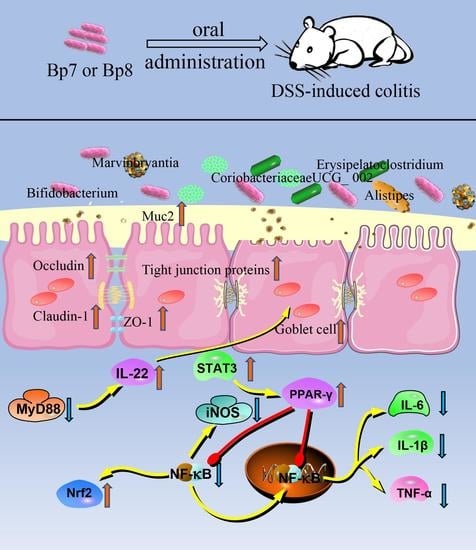
Protective Effects of a Novel Probiotic Bifidobacterium pseudolongum on the Intestinal Barrier of Colitis Mice via Modulating the Pparγ/STAT3 Pathway and Intestinal Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Suspension
2.2. Animal Experiments
2.3. Measurement of Disease Activity Index
2.4. Measurement of Cell Cytokines and Antioxidant System in the Colon
2.5. Histological Analysis
2.6. Immunohistochemical Analysis
2.7. Quantitative RT-PCR
2.8. SCFA Analysis
2.9. Bioinformatics Analysis of 16S rRNA Gene Sequences
2.10. Statistical Analysis
3. Results
3.1. Influence of B. pseudolongum Intervention on Body Weight and Colon Morphology in Colitis Mice
3.2. B. pseudolongum Intervention Relieves Colonic Cell Cytokines and Oxidative Stress in Colitis Mice
3.3. B. pseudolongum Intervention Attenuates Gut Barrier Integrity in Colitis Mice
3.4. B. pseudolongum Intervention Modulates the PPARγ/STAT3 Pathway
3.5. B. pseudolongum Alters Intestinal Microbiota Composition
3.6. Influences of B. pseudolongum on SCFA Levels in Mice with DSS Treatment
3.7. The Associations between Colitis-Related Parameters and Key Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olén, O.; Askling, J.; Sachs, M.C.; Neovius, M.; Smedby, K.E.; Ekbom, A.; Ludvigsson, J.F. Mortality in adult-onset and elderly-onset IBD: A nationwide register-based cohort study 1964–2014. Gut 2020, 69, 453. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Cario, E. Innate immune signalling at intestinal mucosal surfaces: A fine line between host protection and destruction. Curr. Opin. Gastroenterol. 2008, 24, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, K.; Ji, X.; Wang, H.; Zhang, Y. ACE2 suppresses the inflammatory response in LPS-induced porcine intestinal epithelial cells via regulating the NF-κB and MAPK pathways. Peptides 2022, 149, 170717. [Google Scholar] [CrossRef]
- Yuan, Q.; Gu, J.; Zhang, J.; Liu, S.; Wang, Q.; Tian, T.; Chen, Z.; Zhang, J. MyD88 in myofibroblasts enhances colitis-associated tumorigenesis via promoting macrophage M2 polarization. Cell Rep. 2021, 34, 108724. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017, 7, 9523. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, K.; Yu, C.; Wang, L.; Liang, T.; Zhu, H.; Xu, X.; Liu, Y. Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities. Anim. Nutr. 2021, 7, 609–620. [Google Scholar] [CrossRef]
- McLean, L.P.; Cross, R.K. Adverse events in IBD: To stop or continue immune suppressant and biologic treatment. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 223–240. [Google Scholar] [CrossRef] [Green Version]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.C.; Ngan, B.Y.; McKay, D.M.; Soderholm, J.D.; Perdue, M.H.; Sherman, P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Chen, Y.; Wang, G.; Yang, Y.; Song, X.; Xiong, Z.; Zhang, H.; Lai, P.; Wang, S.; Ai, L. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods 2020, 67, 103854. [Google Scholar] [CrossRef]
- Fan, L.; Qi, Y.; Qu, S.; Chen, X.; Li, A.; Hendi, M.; Xu, C.; Wang, L.; Hou, T.; Si, J.; et al. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xiang, Q.; Mao, B.; Tang, X.; Cui, S.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Protective effects of microbiome-derived inosine on lipopolysaccharide-induced acute liver damage and inflammation in mice via mediating the TLR4/NF-κB pathway. J. Agric. Food Chem. 2021, 69, 7619–7628. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, M.; Wang, S.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus ruminis alleviates DSS-induced colitis by inflammatory cytokines and gut microbiota modulation. Foods 2021, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.S.; Pierezan, M.D.; Hassemer, G.S.; Lima, C.M.G.; Lindner, J.D.D.; Miotto, M.; Verruck, S. Effect of prebiotics and synbiotics carried by food over irritable bowel syndrome symptoms: A systematic review. Dairy 2022, 3, 148–162. [Google Scholar] [CrossRef]
- Dendoncker, K.; Timmermans, S.; Vandewalle, J.; Eggermont, M.; Lempiäinen, J.; Paakinaho, V.; Van Hamme, E.; Dewaele, S.; Vandevyver, S.; Ballegeer, M.; et al. TNF-α inhibits glucocorticoid receptor-induced gene expression by reshaping the GR nuclear cofactor profile. Proc. Natl. Acad. Sci. USA 2019, 116, 12942. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, A.J.; Horng, T. IL-6 strikes a balance in metabolic inflammation. Cell Metab. 2014, 19, 898–899. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, Z.; Liu, J.; Zheng, Z.; Li, Q.; Wang, H.; Chen, Z.; Wang, K. Identification of the core active structure of a Dendrobium officinale polysaccharide and its protective effect against dextran sulfate sodium-induced colitis via alleviating gut microbiota dysbiosis. Food Res. Int. 2020, 137, 109641. [Google Scholar] [CrossRef]
- Li, R.; Kim, M.-H.; Sandhu, A.K.; Gao, C.; Gu, L. Muscadine Grape (Vitis rotundifolia) or wine phytochemicals reduce intestinal inflammation in mice with dextran sulfate sodium-induced colitis. J. Agric. Food Chem. 2017, 65, 769–776. [Google Scholar] [CrossRef]
- Guo, T.; Lin, Q.; Li, X.; Nie, Y.; Wang, L.; Shi, L.; Xu, W.; Hu, T.; Guo, T.; Luo, F. Octacosanol attenuates inflammation in both raw264.7 macrophages and a mouse model of colitis. J. Agric. Food Chem. 2017, 65, 3647–3658. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Mahony, C.B.; Petzold, T.; Bordignon, P.; Rougemont, A.-L.; Bertrand, J.Y. A connexin/ifi30 pathway bridges HSCs with their niche to dampen oxidative stress. Nat. Commun. 2021, 12, 4484. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immun. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Pandit, L.; Kolodziejska, K.E.; Zeng, S.; Eissa, N.T. The physiologic aggresome mediates cellular inactivation of iNOS. Proc. Natl. Acad. Sci. USA 2009, 106, 1211. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.P.; Kamra, A.; Kessie, G.; Kalavapudi, S.; Chen, J.H.; Shores, R.; Madairos, L.; Fasano, A.; Nair, P. Markers of inflammation and lineage on exfoliated colonic cells in pediatric inflammatory bowel disease. J. Gastrointest. Dig. Syst. 2011, 8, 1–6. [Google Scholar]
- Menghini, P.; Corridoni, D.; Buttó, L.F.; Osme, A.; Shivaswamy, S.; Lam, M.; Bamias, G.; Pizarro, T.T.; Rodriguez-Palacios, A.; Dinarello, C.A.; et al. Neutralization of IL-1α ameliorates Crohn’s disease-like ileitis by functional alterations of the gut microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 26717. [Google Scholar] [CrossRef]
- Bel, S.; Elkis, Y.; Elifantz, H.; Koren, O.; Ben-Hamo, R.; Lerer-Goldshtein, T.; Rahimi, R.; Ben Horin, S.; Nyska, A.; Shpungin, S.; et al. Reprogrammed and transmissible intestinal microbiota confer diminished susceptibility to induced colitis in TMF-/- mice. Proc. Natl. Acad. Sci. USA 2014, 111, 4964. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, J.; Liu, W.; Zhang, H.; Sun, Z. Metagenomic and metatranscriptomic profiling of Lactobacillus casei Zhang in the human gut. NPJ Biofilms Microbiomes 2021, 7, 55. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Q.; Mi, X.; Qiu, L.; Tao, X.; Zhang, Z.; Xia, J.; Wu, Q.; Wei, H. Ripened pu-erh tea extract promotes gut microbiota resilience against dextran sulfate sodium induced colitis. J. Agric. Food Chem. 2021, 69, 2190–2203. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Zhao, C.; Yang, M.; Zhang, L.; Wei, R.; Meng, K.; Bao, Y.; Zhang, L.; Zheng, J. Four citrus flavanones exert atherosclerosis alleviation effects in ApoE–/– mice via different metabolic and signaling pathways. J. Agric. Food Chem. 2021, 69, 5226–5237. [Google Scholar] [CrossRef]
- Han, R.; Ma, Y.; Xiao, J.; You, L.; Pedisić, S.; Liao, L. The possible mechanism of the protective effect of a sulfated polysaccharide from Gracilaria Lemaneiformis against colitis induced by dextran sulfate sodium in mice. Food Chem. Toxicol. 2021, 149, 112001. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.K.; Xiao, M. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, S.; Xu, Z.; Su, H.; Tian, X.; Han, B.; Shen, B.; Liao, Q.; Xie, Z.; Hong, Y. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res. Int. 2021, 147, 110569. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Mao, B.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H. Protective Effects of a Novel Probiotic Bifidobacterium pseudolongum on the Intestinal Barrier of Colitis Mice via Modulating the Pparγ/STAT3 Pathway and Intestinal Microbiota. Foods 2022, 11, 1551. https://doi.org/10.3390/foods11111551
Guo W, Mao B, Cui S, Tang X, Zhang Q, Zhao J, Zhang H. Protective Effects of a Novel Probiotic Bifidobacterium pseudolongum on the Intestinal Barrier of Colitis Mice via Modulating the Pparγ/STAT3 Pathway and Intestinal Microbiota. Foods. 2022; 11(11):1551. https://doi.org/10.3390/foods11111551
Chicago/Turabian StyleGuo, Weiling, Bingyong Mao, Shumao Cui, Xin Tang, Qiuxiang Zhang, Jianxin Zhao, and Hao Zhang. 2022. "Protective Effects of a Novel Probiotic Bifidobacterium pseudolongum on the Intestinal Barrier of Colitis Mice via Modulating the Pparγ/STAT3 Pathway and Intestinal Microbiota" Foods 11, no. 11: 1551. https://doi.org/10.3390/foods11111551