Preparation of Core-Shell Rare Earth-Doped Upconversion Nanomaterials and Simultaneous Detection of Two Pesticides in Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Upconversion Nanomaterials
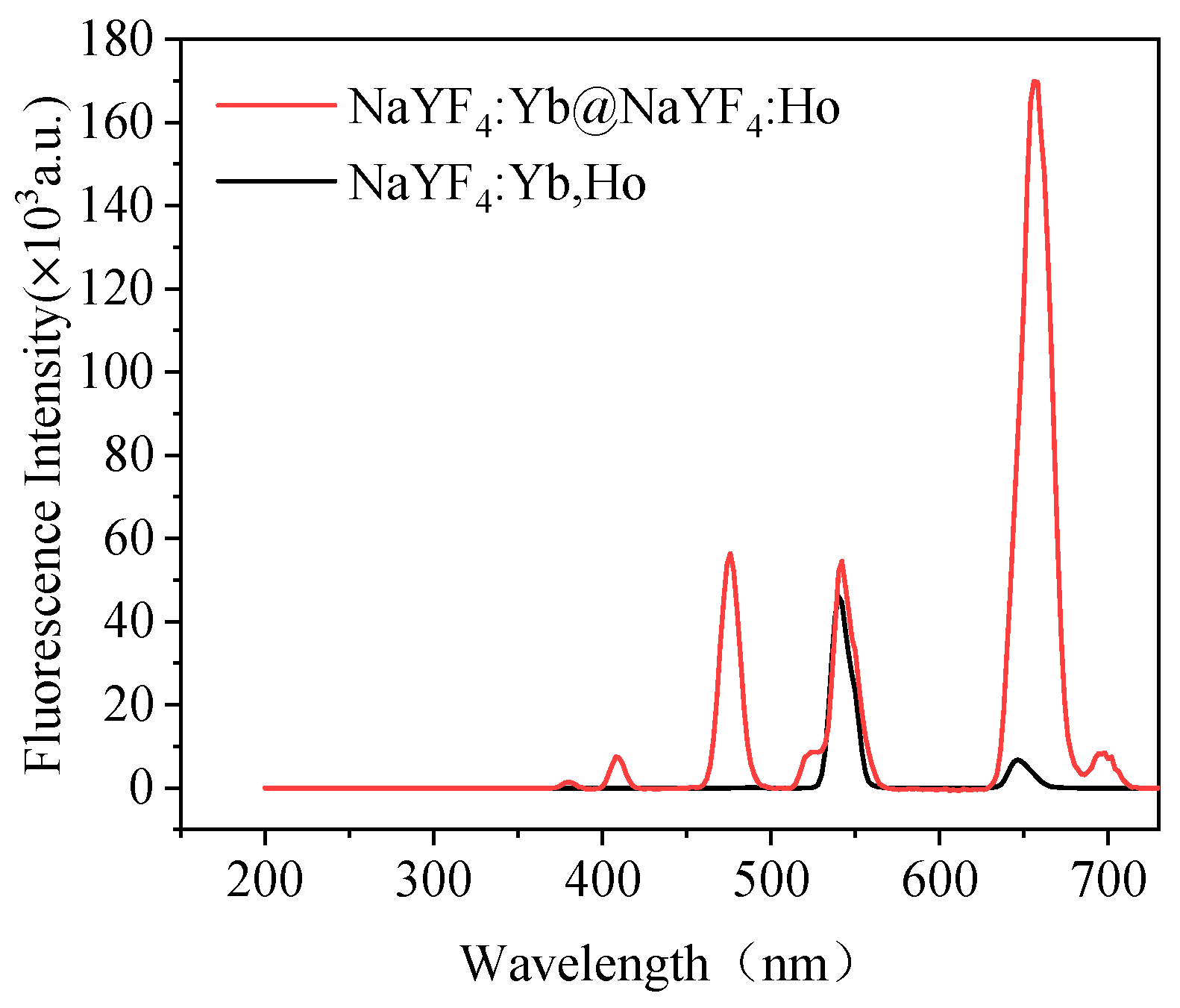
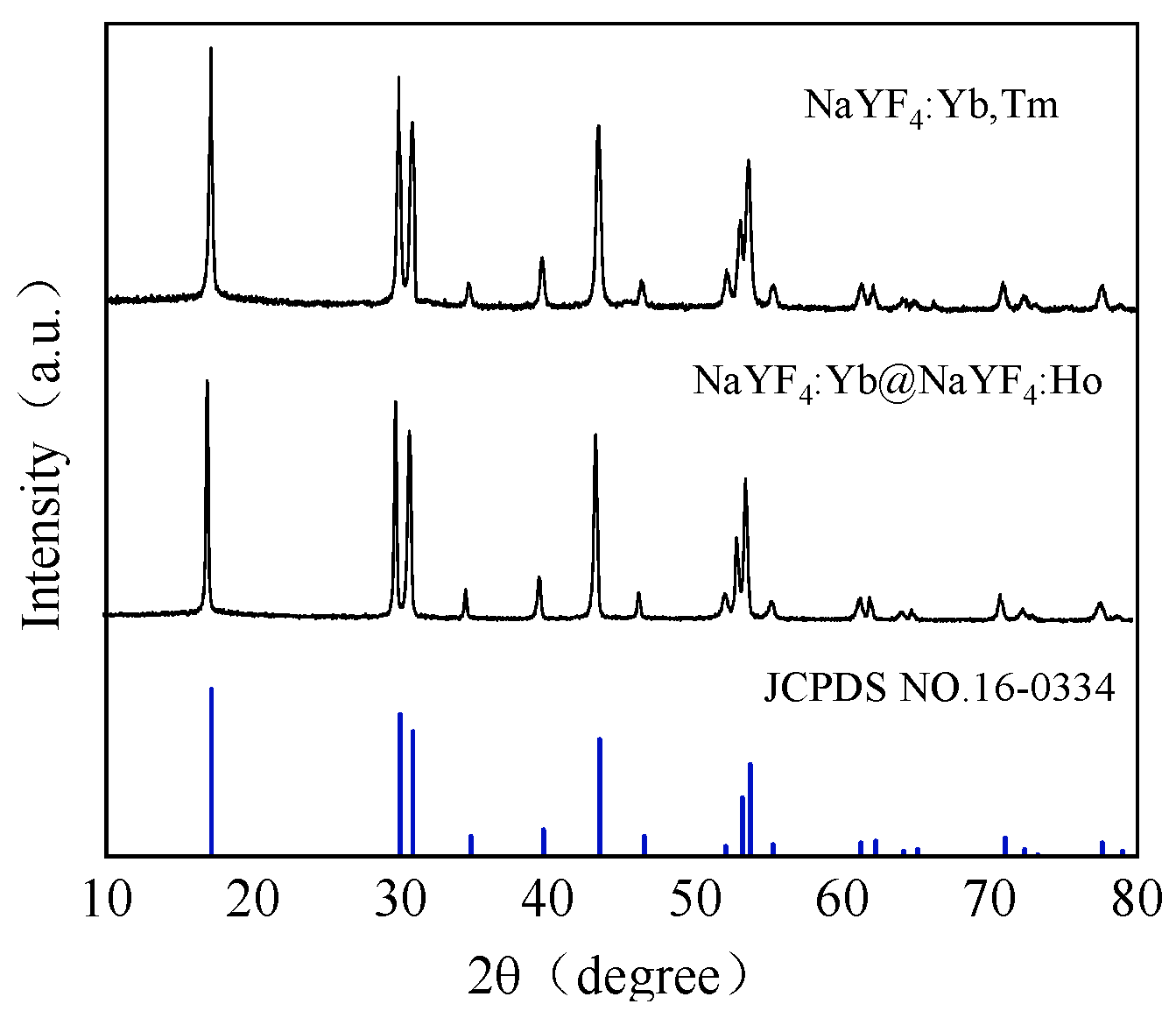
2.2.1. Preparation of NaYF4:Yb@NaYF4:Ho UCNPs
2.2.2. Preparation of NaYF4:Yb,Tm UCNPs
2.2.3. Preparation of NaYF4:Yb,Ho UCNPs
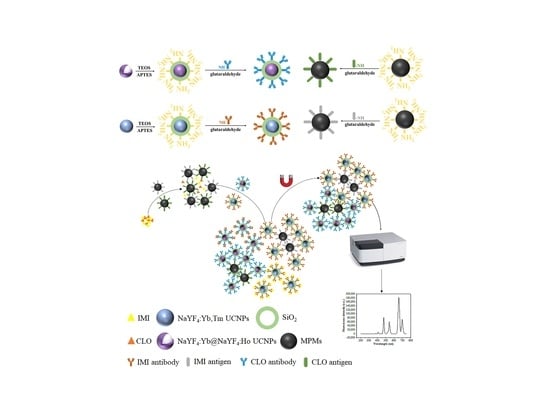
2.3. Surface Modification of Upconversion Nanomaterials
2.4. Preparation of Capture Probes and Signal Probes
2.5. Establishment of Fluorescence Immunoassay
2.6. Specificity
2.7. Sample Handling
2.8. Recovery Experiment
2.9. HPLC Comparison Test
2.10. Actual Sample Detection
3. Results and Discussion
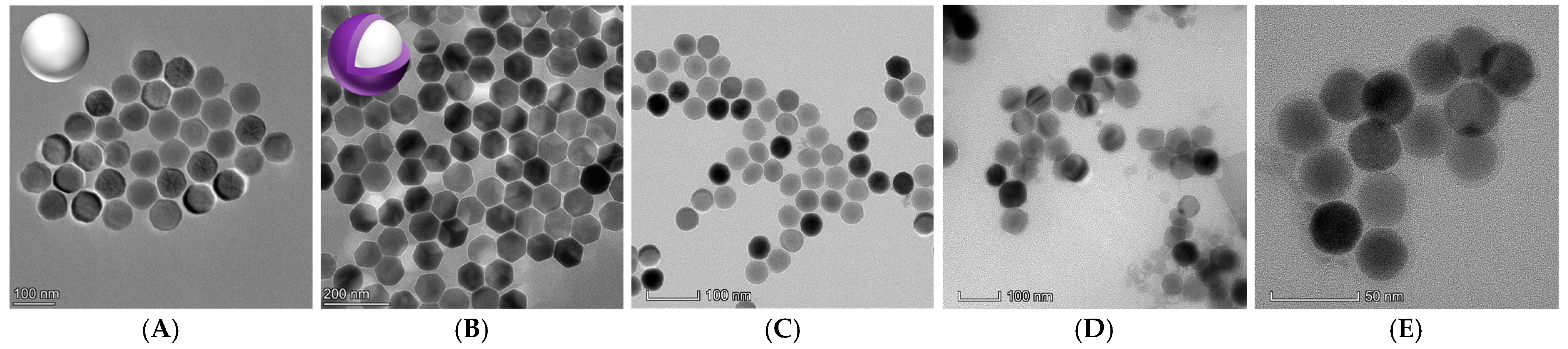
3.1. Characterization of the Prepared UCNPs
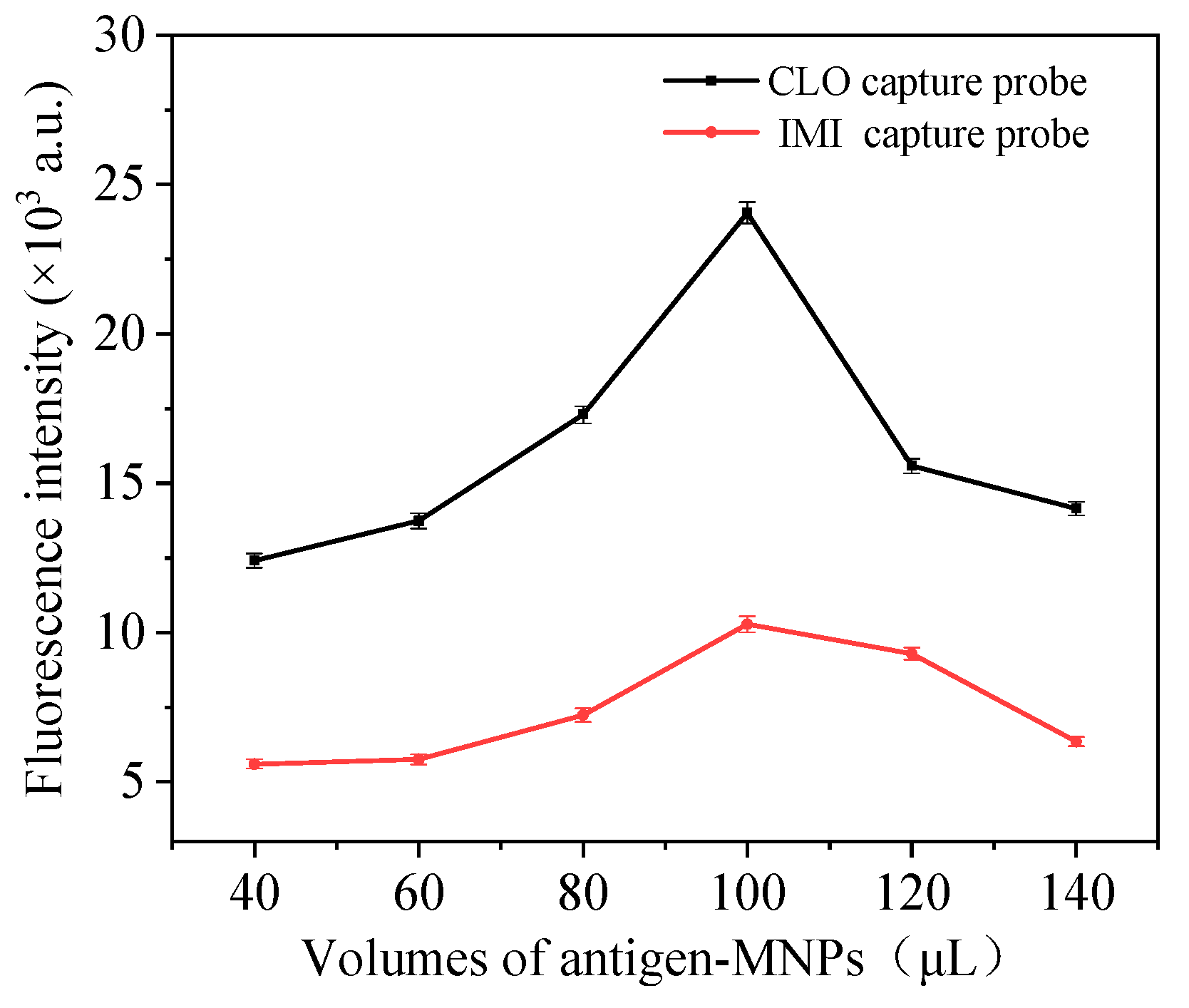
3.2. Optimize Probe Preparation Conditions
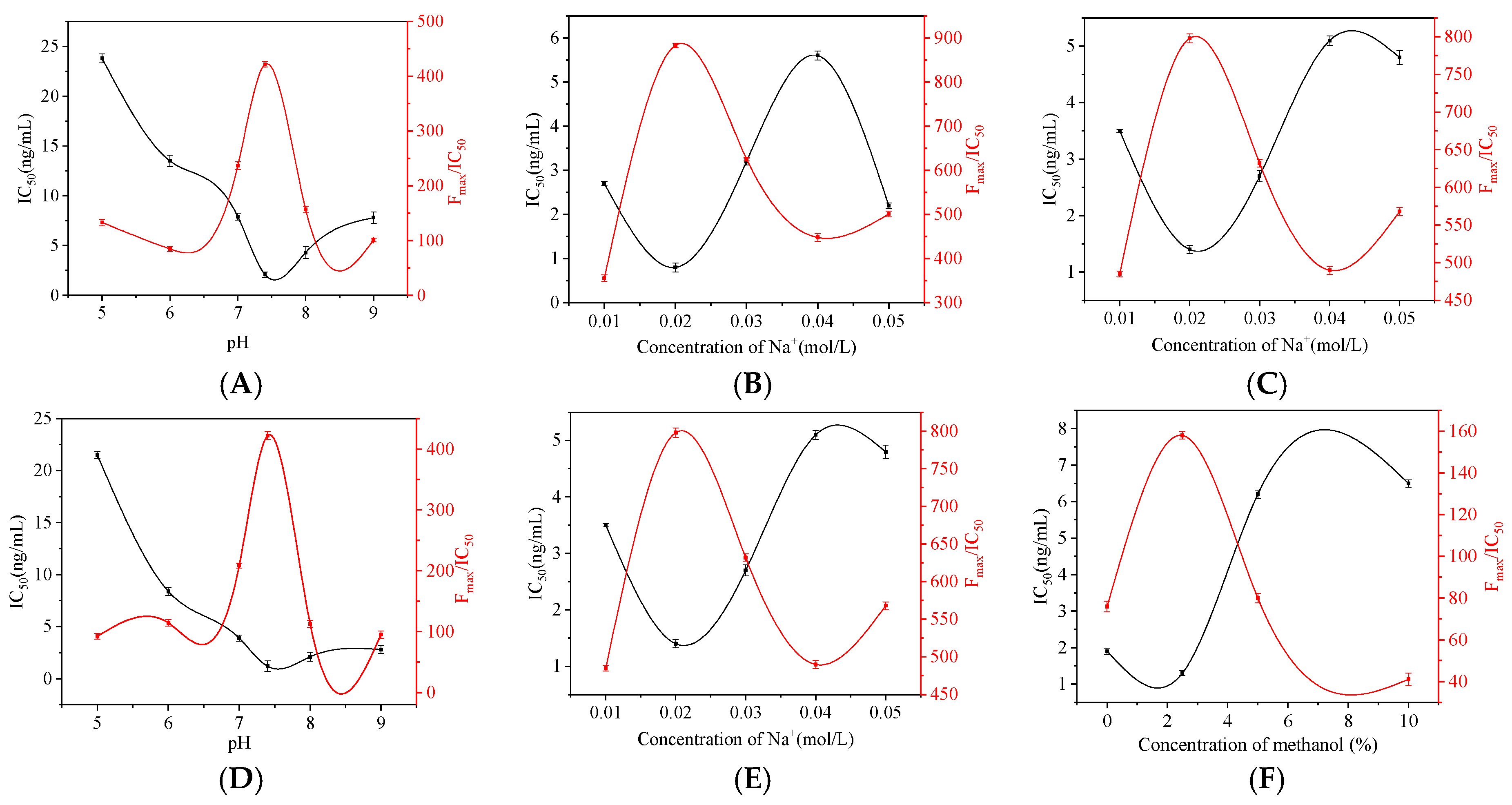
3.3. Optimization of Method Conditions
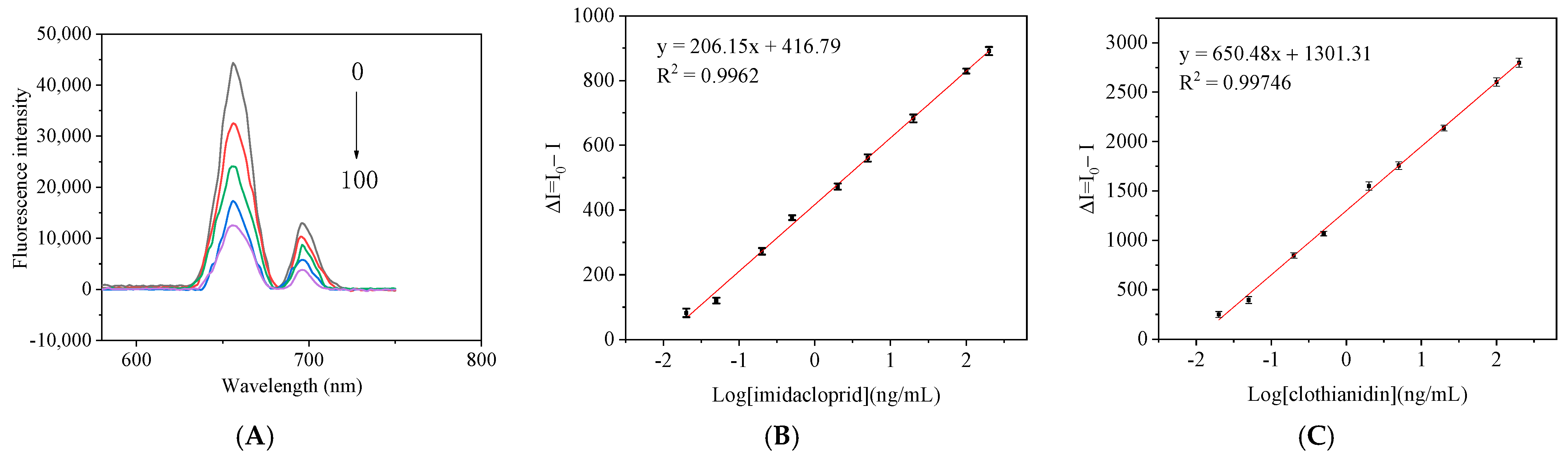
3.4. Determination of the Detection Limit of the Method
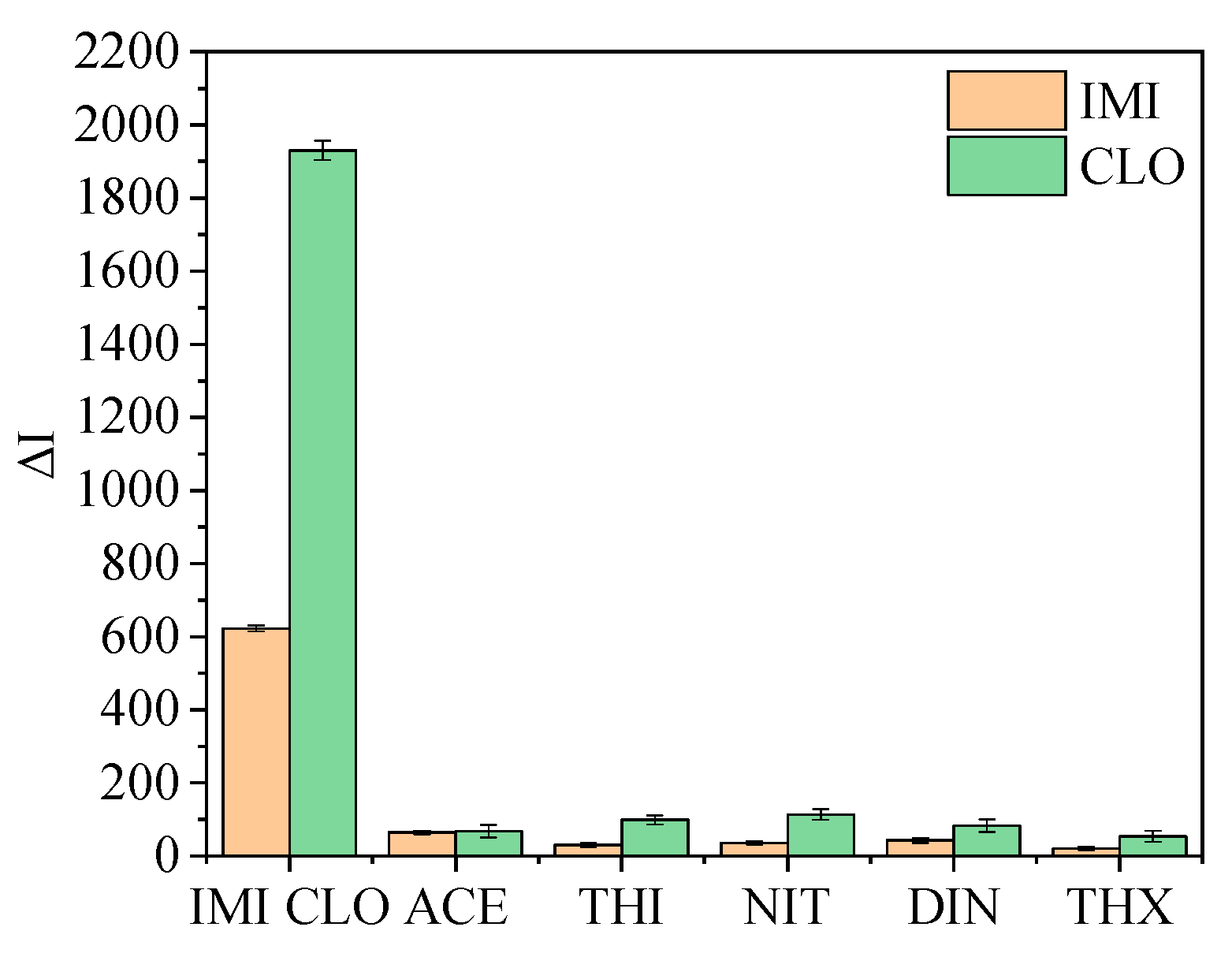
3.5. Specificity
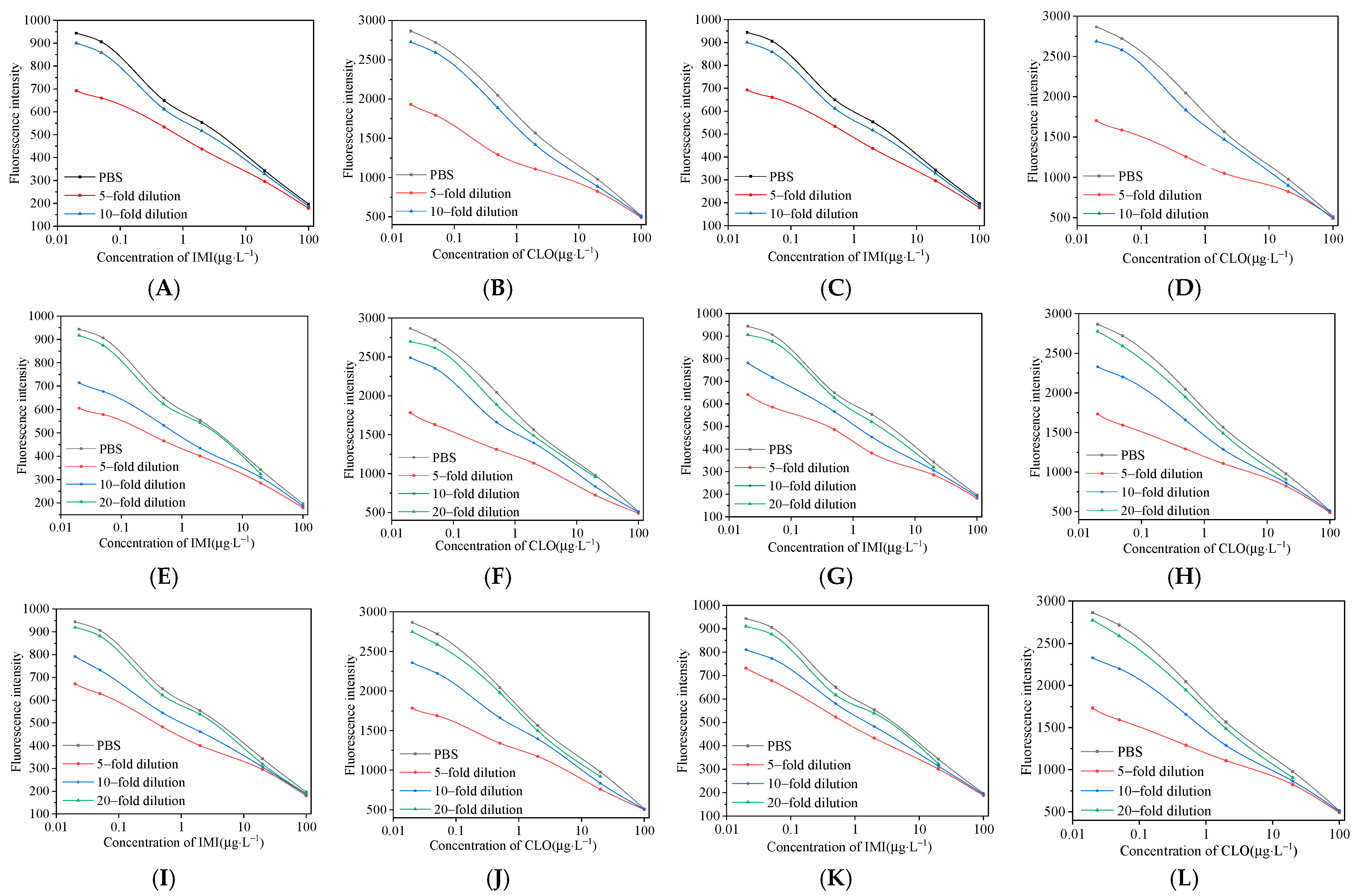
3.6. Elimination of Matrix Effects
3.7. Spiked Recovery Experimental Results
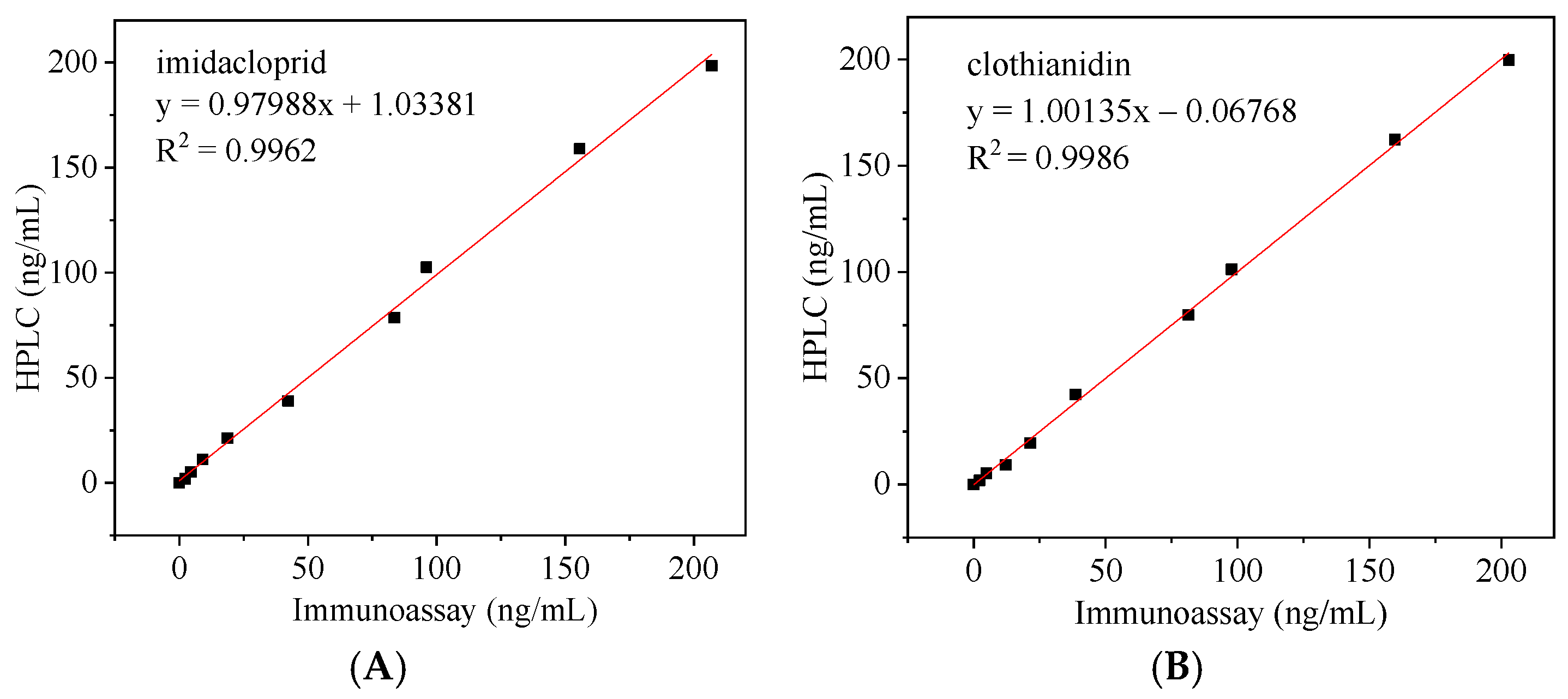
3.8. Correlation of Immunoassays with HPLC
3.9. Test Results of Actual Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez-Lopez, J.; Llorent-Martinez, E.J.; Ortega-Barrales, P.; Ruiz-Medina, A. Analysis of neonicotinoid pesticides in the agri-food sector: A critical assessment of the state of the art. Appl. Spectrosc. Rev. 2020, 55, 613–646. [Google Scholar] [CrossRef]
- Peter, J.; Ralf, N.; Michael, S.; Alfred, E. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar]
- Van der Sluijs, J.P.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.M.; Belzunces, L.P. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Sultana, T.; Murray, C.; Kleywegt, S.; Metcalfe, C.D. Neonicotinoid pesticides in drinking water in agricultural regions of southern Ontario, Canada. Chemosphere 2018, 202, 506–513. [Google Scholar] [CrossRef]
- Longing, S.D.; Peterson, E.M.; Jewett, C.T.; Rendon, B.M.; Discua, S.A.; Wooten, K.J.; Subbiah, S.; Smith, P.N.; McIntyre, N.E. Exposure of Foraging Bees (Hymenoptera) to Neonicotinoids in the U.S. Southern High Plains. Environ. Entomol. 2020, 49, 528–535. [Google Scholar] [CrossRef]
- Bishop, C.A.; Woundneh, M.B.; Maisonneuve, F.; Common, J.; Elliott, J.E.; Moran, A.J. Determination of neonicotinoids and butenolide residues in avian and insect pollinators and their ambient environment in Western Canada (2017, 2018). Sci. Total Environ. 2020, 737, 139386–139429. [Google Scholar] [CrossRef]
- Maria, P.; Alzbeta, S.; Valbona, A.; Caterina, F. Impact of Neonicotinoids to Aquatic Invertebrates—In Vitro Studies on Mytilus galloprovincialis: A Review. J. Mar. Sci. Eng. 2020, 8, 801. [Google Scholar]
- Chen, Z.L.; Dong, F.S.; Ren, X.; Wu, X.J.; Yuan, L.F.; Li, L.; Li, W.; Zheng, Y.Q. Enantioselective fate of dinotefuran from tomato cultivation to home canning for refining dietary exposure. J. Hazard. Mater. 2021, 405, 124254. [Google Scholar] [CrossRef]
- Mikolic, A.; Karaconji, I.B. Imidacloprid as reproductive toxicant and endocrine disruptor: Investigations in laboratory animals. Arch. Ind. Hyg. Toxicol. 2018, 69, 103. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.Y.; Huang, L.M.; Xiao, Y.Z.; Chao, L.; Jia, W.; Fan, L.Y.; Wang, S.; Zhao, Y.H. Bio-uptake, tissue distribution and metabolism of a neonicotinoid insecticide clothianidin in zebrafish. Environ. Pollut. 2021, 292, 118317. [Google Scholar]
- GB 2763-2021. National Food Safety Standard Maximum Residue Limit of Pesticides in Food. Available online: http://down.foodmate.net/wap/index.php?itemid=97819&moduleid=23 (accessed on 31 March 2022).
- Himdata, A.; Maria, A.; Alba, B.; Daniela, B.; Luis, C.C.; Chloe, D.L.; Lucien, F.; Luna, G.; Samira, J.; Dimitra, K.; et al. Review of the existing maximum residue levels for imidacloprid according to Article 12 of Regulation (EC) No 396/2005. Eur. Food Saf. Auth. 2019, 17, 5570. [Google Scholar]
- European Food Safety Authority. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for clothianidin and thiamethoxam according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2014, 12, 3918. [Google Scholar]
- Erickson, B. Neonicotinoids can stay in US market. Chem. Eng. News 2020, 98, 17. [Google Scholar] [CrossRef]
- Science—Analytical Science. Studies from Agriculture and Agri-Food Canada Update Current Data on Analytical Science (High-Throughput Quantitation of Neonicotinoids in Lyophilized Surface Water by LC-APCI-MS/MS). Sci. Lett. 2018. Available online: https://schlr.cnki.net/en/Detail/index/GARJ2018/SPQD29D2BA7ABBA867452A99D741578592D8 (accessed on 31 March 2022).
- Hem, L.; Abd El-Aty, A.M.; Park, J.H.; Shim, J.H. Determination of dinotefuran in pepper using liquid chromatography: Contribution to safety evaluation. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 765–768. [Google Scholar] [CrossRef]
- Rok, T.; David, H.; Ester, H.; Jernej, M.; Andreja, K.B.; Helena, P. Determination of Neonicotinoid Pesticides in Propolis with Liquid Chromatography Coupled to Tandem Mass Spectrometry. Molecules 2020, 25, 5870. [Google Scholar]
- Watanabe, E.K.; Miyake, S. Direct determination of neonicotinoid insecticides in an analytically challenging crop such as Chinese chives using selective ELISAs. J. Environ. Sci. Health 2018, 53, 707–712. [Google Scholar] [CrossRef]
- Li, D.P.; Gao, Y.J.; Ren, S.M.; Gao, H. Detection of pesticide residues in Honeysuckle by QEChERS extraction and LC-MS/MS technology. Guangdong Chem. Ind. 2021, 48, 245–248. [Google Scholar]
- Hua, X.D.; You, H.J.; Luo, P.W.; Tao, Z.X.; Chen, H.; Liu, F.Q.; Wang, M.H. Upconversion fluorescence immunoassay for imidaclothiz by magnetic nanoparticle separation. Anal. Bioanal. Chem. 2017, 409, 6885–6892. [Google Scholar] [CrossRef]
- Kang, D.K.; Jeon, E.; Kim, S.; Lee, J. Lanthanide-Doped Upconversion Nanomaterials: Recent Advances and Applications. BioChip J. 2020, 14, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Kumar, K.R.; Liu, C.G.; Wang, S.B.; Chen, A.Z.; Zhang, Y. Lanthanides-doped near-infrared active upconversion nanocrystals: Upconversion mechanisms and synthesis. Coord. Chem. Rev. 2021, 438, 17. [Google Scholar] [CrossRef]
- Damien, H.; Howard, I.A.; Popescu, R.; Dagmar, G.; Richards, B.S. Structure-Property Relationships in Lanthanide-Doped Upconverting Nanocrystals: Recent Advances in Understanding Core-Shell Structures. Adv. Mater. 2019, 31, 25. [Google Scholar]
- Ye, S.; Chen, G.Y.; Shao, W.; Qu, J.L.; Prasad, P.N. Tuning upconversion through a sensitizer/activator-isolated NaYF₄ core/shell structure. Nanoscale 2015, 7, 3976–3984. [Google Scholar] [CrossRef]
- Ye, S.; Song, J.; Wang, D.; Tian, Y.L.; Qu, J.L.; Niu, H.B. Reduced photon quenching in Ce-doped NaYF4:Yb/Ho upconversion nanoparticles with core/shell structure. Chin. Opt. Lett. 2016, 14, 67–71. [Google Scholar]
- Zhang, R. Two Color Upconversion NaYF4:Yb3+/Ln3+ (Ln = Er, Tm) Nanorods and Their Anti-Counterfeiting Applications. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2021. [Google Scholar]
- GBT 20769-2008. Determination of 450 Pesticides and Related Chemicals Residues in Fruits and Vegetables—LC-MS-MS Method. Available online: https://max.book118.com/html/2019/0107/7052156036002000.shtm (accessed on 31 March 2022).
- Tao, Z.X. Study on Immunoassay of Imidacloprid and Thiacloprid Based on Nano Materials. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2019. [Google Scholar]



| Sample | Scalar (μg/kg) | IMI | CLO | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (μg/kg) | Recovery Rate (%) | Coefficient of Variation (%) | Mean ± SD (μg/kg) | Recovery Rate (%) | Coefficient of Variation (%) | ||
| pepper | 2 | 1.71 ± 0.15 | 85.34 | 8.6 | 1.99 ± 0.18 | 99.28 | 9.0 |
| 20 | 20.72 ± 1.43 | 103.58 | 6.9 | 21.33 ± 1.30 | 106.64 | 6.1 | |
| 200 | 182.50 ± 7.85 | 91.25 | 4.3 | 168.98 ± 6.08 | 84.49 | 3.6 | |
| cucumber | 2 | 1.90 ± 0.17 | 95.10 | 9.2 | 1.93 ± 0.17 | 96.27 | 8.9 |
| 20 | 17.98 ± 0.91 | 89.89 | 5.1 | 20.55 ± 0.97 | 102.75 | 4.7 | |
| 200 | 180.47 ± 3.43 | 90.24 | 1.9 | 175.94 ± 4.57 | 87.97 | 2.6 | |
| tomato | 5 | 4.29 ± 0.35 | 85.74 | 8.1 | 5.05 ± 0.35 | 100.93 | 6.9 |
| 50 | 47.11 ± 2.12 | 94.23 | 4.5 | 43.62 ± 1.57 | 87.24 | 3.6 | |
| 500 | 480.43 ± 11.53 | 96.09 | 2.4 | 444.02 ± 7.99 | 88.80 | 1.8 | |
| orange | 5 | 4.17 ± 0.30 | 83.33 | 7.2 | 4.65 ± 0.35 | 92.94 | 7.5 |
| 50 | 50.15 ± 2.81 | 100.31 | 5.6 | 50.38 ± 1.66 | 100.76 | 3.3 | |
| 500 | 425.36 ± 13.19 | 85.07 | 3.1 | 476.60 ± 7.63 | 95.32 | 1.6 | |
| peach | 5 | 4.31 ± 0.29 | 86.12 | 6.8 | 4.37 ± 0.27 | 87.48 | 6.2 |
| 50 | 47.96 ± 1.97 | 95.92 | 4.1 | 49.18 ± 1.67 | 98.36 | 3.4 | |
| 500 | 570.60 ± 15.41 | 114.12 | 2.7 | 489.07 ± 9.78 | 97.81 | 2.0 | |
| apple | 5 | 4.40 ± 0.33 | 87.97 | 7.5 | 4.37 ± 0.34 | 87.36 | 7.7 |
| 50 | 55.96 ± 2.80 | 111.91 | 5.0 | 49.69 ± 1.89 | 99.38 | 3.8 | |
| 500 | 575.08 ± 19.55 | 115.02 | 3.4 | 522.91 ± 6.27 | 104.58 | 1.2 | |
| Sample Serial Number | This Experimental Method | HPLC | ||
|---|---|---|---|---|
| IMI (ng/mL) | CLO (ng/mL) | IMI (ng/mL) | CLO (ng/mL) | |
| 1#pepper | - | - | - | - |
| 2#pepper | - | - | - | - |
| 3#pepper | 22.7 | - | 23.2 | - |
| 4#cucumber | - | 82.4 | - | 85.2 |
| 5#cucumber | - | - | - | - |
| 6#cucumber | 61.8 | 103.4 | 63.7 | 101.1 |
| 7#tomato | 92.1 | - | 94.8 | - |
| 8#tomato | - | - | - | - |
| 9#tomato | - | - | - | - |
| 10#orange | - | 19.2 | - | 20.3 |
| 11#orange | 136.4 | - | 132.8 | - |
| 12#orange | - | - | - | - |
| 13#peach | 31.6 | 54.7 | 30.2 | 55.8 |
| 14#peach | - | - | - | - |
| 15#peach | - | - | - | - |
| 16#apple | - | 36.7 | - | 38.1 |
| 17#apple | 48.7 | - | 47.9 | - |
| 18#apple | - | 112.3 | - | 114.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Zhao, L.; Jin, J.; Song, Y. Preparation of Core-Shell Rare Earth-Doped Upconversion Nanomaterials and Simultaneous Detection of Two Pesticides in Food. Foods 2022, 11, 1485. https://doi.org/10.3390/foods11101485
Zhu W, Zhao L, Jin J, Song Y. Preparation of Core-Shell Rare Earth-Doped Upconversion Nanomaterials and Simultaneous Detection of Two Pesticides in Food. Foods. 2022; 11(10):1485. https://doi.org/10.3390/foods11101485
Chicago/Turabian StyleZhu, Wenbo, Lingyan Zhao, Jingyi Jin, and Yang Song. 2022. "Preparation of Core-Shell Rare Earth-Doped Upconversion Nanomaterials and Simultaneous Detection of Two Pesticides in Food" Foods 11, no. 10: 1485. https://doi.org/10.3390/foods11101485
APA StyleZhu, W., Zhao, L., Jin, J., & Song, Y. (2022). Preparation of Core-Shell Rare Earth-Doped Upconversion Nanomaterials and Simultaneous Detection of Two Pesticides in Food. Foods, 11(10), 1485. https://doi.org/10.3390/foods11101485