Construction of an ECL Detection Platform for Sensitive Detection of Carbaryl Based on an Eu3+-Functionalized Metal–Organic Framework Encapsulated with Nanogold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. ECL Measurement
2.3. Preparation of Eu3+-Au@MOF-253 Modified Electrodes
2.4. Sample Preparation
3. Results and Discussion
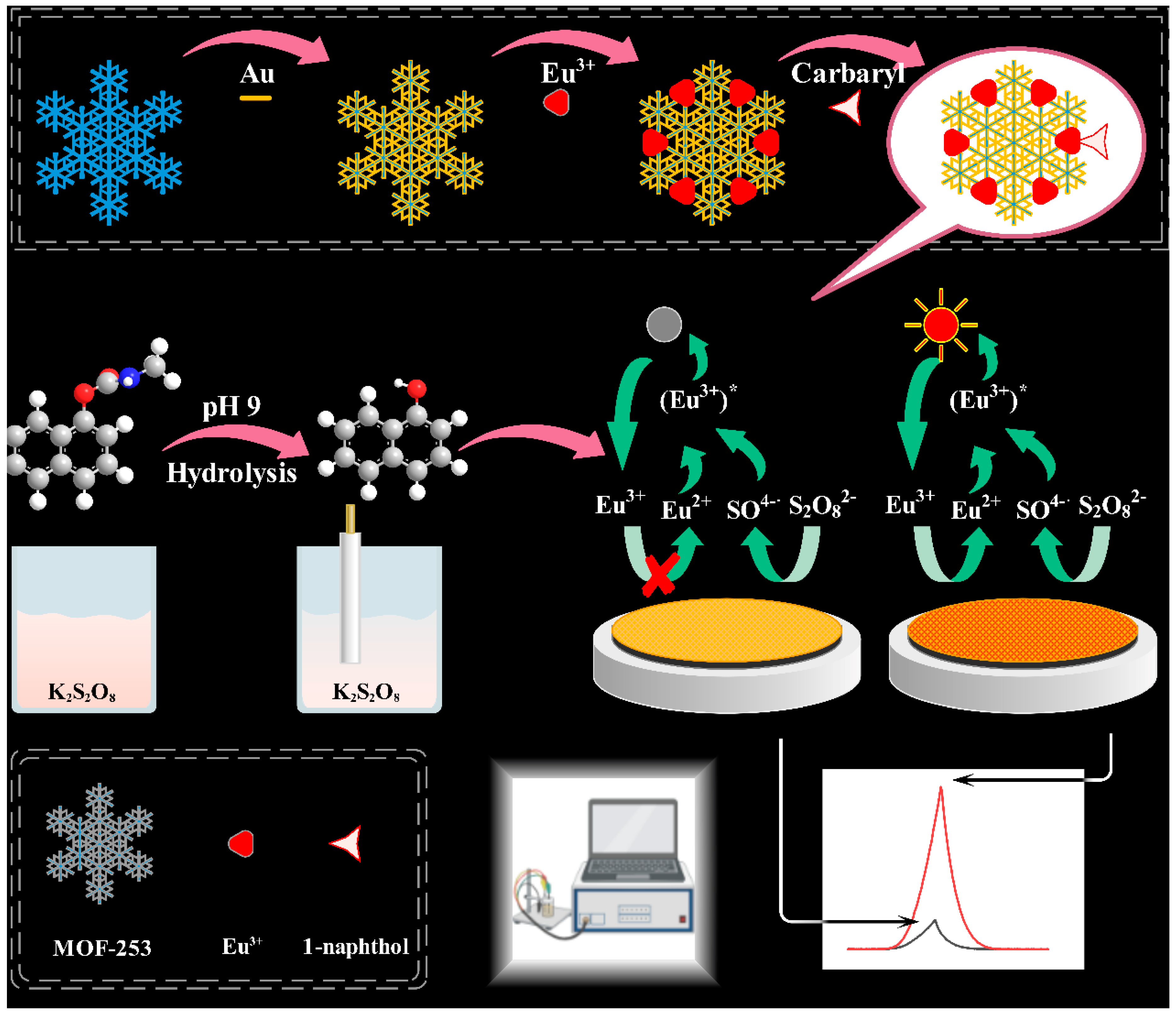
3.1. Preparation of Electrodes and Detection of Carbaryl
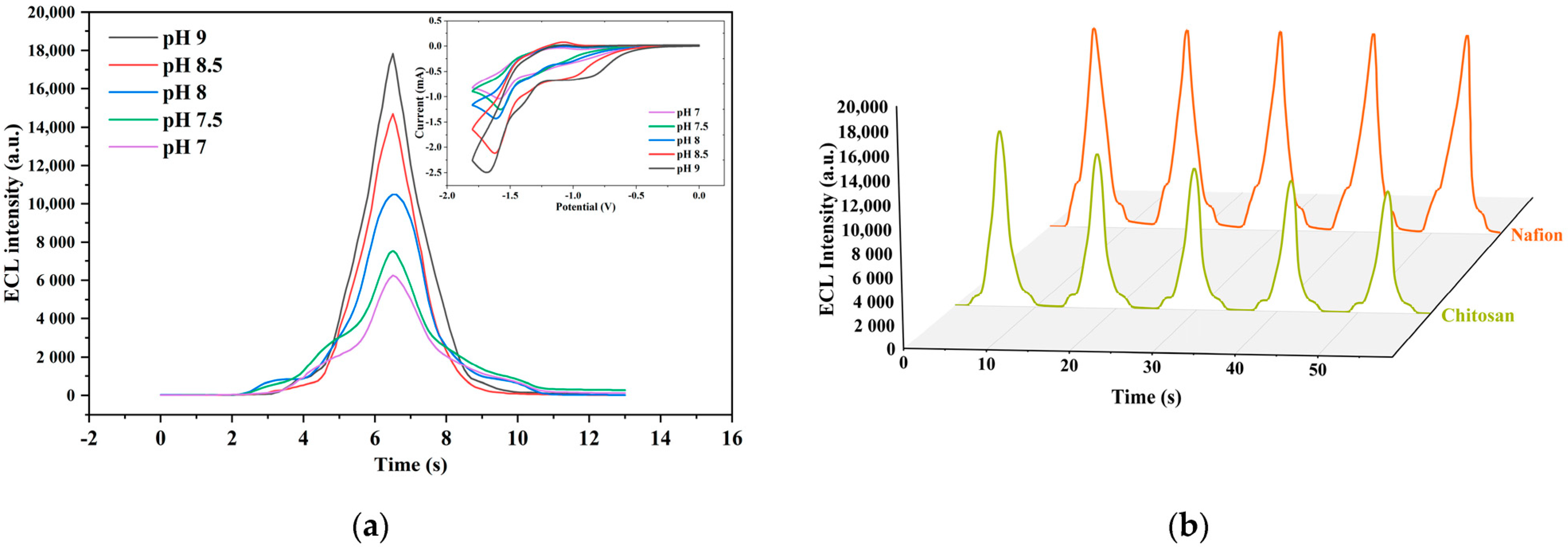
3.2. Optimization of ECL Detection Platform
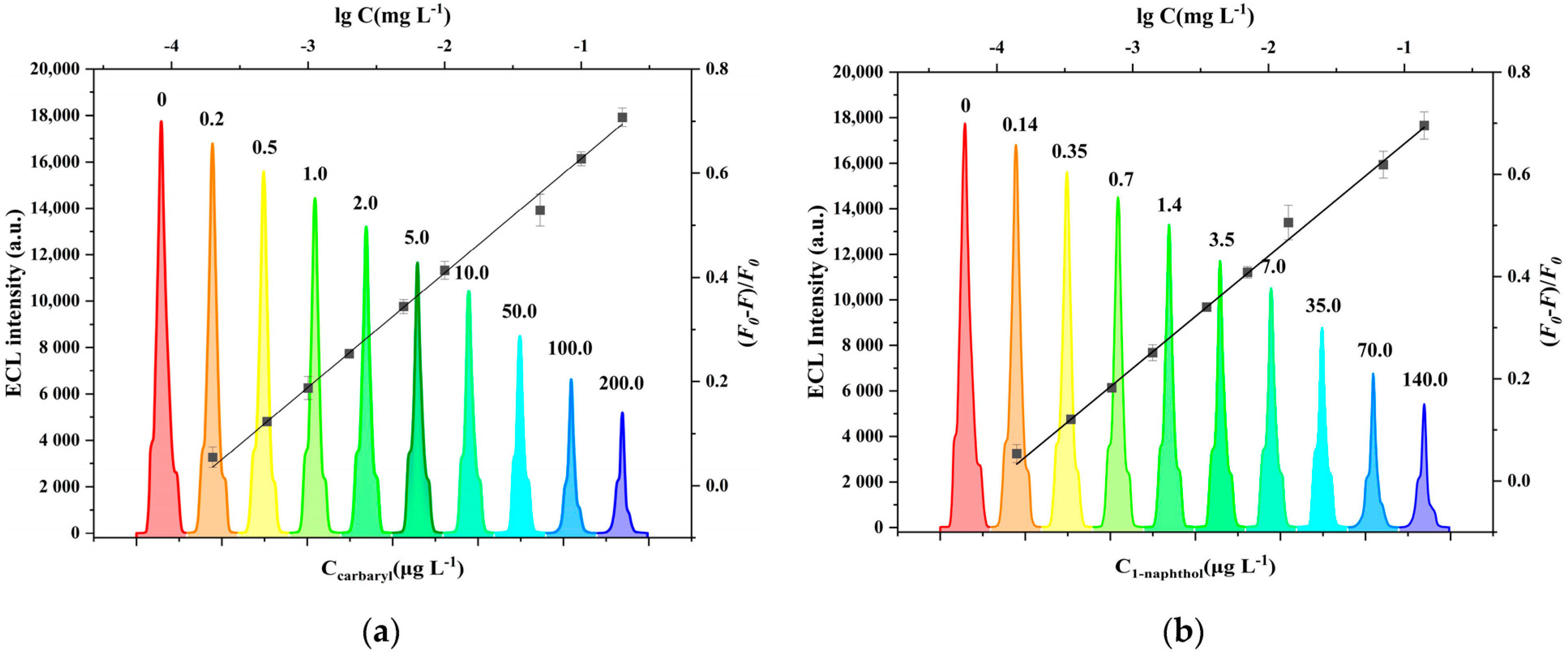
3.3. Performance of ECL Detection Platform
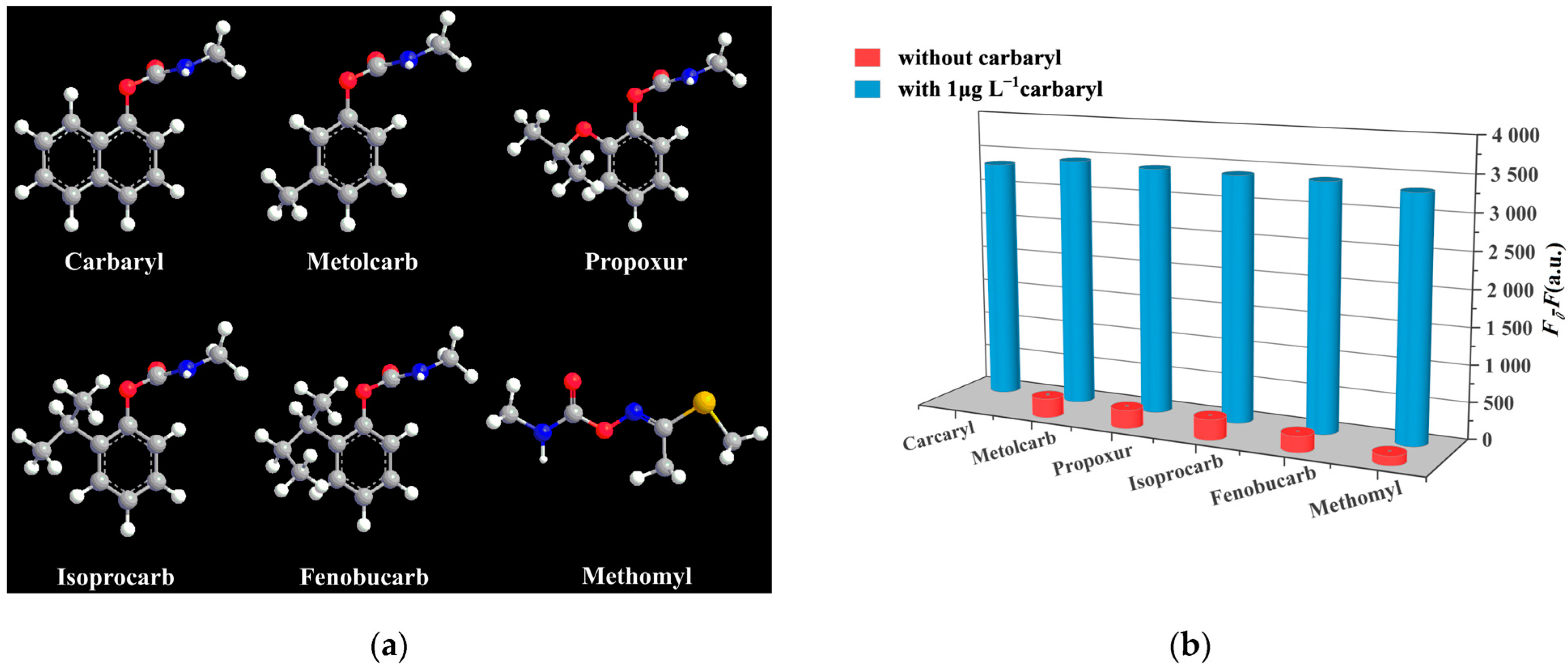
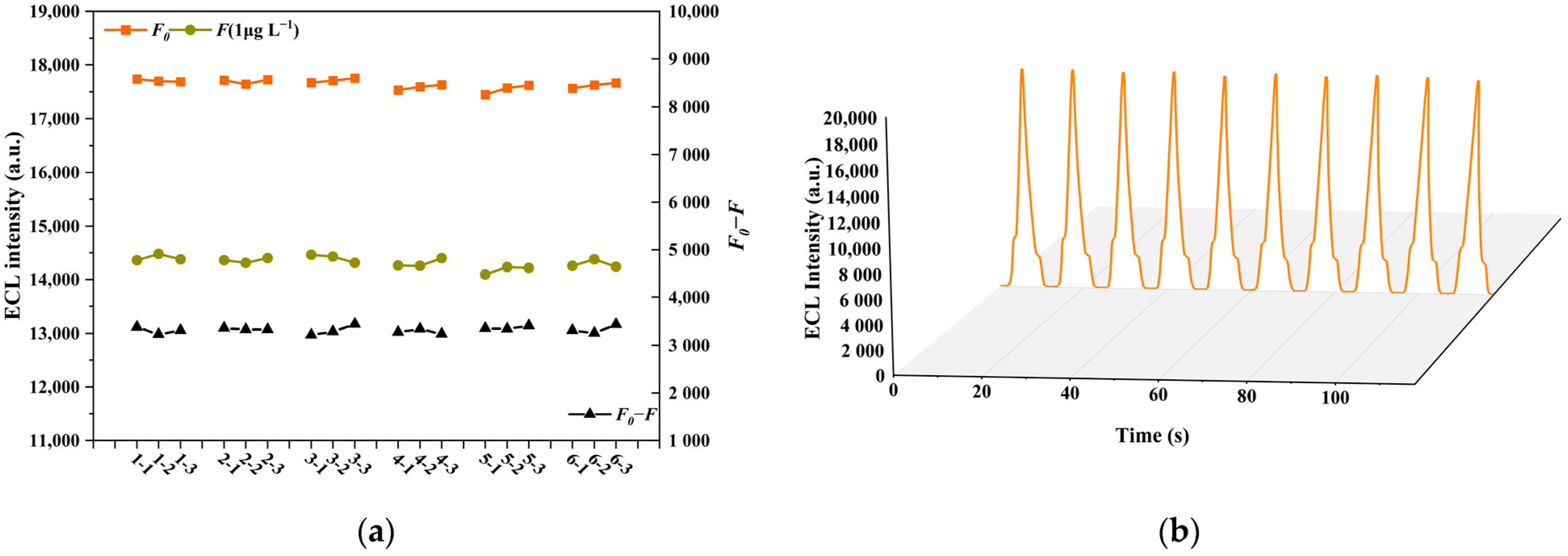
3.4. Selectivity, Precision and Stability
3.5. Real Sample Analysis
3.6. Comparison of Different Detection Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, S.P.; Luo, Q.X.; Li, Y.J.; Liang, R.P.; Qiu, J.D. Gold nanoparticles decorated carbon nitride nanosheets as a coreactant regulate the conversion of the dual-potential electrochemiluminescence of Ru(bpy)32+ for Hg2+ detection. Chem. Commun. 2020, 56, 5625–5628. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, X.; Pang, C.; Wang, M.; Luo, J. Novel chloramphenicol sensor based on aggregation-induced electrochemiluminescence and nanozyme amplification. Biosens. Bioelectron. 2018, 176, 112944. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Liu, J.; Chai, Y.; Yuan, R.; Zhuo, Y. DNA Structure Transition-Induced Affinity Switch for Biosensing Based on the Strong Electrochemiluminescence Platform from Organic Microcrystals. Anal. Chem. 2020, 92, 3940–3948. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, Z.; Dong, S. Electrogenerated chemiluminescence biosensor with alcohol dehydrogenase and tris(2,2′-bipyridyl)ruthenium (II) immobilized in sol–gel hybrid material. Biosens. Bioelectron. 2005, 21, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qi, W.; Xu, G. ChemInform Abstract: Recent Advances in Electrochemiluminescence. Cheminform 2015, 46, 3117–3142. [Google Scholar] [CrossRef]
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Martin, J.E.; Hart, E.J.; Adamson, A.W.; Gafney, H.; Halpern, J. Chemiluminescence from the reaction of the hydrated electron with tris(bipyridyl)ruthenium(III). J. Am. Chem. Soc. 1972, 94, 9238–9240. [Google Scholar] [CrossRef]
- Xu, G.; Zeng, X.; Lu, S.; Dai, H.; Gong, L.; Lin, Y.; Wang, Q.; Tong, Y.; Chen, G. Electrochemiluminescence of luminol at the titanate nanotubes modified glassy carbon electrode. Luminescence 2013, 28, 456–460. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Tang, L.; Chang, H.; Li, J. Graphene oxide amplified electrogenerated chemiluminescence of quantum dots and its selective sensing for glutathione from thiol-containing compounds. Anal. Chem. 2009, 81, 9710–9715. [Google Scholar] [CrossRef]
- Myung, N.; Ding, Z.; Bard, A.J. Electrogenerated Chemiluminescence of CdSe Nanocrystals. Nano Lett. 2002, 2, 1315–1319. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Fu, L.; Fu, K.; Zou, G. Efficient and Monochromatic Electrochemiluminescence of Aqueous-Soluble Au Nanoclusters via Host–Guest Recognition. Angew. Chem. Int. Ed. 2019, 131, 6975–6979. [Google Scholar] [CrossRef]
- Chen, S.; Lv, Y.; Shen, Y.; Ji, J.; Zhou, Q.; Liu, S.; Zhang, Y. Highly Sensitive and Quality Self-Testable Electrochemiluminescence Assay of DNA Methyltransferase Activity Using Multifunctional Sandwich-Assembled Carbon Nitride Nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 6887. [Google Scholar] [CrossRef] [PubMed]
- Danis, A.S.; Potts, K.P.; Perry, S.C.; Mauzeroll, J. Combined Spectroelectrochemical and Simulated Insights into the Electrogenerated Chemiluminescence Coreactant Mechanism. Anal. Chem. 2018, 90, 7377–7382. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Eddaoudi, M.M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly Porous Metal-Organic Framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Jia, J.; Hubberstey, P.; Schrder, M.; Champness, N.R. Hydrogen storage in metal–organic frameworks. Crystengcomm 2007, 9, 438–448. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regi, M.; Sebban, M.; Taulelle, F.; Ferey, G. Flexible Porous Metal-Organic-Frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273, 76–86. [Google Scholar] [CrossRef]
- Jin, Z.; Zhu, X.; Wang, N.; Li, Y.; Lei, J. Electroactive Metal Organic Frameworks as Emitters for Self-Enhanced Electrochemiluminescence in Aqueous Medium. Angew. Chem. 2020, 132, 10532–10536. [Google Scholar] [CrossRef]
- Hu, G.B.; Xiong, C.Y.; Liang, W.B.; Zeng, X.S.; Xu, H.L.; Yang, Y.; Yao, L.Y.; Yuan, R.; Xiao, D.R. Highly Stable Mesoporous Luminescence-Functionalized MOF with Excellent Electrochemiluminescence Property for Ultrasensitive Immunosensor Construction. ACS Appl. Mater. Interfaces 2018, 10, 15913–15919. [Google Scholar] [CrossRef]
- Castorina, R.; Harnly, M.; Eskenazi, B.; Barr, D.B.; Bradman, A. Carbaryl and naphthalene exposures among a pregnant Latina population living in an agricultural area. Epidemiology 2008, 19, S207–S208. [Google Scholar] [CrossRef]
- Karami-Mohajeri, S.; Abdollahi, M. Toxic influence of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: A systematic review. Hum. Exp. Toxicol. 2011, 30, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, X.; Wu, Q.; Wang, C.; Wang, Z. Solid phase extraction of carbamate pesticides with porous organic polymer as adsorbent followed by high performance liquid chromatography-diode array detection. J. Chromatogr. A 2019, 1600, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Yu, T.; Luo, H.; Zhu, T.; Huang, X.; Luo, L. Direct detection of multiple pesticides in honey by neutral desorption-extractive electrospray ionization mass spectrometry. Int. J. Mass Spectrom. 2017, 422, 111–118. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Qu, R.; Xu, Y.; Shang, S.; Hui, N. Non-Enzymatic Electrochemical Sensors Based on Conducting Polymer Hydrogels for Ultrasensitive Carbaryl Pesticide Detection. J. Electrochem. Soc. 2021, 168, 047506. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Li, T.; Deng, Y.; He, N. Selection of a DNA aptamer for the development of fluorescent aptasensor for carbaryl detection. Chin. Chem. Lett. 2021, 32, 1957–1962. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Tian, Y.; Gu, C.; Jiang, T. Contrastive Study of In Situ Sensing and Swabbing Detection Based on SERS-Active Gold Nanobush–PDMS Hybrid Film. J. Agric. Food Chem. 2021, 69, 1975–1983. [Google Scholar] [CrossRef]
- Lee, M.G.; Patil, V.; Na, Y.C.; Lee, D.S.; Lim, S.H.; Yi, G.R. Highly stable, rapid colorimetric detection of carbaryl pesticides by azo coupling reaction with chemical pre-treatment. Sens. Actuators B Chem. 2018, 261, 489–496. [Google Scholar] [CrossRef]
- Staninski, K.; Lis, S. Ultraweak emission of the Eu(III) ions in cathodic generated electrochemiluminescence. Opt. Mater. 2011, 33, 1540–1543. [Google Scholar] [CrossRef]
- Yu, H.X.; Cui, H.; Guan, J.B. Cathodic electrochemiluminescence of acetonitrile, acetonitrile-1,10-phenanthroline and acetonitrile-ternary Eu(III) complexes at a gold electrode. Luminescence 2010, 21, 81–89. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.Y.; Liu, J.; Xu, J.J.; Chen, H.Y. Electrochemiluminescence Resonance Energy Transfer between CdS:Eu Nancrystals and Au Nanorods for Sensitive DNA Detection. J. Phys. Chem. C 2012, 116, 17773–17780. [Google Scholar] [CrossRef]
- Zhao, J.J.; Liu, P.Y.; Dong, Z.P.; Liu, Z.L.; Wang, Y.Q. Eu(III)-organic framework as a multi-responsive photoluminescence sensor for efficient detection of 1-naphthol, Fe3+ and MnO4− in water. Inorg. Chim. Acta 2020, 511, 119843. [Google Scholar] [CrossRef]
- Li, D.; Shan, Y.; Xu, J.J.; Chen, H.Y. Electrochemiluminescence behaviors of Eu3+-doped CdS nanocrystals film in aqueous solution. Nanoscale 2012, 4, 831–836. [Google Scholar] [CrossRef]
- Bloch, E.D.; Britt, R.; Lee, R.; Doonan, R.J.; Uribe-Romo, R.J.; Furukawa, H.; Long, R.R.; Yaghi, R.M. Metal insertion in a microporous metal-organic framework lined with 2,2′-bipyridine. J. Am. Chem. Soc. 2010, 132, 14382. [Google Scholar] [CrossRef]
- Deng, X.; Qin, Y.; Hao, M.; Li, Z. MOF-253-Supported Ru Complex for Photocatalytic CO2 Reduction by Coupling with Semidehydrogenation of 1,2,3,4-Tetrahydroisoquinoline (THIQ). Inorg. Chem. 2019, 58, 16574–16580. [Google Scholar] [CrossRef]
- Shi, L.; Xiang, Z.; Liu, T.; Zhao, H.; Lan, M. Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Si-Jia; Qin; Bing; Yan, A facile indicator box based on Eu3+ functionalized MOF hybrid for the determination of 1-naphthol, a biomarker for carbaryl in urine. Sens. Actuators B Chem. 2018, 259, 125–132. [CrossRef]
- GB 23200.90-2016; National Food Safety Standards-Determination of Multiple Carbamate Pesticides Residues in Milk and Dairy Products Liquid Chromatography-Mass Spectrometry. National Health and Family Planning Commission of the People’s Republic of China, Ministry of Agriculture of the People’s Republic of China, State Administration for Market Regulation: Beijing, China, 2016; p. 2.
- GB 23200.112-2018; National Food Safety Standards-Determination of 9 Carbamate Pesticides and Metabolites Residues in Foods of Plant Origin-Liquid Chromatography-Post-Colum Derivatization. National Health Commission of the People’s Republic of China, Ministry of Agriculture and Rural Affairs of the People’s Republic of China, State Administration for Market Regulation: Beijing, China, 2018; p. 3.
- Shi, X.; Liu, H.; Zhang, M.; Yang, F.; Li, J.; Guo, Y.; Sun, X. Ultrasensitive electrochemiluminescence aptasensor based on AuNPs@MWCNTs and Au@AgNPs for detection of profenofos residues. Sens. Actuators B Chem. 2021, 348, 130663. [Google Scholar] [CrossRef]
- Wang, D.; Liang, Y.; Su, Y.; Shang, Q.; Zhang, C. Sensitivity enhancement of cloth-based closed bipolar electrochemiluminescence glucose sensor via electrode decoration with chitosan/multi-walled carbon nanotubes/graphene quantum dots-gold nanoparticles. Biosens. Bioelectron. 2019, 130, 55–64. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Zhang, Y.; Fang, J.; Xu, P.; Xu, J.; Li, X.; Liud, C.-C.; Wen, W. Highly effective and specific way for the trace analysis of carbaryl insecticides based on Au42Rh58 alloy nanocrystals. J. Mater. Chem. A 2017, 5, 7064–7071. [Google Scholar] [CrossRef]
- Zhang, F.; Yi, W.; Chu, T.S.; Wang, Z.H.; Yang, Y.Y. A facile fabrication of electrodeposited luminescent MOF thin films for selective and recyclable sensing of nitroaromatic explosives. Analyst 2016, 141, 4502. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.L.; Chen, B.; Liu, X.G.; Zhou, L.J.; Zhu, X.L.; Cao, J.; Gu, Z.G.; Zhao, Z.; Shen, L.; Tang, B.Z. A highly luminescent entangled metal–organic framework based on pyridine-substituted tetraphenylethene for efficient pesticide detection. Chem. Commun. 2017, 53, 9975–9978. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shi, F.; Zhang, W.; Zang, S.; Mak, T.C.W. Selective Sensing of Fe(3+) and Al(3+) Ions and Detection of 2,4,6-Trinitrophenol by a Water-Stable Terbium-Based Metal-Organic Framework. Chemistry 2015, 21, 15705–15712. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Zhao, F.; Liu, J.J.; Liu, Z.; Wang, Y.Q. An ultrastable zinc(II)–organic framework as a recyclable multi-responsive luminescent sensor for Cr(III), Cr(VI) and 4-nitrophenol in the aqueous phase with high selectivity and sensitivity. J. Mater. Chem. A 2017, 5, 20035–20043. [Google Scholar] [CrossRef]
- 1096/2014, R.E.N. EU Pesticides Database, Pesticide Residues. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/mrls/?event=search.pr (accessed on 1 April 2021).
- GB2763-2021; State Administration of Market Supervision and Administration, National Food Safety Standard-Maximum Residue Limits for Pesticides in Food. National Health Commission of the People’s Republic of China, Ministry of Agriculture and Rural Affairs of the People’s Republic of China, State Administration for Market Regulation: Beijing, China, 2021.
- Luo, J.; Zhou, C.; Shi, Y.; Zhang, L.; Xiao, D. The self-assembled Ru(bpy)3(PF6)2 nanoparticle on polystyrene microfibers and its application for ECL sensing. Analyst 2013, 138, 6171–6176. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, L.; Liu, X.; You, T. Ultrasensitive self-enhanced electrochemiluminescence sensor based on novel PAN@Ru@PEI@Nafion nanofiber mat. J. Mater. Chem. B 2020, 8, 3590–3597. [Google Scholar] [CrossRef]
- Wang, S.; Lv, S.; Wu, W.; Guo, Z. A Novel Electrochemiluminescence Sensor for Sensitive Determination of Carbaryl Based on Solid Phase Microextraction at NH2–Graphene–Nafion Modified Electrode. Aust. J. Chem. 2014, 68, 793. [Google Scholar] [CrossRef]


| Sample | Added (μg kg−1 /μg L−1) | HPLC | ECL | ||
|---|---|---|---|---|---|
| Found (μg kg−1 /μg L−1) | Recovery (Mean ± SD, n = 3) | Found (μg kg−1 /μg L−1) | Recovery (Mean ± SD, n = 3) | ||
| Milk | 0 | not detected | — | not detected | — |
| 1 | 0.87 | 87.1 ± 1.5 | 0.82 | 82.4 ± 2.4 | |
| 50 | 47.42 | 94.8 ± 3.4 | 47.57 | 95.1 ± 3.6 | |
| 100 | 97.73 | 97.7 ± 1.9 | 95.42 | 95.4 ± 2.2 | |
| Soybean oil | 0 | not detected | — | not detected | — |
| 1 | 0.83 | 82.7 ± 2.6 | 0.77 | 76.5 ± 2.5 | |
| 50 | 44.12 | 89.1 ± 2.6 | 41.53 | 83.1 ± 3.1 | |
| 100 | 93.51 | 93.5 ± 1.8 | 86.33 | 86.3 ± 1.7 | |
| Method | Detection Range | LOD | Sample | Recovery (%) | RSD (%) | Year | References |
|---|---|---|---|---|---|---|---|
| High performance liquid chromatography-diode array | 1.0 to 320.0 ng mL−1 | 0.12–0.40 ng mL−1 | Milk, White wine | 86.5–99.2 | 3.2–5.7 | 2019 | [23] |
| 0.5 to 160.0 ng mL−1 | 0.06–0.20 ng mL−1 | Juice | 82.0–104.7 | 2.8–4.5 | |||
| Electrospray ionization mass spectrometry | 20.00–1000.00 ng mL−1 | 1.16–4.18 ng g−1 | Honey | 87.00–114.98 | 0.97–6.22 | 2017 | [24] |
| DNA aptamer | 100–1500 nmol L−1 | 15.23 nmol L−1 | Tap water, Xuanwu Hu | 97.7–107.3% | 0.8–2.5 | 2021 | [26] |
| Surface-enhanced Raman scattering | 10−4 − 10−9 g mL−1 (situ detection) | 0.77 ng mL−1 (situ detection) | Cherry | -- | -- | 2021 | [27] |
| 10−3 − 10−8 g mL−1 (swabbing detection) | 5.8 ng mL−1 (swabbing detection) | -- | -- | ||||
| Colorimetric | 10 μM-5 mM | 10 ppm | Apple | -- | -- | 2018 | [28] |
| ECL | 0–18 μM | 1.0 nM | -- | 96.9–101.7 | 1.87–3.02 | 2013 | [49] |
| ECL | 1.0 × 10−12–1.0 × 10−7 M | 1.0 × 10−12 M | Tap water | 94.0–95.8% | 3.53–4.62 | 2020 | [50] |
| ECL | 5 × 10−4–10 mg mL−1 | 2 × 10−4 μg mL−1 | River water | 99.0–108.0 | 5%< | 2015 | [51] |
| ECL | 0.2–200 μg L−1 | 0.14 μg L−1 | Milk, Soybean oil | 76.5–95.4% | 2.0–3.8 | 2021 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, H.; Hu, X.; Cao, Y.; Fang, G. Construction of an ECL Detection Platform for Sensitive Detection of Carbaryl Based on an Eu3+-Functionalized Metal–Organic Framework Encapsulated with Nanogold. Foods 2022, 11, 1487. https://doi.org/10.3390/foods11101487
Liu C, Wang H, Hu X, Cao Y, Fang G. Construction of an ECL Detection Platform for Sensitive Detection of Carbaryl Based on an Eu3+-Functionalized Metal–Organic Framework Encapsulated with Nanogold. Foods. 2022; 11(10):1487. https://doi.org/10.3390/foods11101487
Chicago/Turabian StyleLiu, Chang, Haiyang Wang, Xuelian Hu, Yichuan Cao, and Guozhen Fang. 2022. "Construction of an ECL Detection Platform for Sensitive Detection of Carbaryl Based on an Eu3+-Functionalized Metal–Organic Framework Encapsulated with Nanogold" Foods 11, no. 10: 1487. https://doi.org/10.3390/foods11101487
APA StyleLiu, C., Wang, H., Hu, X., Cao, Y., & Fang, G. (2022). Construction of an ECL Detection Platform for Sensitive Detection of Carbaryl Based on an Eu3+-Functionalized Metal–Organic Framework Encapsulated with Nanogold. Foods, 11(10), 1487. https://doi.org/10.3390/foods11101487





