Preparation of Magnetic Metal-Organic Frameworks@Molecularly Imprinted Nanoparticles for Specific Extraction and Enrichment of Bisphenol A in Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
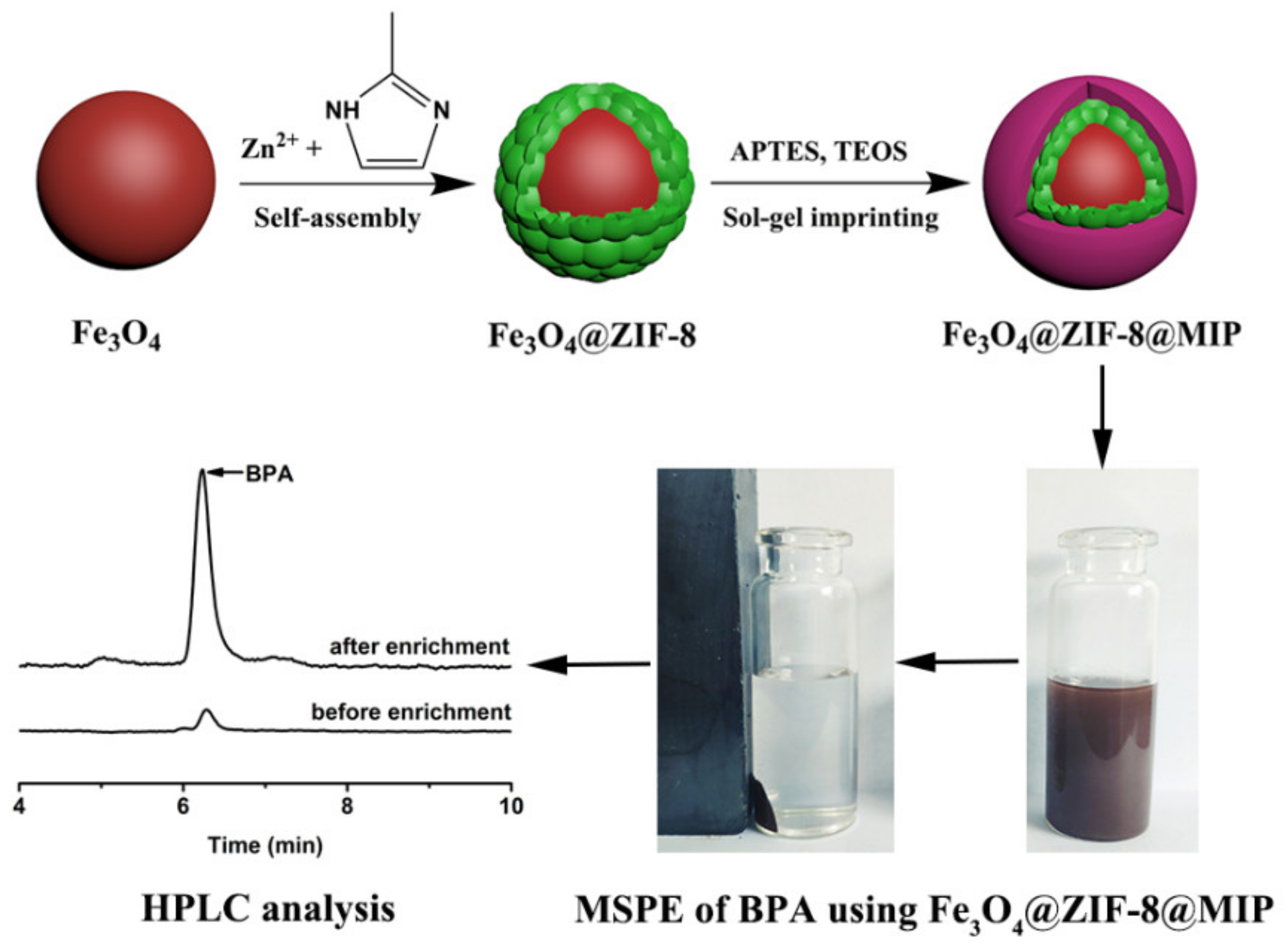
2.2. Preparation of Core–Shell MMOF@MIP (Fe3O4@ZIF-8@MIP)
2.3. Extraction and Detection of BPA in Real Samples
2.3.1. Sample Pretreatment
2.3.2. MSPE of BPA from Samples Using MMOF@MIP
2.3.3. HPLC Analysis
3. Results and Discussion
3.1. Construction of Core–Shell Fe3O4@ZIF-8@MIP
3.2. Characterization of Fe3O4@ZIF-8@MIP
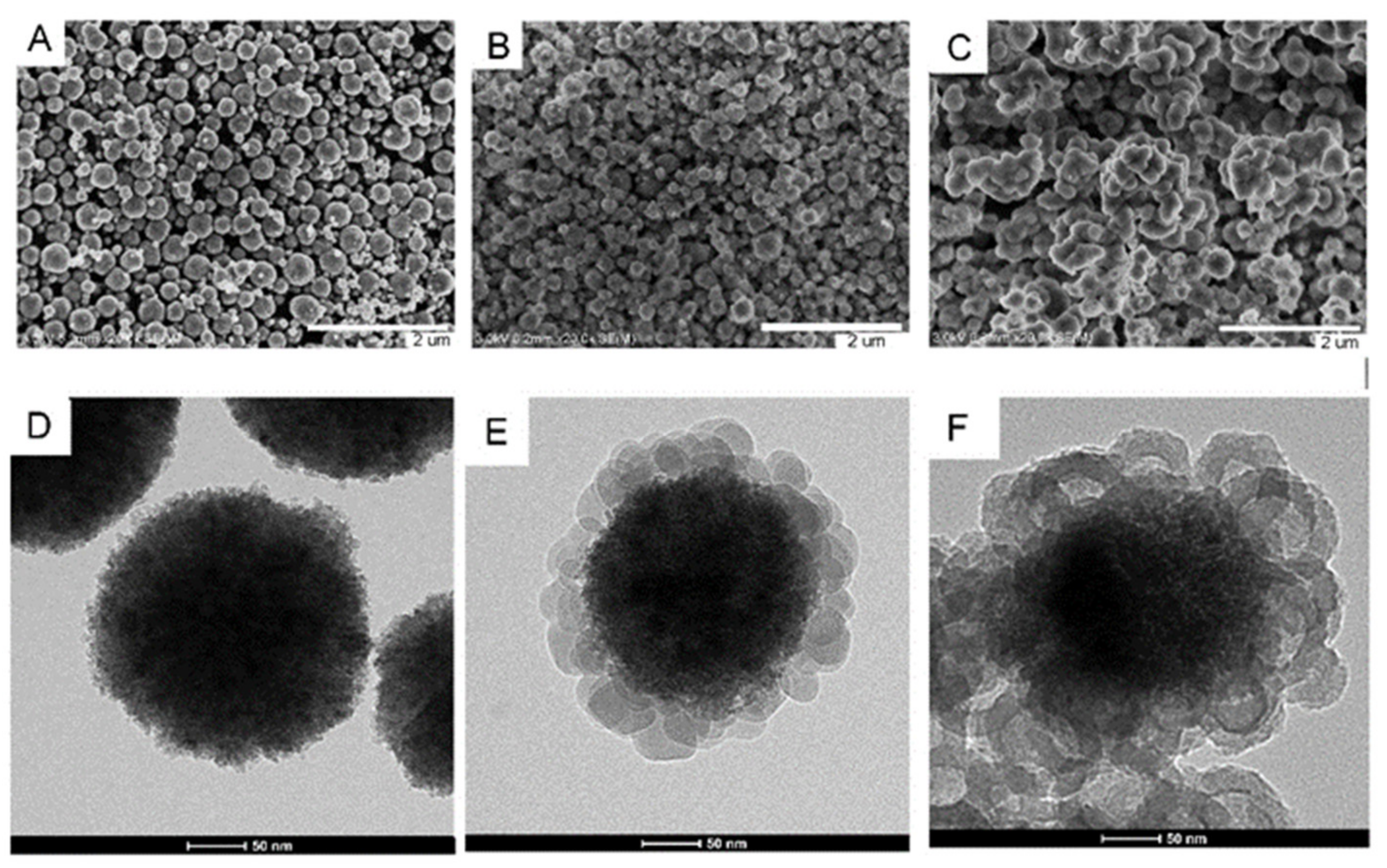
3.2.1. SEM and TEM Characterization
3.2.2. FT−IR Measurements
3.2.3. VSM Analysis
3.2.4. XRD Analysis
3.2.5. TG Analysis
3.2.6. BET Measurements
3.3. Adsorption Behaviors of MMOFs@MIP for BPA
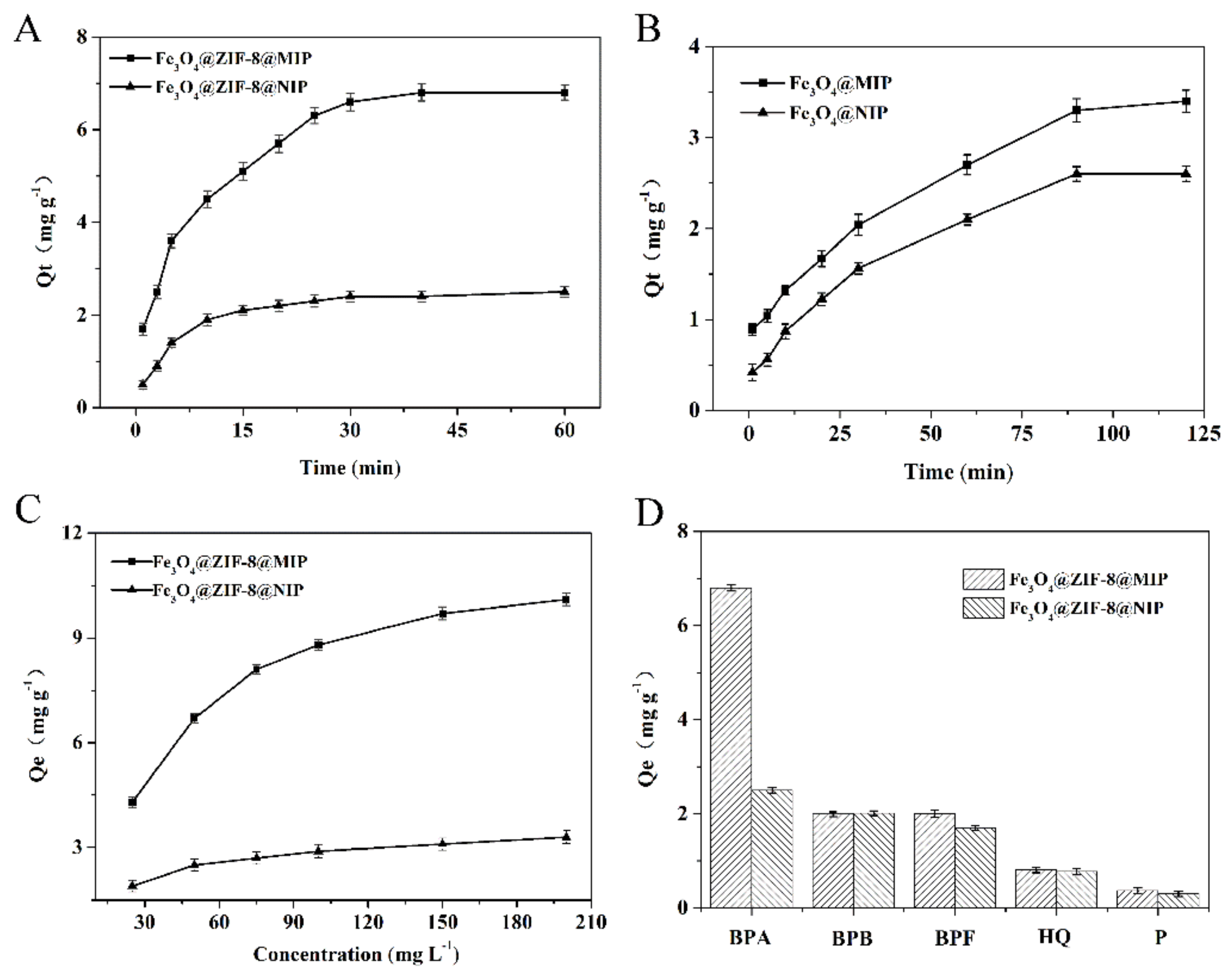
3.3.1. Adsorption Kinetic Experiment
3.3.2. Equilibrium Binding Experiment
3.3.3. The Selectivity Evaluation for BPA
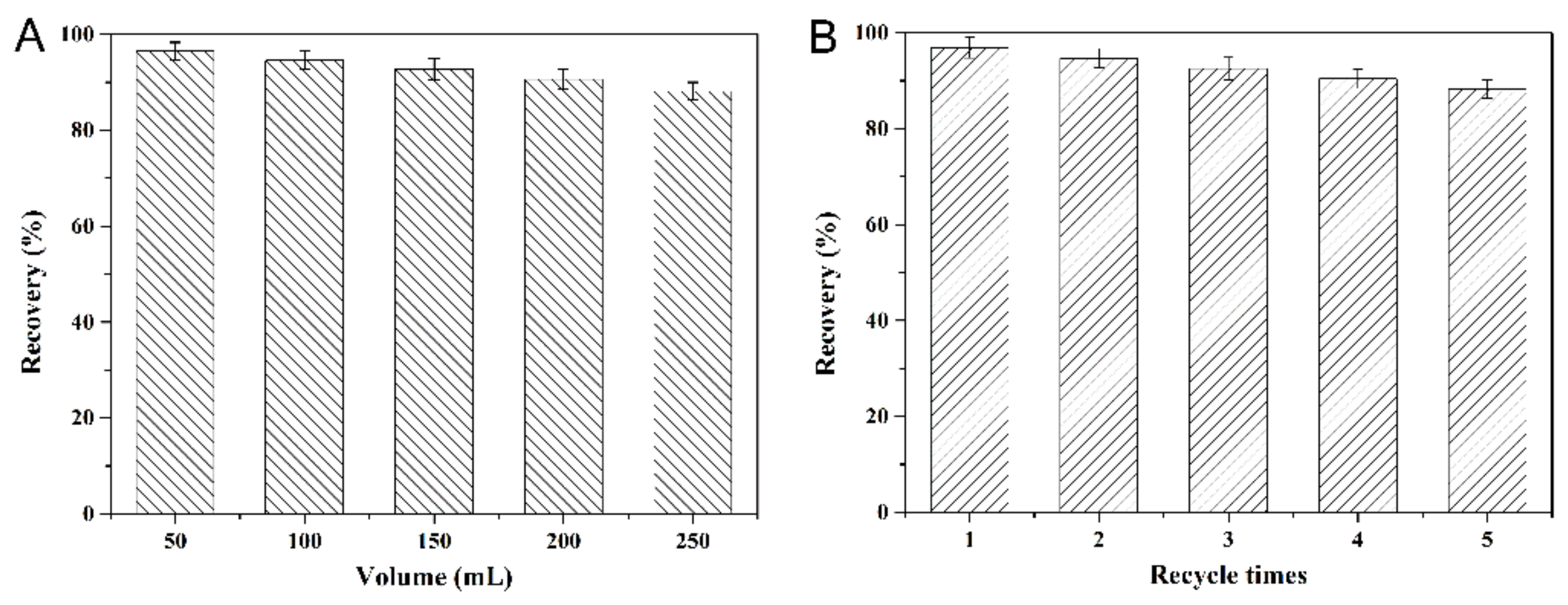
3.4. MSPE of BPA Using Fe3O4@ZIF-8@MIP
3.5. MSPE-HPLC for BPA Analysis in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vilarinho, F.; Sendon, R.; Van der Kellen, A.; Vaz, M.F.; Sanches Silva, A. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019, 91, 33–65. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A-Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nakagawa, Y.; Takano, I.; Yaguchi, K.; Yasuda, K. Environmental fate of bisphenol A and its biological metabolites in river water and their xeno-estrogenic activity. Environ. Sci. Technol. 2004, 38, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Ricco, R.; Malfatti, L.; Takahashi, M.; Hill, A.J.; Falcaro, P. Applications of magnetic metal-organic framework composites. J. Mater. Chem. A 2013, 1, 13033–13045. [Google Scholar] [CrossRef]
- Huo, S.H.; Yan, X.P. Facile magnetization of metal-organic framework MIL-101 for magnetic solid-phase extraction of poycyclic aromatic hydrocarbons in environmental water samples. Analyst 2012, 137, 3445–3451. [Google Scholar] [CrossRef]
- Hu, Y.L.; Huang, Z.L.; Liao, J.; Li, G.K. A chemical bonding approach for fabrication of hybrid magnetic metal-organic framework-5: High efficient adsorbents for magnetic enrichment of trace analytes. Anal. Chem. 2013, 85, 6885–6893. [Google Scholar] [CrossRef]
- Wu, Y.H.; Li, B.Y.; Wang, X.X.; Yu, S.J.; Pang, H.W.; Liu, Y.; Liu, X.Y.; Wang, X.K. Magnetic metal-organic frameworks (Fe3O4@ZIF-8) composites for U (VI) and Eu (III) elimination: Simultaneously achieve favorable stability and functionality. Chem. Eng. J. 2019, 378, 122105–122116. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel metal-organic framework (MOF) based composite material for the sequestration of U (VI) and Th (IV) metal ions from aqueous environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, Z.C.; Peng, L.; Gan, Y.Y.; Zhao, Y.M.; Shen, J.; Qan, J.H.; Zhang, L.Y.; Zhang, W.B. Facile preparation of core-shell magnetic metal-organic framework nanoparticles for the selective capture of phosphopeptides. ACS Appl. Mater. Interfaces 2015, 7, 16338–16347. [Google Scholar] [CrossRef]
- Xiao, R.L.; Pan, Y.N.; Li, J.; Zhang, L.Y.; Zhang, W.B. Layer-by-layer assembled magnetic bimetallic metal-organic framework composite for global phosphopeptide enrichment. J. Chromatogr. A 2019, 1601, 45–52. [Google Scholar] [CrossRef]
- Ke, F.; Jiang, J.; Li, Y.Z.; Liang, J.; Wan, X.C.; Ko, S. Highly selective removal of Hg2+ and Pb2+ by thiol-functionalized Fe3O4@metal-organic framework core-shell magnetic microspheres. Appl. Surf. Sci. 2017, 413, 266–274. [Google Scholar] [CrossRef]
- Liu, G.Y.; Li, L.Y.; Gao, Y.H.; Gao, M.K.; Huang, X.D.; Lv, J.; Xu, D.H. A beta-cyclodextrin-functionalized magnetic metal organic framework for efficient extraction and determination of prochloraz and triazole fungicides in vegetables samples. Ecotoxicol. Environ. Saf. 2019, 183, 109546. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, G.; Liu, Z.; Meng, M.; Liu, F.; Ni, L. Facile synthesis of novel photoresponsive mesoporous molecularly imprinted polymers for photo-regulated selective separation of bisphenol A. Chem. Eng. J. 2016, 296, 437–446. [Google Scholar] [CrossRef]
- Mao, Y.L.; Kang, H.Y.; Guo, Y.F.; Chen, S.T.; Wang, Z.X. Synthesis of surface imprinted polymer upon modified kaolinite and study on the selective adsorption of BPA. Desalin. Water Treat. 2016, 57, 3947–3956. [Google Scholar] [CrossRef]
- Matsumoto, K.; Tiu, B.D.B.; Kawamura, A.; Advincula, R.C.; Miyata, T. QCM sensing of bisphenol A using molecularly imprinted hydrogel/conducting polymer matrix. Polym. J. 2016, 48, 525–532. [Google Scholar] [CrossRef]
- Poliwoda, A.; Moscipan, M.; Wieczorek, P.P. Application of molecular imprinted polymers for selective solid phase extraction of bisphenol A. Ecol. Chem. Eng. S. 2016, 23, 651–664. [Google Scholar] [CrossRef][Green Version]
- Luo, L.J.; Tan, W.; Xong, H.B.; Barrow, C.J.; He, P.; Yang, W.R.; Wang, H.B. TiO2@ phenyl-functionalized mesoporous silica for removal of bisphenol A from water. Desalin. Water Treat. 2017, 72, 182–189. [Google Scholar] [CrossRef]
- Qian, K.; Fang, G.Z.; Wang, S. A novel core-shell molecularly imprinted polymer based on metal-organic frameworks as a matrix. Chem. Commun. 2011, 47, 10118–10120. [Google Scholar] [CrossRef]
- Iskierko, Z.; Sharma, P.S.; Prochowicz, D.; Fronc, K.; D’Souza, F.; Toczydlowska, D.; Stefaniak, F.; Noworyta, K. Molecularly imprinted polymer (MIP) film with improved surface area developed by using metal-organic framework (MOF) for sensitive lipocalin (NGAL) determination. ACS Appl. Mater. Interfaces 2016, 8, 19860–19865. [Google Scholar] [CrossRef]
- Liu, H.; Mu, L.; Chen, X.; Wang, J.; Wang, S.; Sun, B. Core-Shell metal-organic frameworks/molecularly imprinted nanoparticles as absorbents for the detection of pyrraline in milk and milk powder. J. Agric. Food Chem. 2017, 65, 986–992. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.K.; Deng, Y.H.; Zou, Y.; Li, C.Y.; Guo, X.H.; Xiong, L.Q.; Gao, Y.; Li, F.Y.; Zhao, D.Y. Highly water-dispersible biocompatible magnetite particles with low cytotoxicity stabilized by citrate groups. Angew. Chem. Int. Ed. Engl. 2009, 48, 5875–5879. [Google Scholar] [CrossRef]
- Zheng, J.N.; Lin, Z.; Lin, G.; Yang, H.H.; Zhang, L. Preparation of magnetic metal-organic framework nanocomposites for highly specific separation of histidine-rich proteins. J. Mater. Chem. B 2015, 3, 2185–2191. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, D.C.; Hua, S.H.; Chun, H.; Kim, Y.S. Sonochemical Preparation of a Magnet-Responsive Fe3O4@ ZIF-8 Adsorbent for Efficient Cu2+ Removal. Nanomaterials 2022, 12, 753–764. [Google Scholar] [CrossRef]
- Xiong, Z.; Zheng, H.; Hu, Y.; Hu, X.; Ding, W.; Ma, J.; Li, Y. Selective adsorption of Congo red and Cu (II) from complex wastewater by core-shell structured magnetic carbon@ zeolitic imidazolate frameworks-8 nanocomposites. Sep. Purif. Technol. 2021, 277, 119053–119070. [Google Scholar] [CrossRef]
- Rostkowska, H.; Szczepaniak, K.; Nowak, M.J.; Leszczynski, J.; KuBulat, K.; Person, W.B. Thiouracils. 2. Tautomerism and infrared spectra of thiouracils. Matrix-isolation and ab initio studies. J. Am. Chem. Soc. 1990, 112, 2147–2160. [Google Scholar] [CrossRef]
- Liu, J.Z.; Wang, W.Z.; Xie, Y.F.; Huang, Y.Y.; Liu, Y.L.; Liu, X.J.; Zhao, R.; Liu, G.Q.; Chen, Y. A novel polychloromethylstyrene coated superparamagnetic surface molecularly imprinted core–shell nanoparticle for bisphenol A. J. Mater. Chem. 2011, 21, 9232–9238. [Google Scholar] [CrossRef]
- Xu, Z.G.; Yang, Z.L.; Liu, Z.M. Development of dual-templates molecularly imprinted stir bar sorptive extraction and its application for the analysis of environmental estrogens in water and plastic samples. J. Chromatogr. A 2014, 1358, 52–59. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.B.; Liu, Y.X.; Tong, H.W.; Xu, Y.P.; Liu, S.M. Preparation of a hollow porous molecularly imprinted polymer using tetrabromobisphenol A as a dummy template and its application as SPE sorbent for determination of bisphenol A in tap water. Talanta 2013, 117, 281–287. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, Y.H.; Ren, S.Y.; Li, S.; Wang, Y.; Han, D.P.; Qin, K.; Peng, Y.; Han, T.; Gao, Z.X.; et al. Fabrication of magnetic Al-based Fe3O4@MIL-53 metal organic framework for capture of multi-pollutants residue in milk followed by HPLC-UV. Molecules 2022, 27, 2088–2098. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zhu, X.; He, C.; Wang, Q.; Liu, S. Dummy molecularly imprinted magnetic nanoparticles for dispersive solid-phase extraction and determination of bisphenol A in Water Samples and Orange Juice. Talanta 2017, 162, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, X.; Liu, Z.; Xue, S.; Zhang, L. Novel functionalized magnetic ionic liquid green separation technology coupled with high performance liquid chromatography: A rapid approach for determination of estrogens in milk and cosmetics. Talanta 2020, 209, 120542–120551. [Google Scholar] [CrossRef] [PubMed]


| Sample | Spiked (μg L−1, n = 3) | Recovery (%, n = 3) | RSD (%) |
|---|---|---|---|
| Lemon juice beverage | 0.5 | 88.5 | 3.0 |
| 1.0 | 89.3 | 2.3 | |
| 10.0 | 89.8 | 2.1 | |
| Canned Hawthorn | 0.5 | 89.3 | 2.9 |
| 1.0 | 88.3 | 3.3 | |
| 10.0 | 89.5 | 1.9 | |
| Mineral water | 0.5 | 91.3 | 2.9 |
| 1.0 | 92.3 | 3.6 | |
| 10.0 | 91.9 | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Wang, H.; Zhang, Y.; Xu, Z.; Xu, L. Preparation of Magnetic Metal-Organic Frameworks@Molecularly Imprinted Nanoparticles for Specific Extraction and Enrichment of Bisphenol A in Food. Foods 2022, 11, 1408. https://doi.org/10.3390/foods11101408
Zhang Q, Wang H, Zhang Y, Xu Z, Xu L. Preparation of Magnetic Metal-Organic Frameworks@Molecularly Imprinted Nanoparticles for Specific Extraction and Enrichment of Bisphenol A in Food. Foods. 2022; 11(10):1408. https://doi.org/10.3390/foods11101408
Chicago/Turabian StyleZhang, Qi, Haiyang Wang, Yongju Zhang, Zhixiang Xu, and Longhua Xu. 2022. "Preparation of Magnetic Metal-Organic Frameworks@Molecularly Imprinted Nanoparticles for Specific Extraction and Enrichment of Bisphenol A in Food" Foods 11, no. 10: 1408. https://doi.org/10.3390/foods11101408
APA StyleZhang, Q., Wang, H., Zhang, Y., Xu, Z., & Xu, L. (2022). Preparation of Magnetic Metal-Organic Frameworks@Molecularly Imprinted Nanoparticles for Specific Extraction and Enrichment of Bisphenol A in Food. Foods, 11(10), 1408. https://doi.org/10.3390/foods11101408








