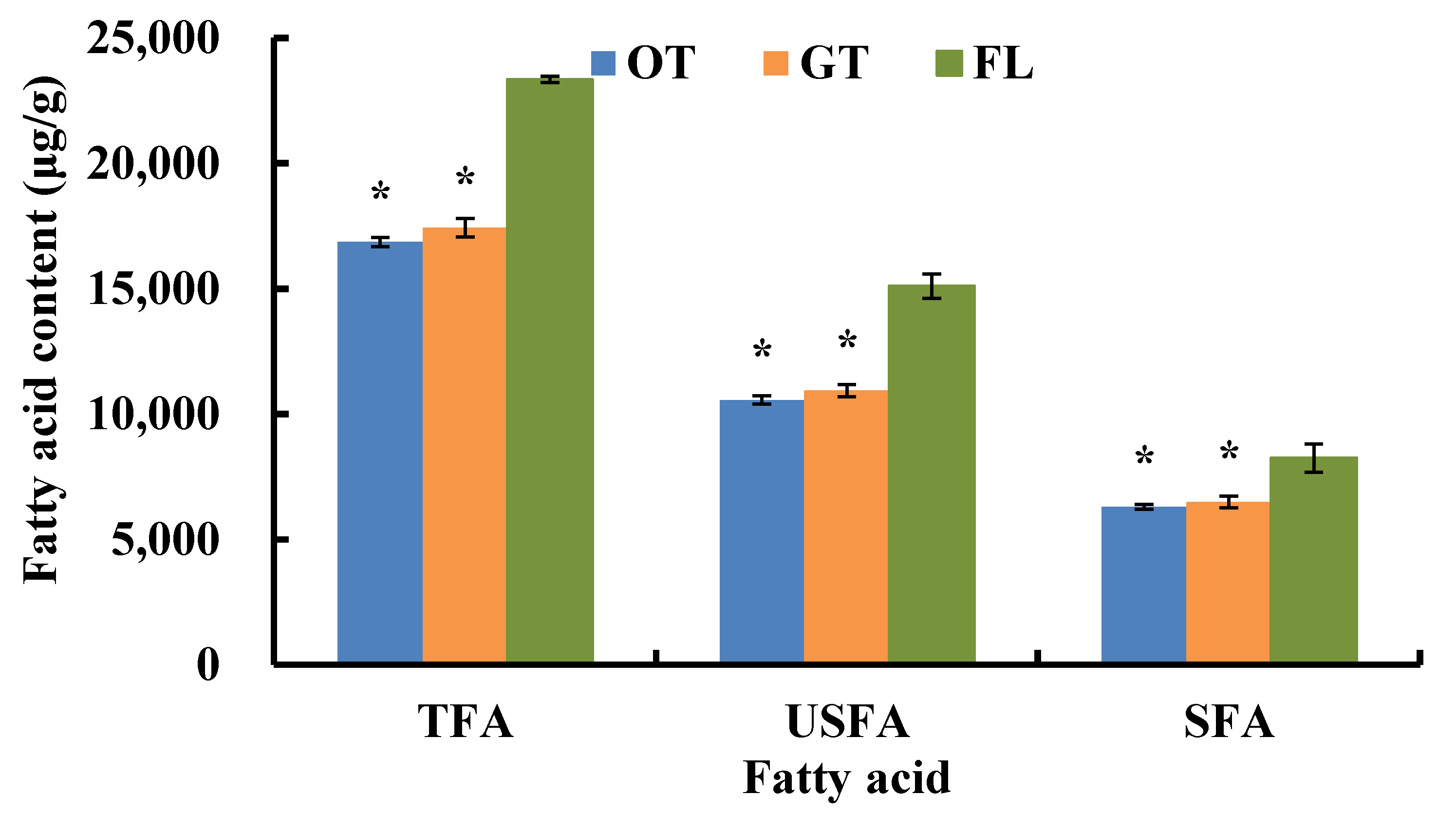
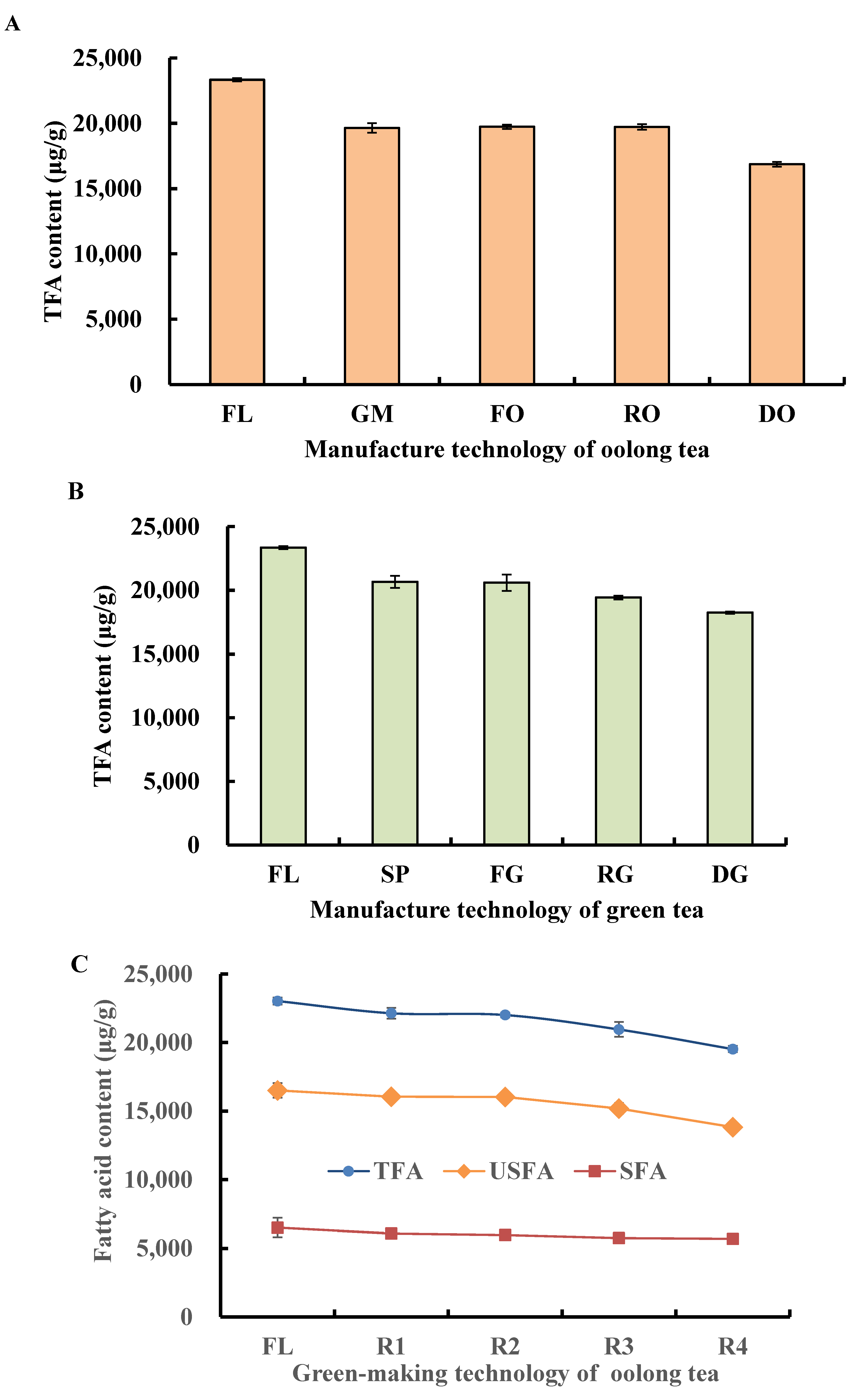
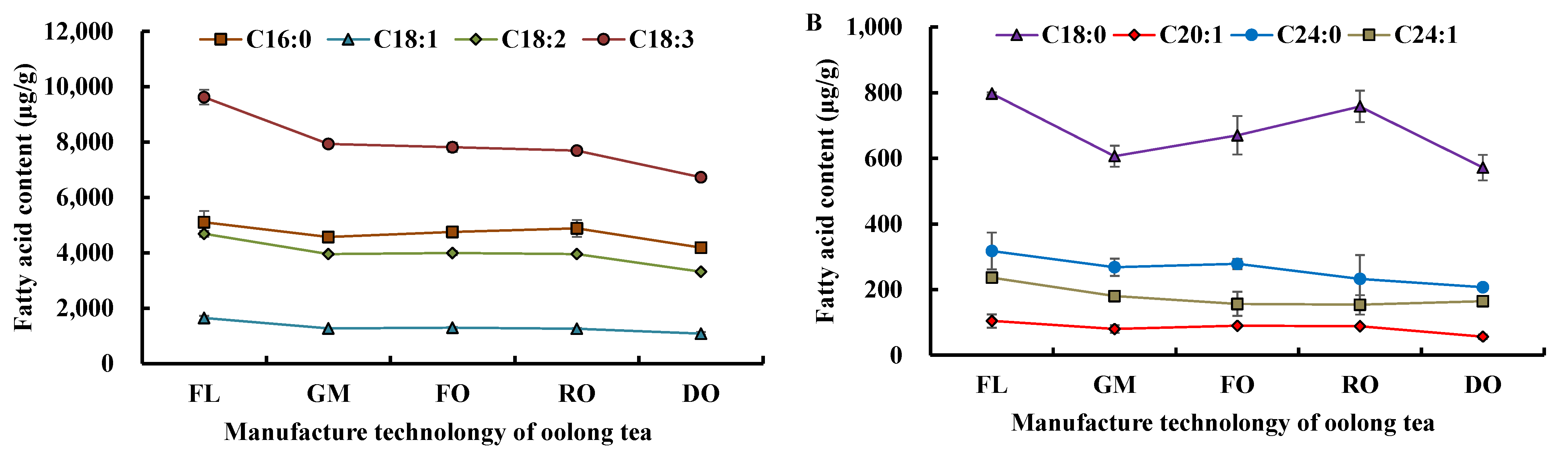
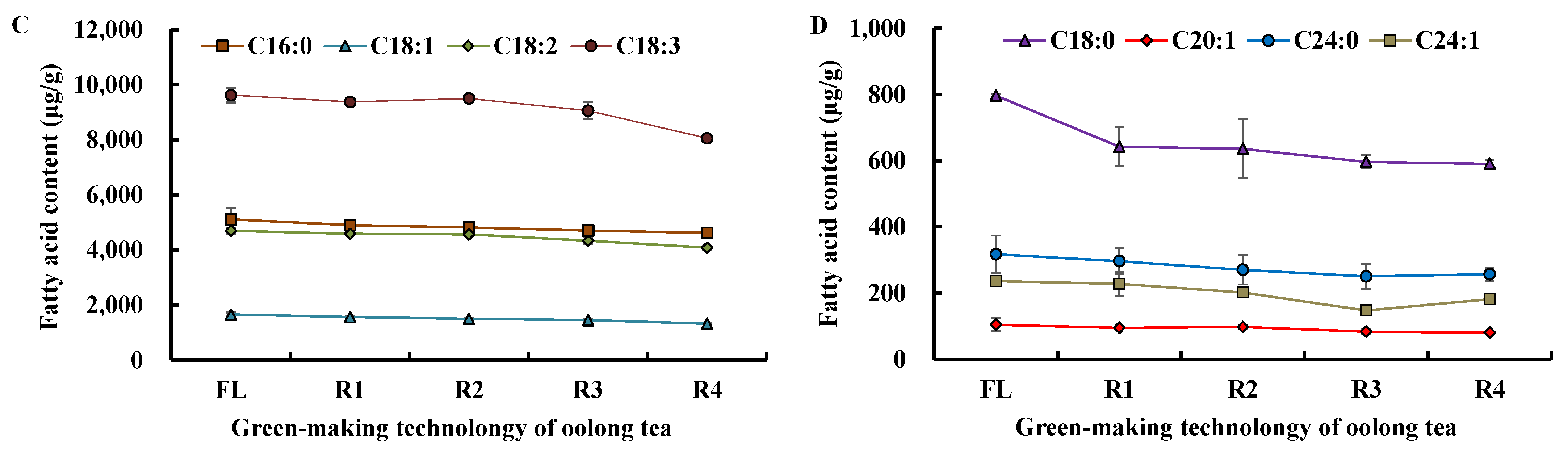
| Fatty Acid (Cm:n) | RT (min) | Fresh Leaves (μg/g) | Oolong Tea (μg/g) | Green Tea (μg/g) |
|---|---|---|---|---|
| C12:0 | 10.412 | 46.1 ± 31.4 | 33.2 ± 6.3 | 20.8 ± 5.7 |
| C14:0 | 13.494 | 53.8 ± 12.7 | 48.3 ± 3.7 | 45.7 ± 1.7 |
| C16:0 | 17.198 | 5105.5 ± 411.4 | 4192.8 ± 15.4 | 4267.4 ± 184.4 |
| C16:1 | 17.854 | 59.2 ± 5.8 | 35.3 ± 2.3 | 188.1 ± 5.0 |
| C18:0 | 21.279 | 796.9 ± 301.5 | 571.5 ± 38.8 | 615.5 ± 85.8 |
| C18:1 | 21.906 | 241.1 ± 24.2 | 188.3 ± 39.7 | 155.1 ± 11.9 |
| C18:2 | 23.009 | 4694.5 ± 111.0 | 3320.1 ± 45.8 | 3410.7 ± 85.5 |
| C18:3 | 24.415 | 9622.6 ± 266.7 | 6734.1 ± 109.2 | 6833.0 ± 189.8 |
| C20:0 | 25.600 | 57.8 ± 13.1 | 51.2 ± 10.7 | 46.9 ± 8.2 |
| C20:1 | 26.320 | 104.5 ± 20.5 | 56.8 ± 23.7 | 72.0 ± 9.7 |
| C20:2 | 27.760 | 38.8 ± 16.7 | 29.1 ± 5.6 | 22.6 ± 10.0 |
| C20:4 | 29.314 | 43.3 ± 26.1 | ND | 54.0 ± 14.2 |
| C20:3 | 29.701 | 57.8 ± 19.5 | 32.3 ± 7.5 | 27.4 ± 22.0 |
| C24:0 | 38.738 | 396.2 ± 143.5 | 207.6 ± 10.0 | 285.2 ± 9.9 |
| C24:1 | 39.838 | 236.3 ± 13.5 | 165.1 ± 6.0 | 173.3 ± 26.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Chen, M.; Guo, Y.; Lin, Z. Variations in Fatty Acids Affected Their Derivative Volatiles during Tieguanyin Tea Processing. Foods 2022, 11, 1563. https://doi.org/10.3390/foods11111563
Guo L, Chen M, Guo Y, Lin Z. Variations in Fatty Acids Affected Their Derivative Volatiles during Tieguanyin Tea Processing. Foods. 2022; 11(11):1563. https://doi.org/10.3390/foods11111563
Chicago/Turabian StyleGuo, Li, Mingjie Chen, Yaling Guo, and Zhi Lin. 2022. "Variations in Fatty Acids Affected Their Derivative Volatiles during Tieguanyin Tea Processing" Foods 11, no. 11: 1563. https://doi.org/10.3390/foods11111563
APA StyleGuo, L., Chen, M., Guo, Y., & Lin, Z. (2022). Variations in Fatty Acids Affected Their Derivative Volatiles during Tieguanyin Tea Processing. Foods, 11(11), 1563. https://doi.org/10.3390/foods11111563







