Sprouts Use as Functional Foods. Optimization of Germination of Wheat (Triticum aestivum L.), Alfalfa (Medicago sativa L.), and Radish (Raphanus sativus L.) Seeds Based on Their Nutritional Content Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Germination Process
2.2.1. Germination Conditions
2.2.2. Determination of the Optimal Imbibition Time for Each Type of Seed
2.3. Drying and Dry Matter Content of Seeds and Sprouts
2.4. Crude Protein and Fat Contents Determination
2.5. Total Soluble Phenolic Content and HPLC Identification and Quantification of the Free Phenolic Acids
2.5.1. Extraction
2.5.2. Total Phenolic Content
2.5.3. HPLC Identification and Quantification of the Free Phenolic Compounds
2.6. DPPH Radical Scavenging Activity (RSA)
2.7. Vitamin Content Analysis
2.7.1. Extractions of Vitamins
2.7.2. Assay of Vitamins by HPLC
3. Results
3.1. Determination of the Optimal Imbibition Time for Each Type of Seed
3.2. Crude Fat and Protein Content Evolution with Germination
3.3. Total Soluble Phenolic Content Evolution during Germination (TPC)
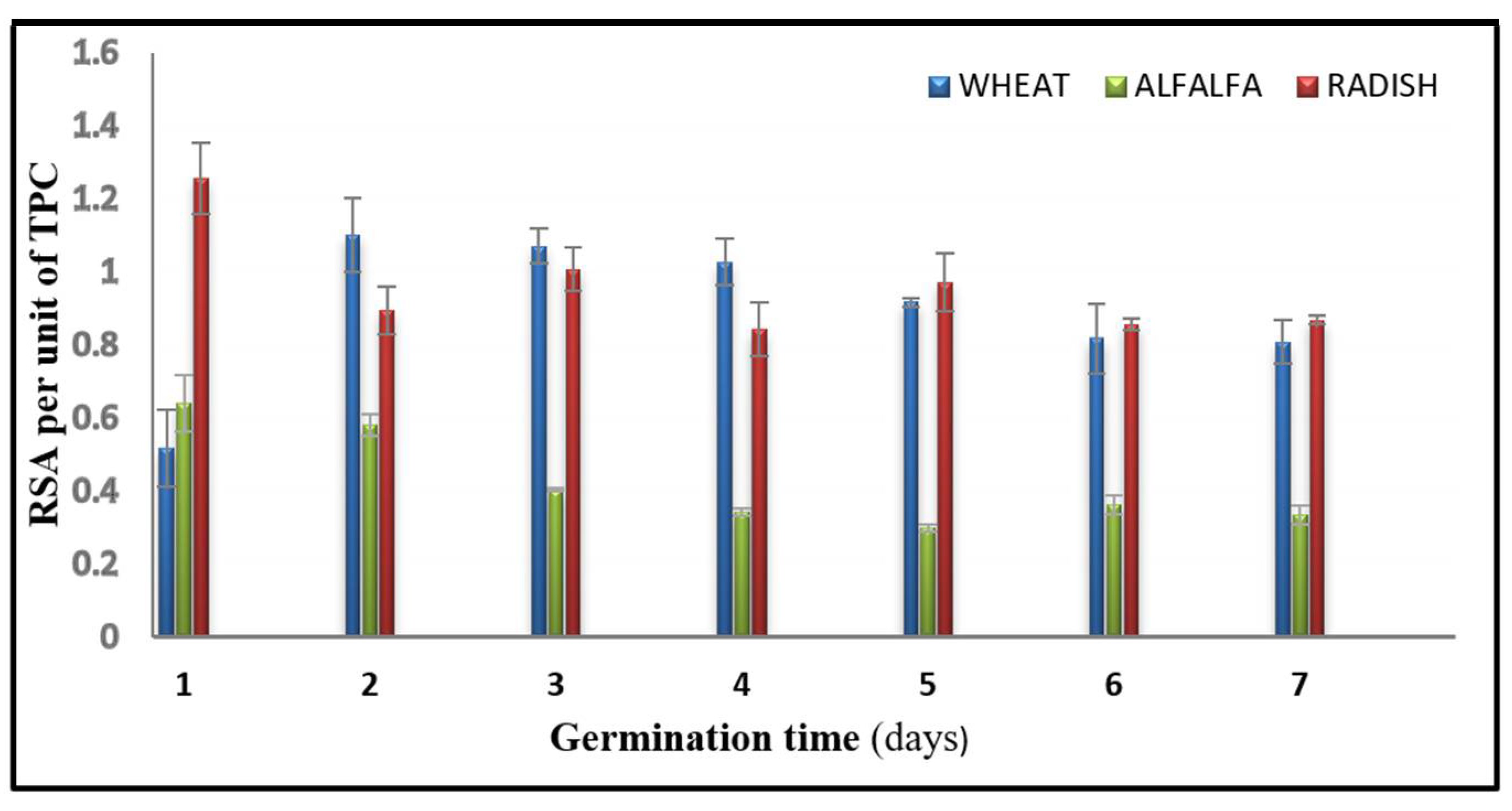
3.4. DPPH Radical Scavenging Activity (RSA)
3.5. HPLC Identification and Quantification of Some Phenolic Compounds
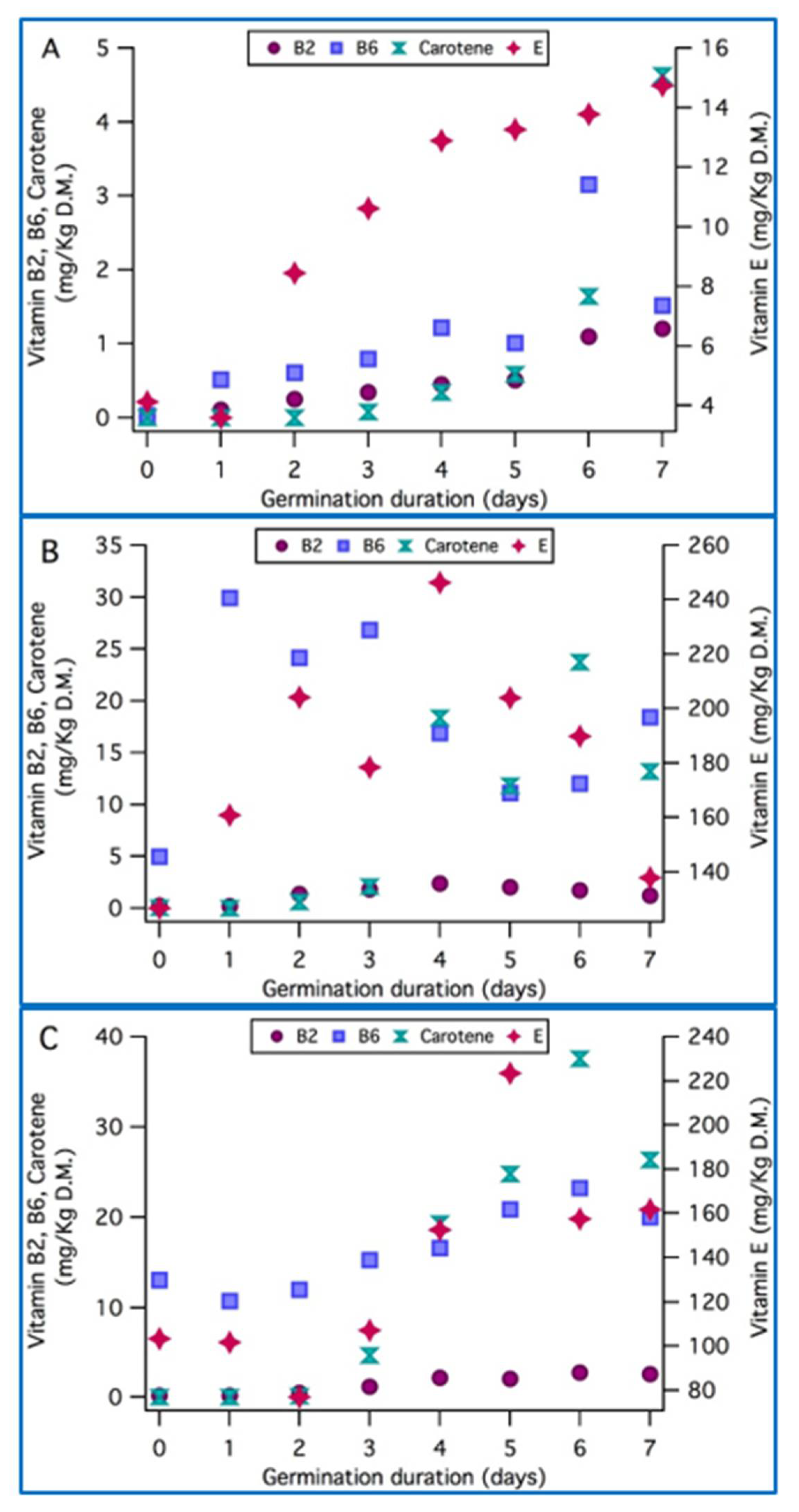
3.6. Vitamins Content Evolution with Germination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aloo, S.O.; Ofosu, F.K.; Oh, D.H. Effect of Germination on Alfalfa and Buckwheat: Phytochemical Profiling by UHPLC-ESI-QTOF-MS/MS, Bioactive Compounds, and in-Vitro Studies of Their Diabetes and Obesity-Related Functions. Antioxidants 2021, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Penãs, E.; Martínez-Villaluenga, C. Advances in Production, Properties and Applications of Sprouted Seeds. Foods 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Peñas, E.; Garc, C.; Mart, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Mart, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Kim-Anne, L.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [Green Version]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a Mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Singh, A.K.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive Compounds and Beneficial Functions of Sprouted Grains; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128115251. [Google Scholar]
- Naveena, N.; Bhaskarachary, K. Effects of Soaking and Germination of Total and Individual Polyphenols Content in the Commonly Consumed Millets and Legumes in India. Int. J. Food Nutr. Sci. 2013, 2, 12–19. [Google Scholar]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Hussain, M.; Anjum, F.M. Nutritional and End-Use Perspectives of Sprouted Grains: A Comprehensive Review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, 2100670. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic Acids Composition, Total Polyphenols Content and Antioxidant Activity of Triticum Monococcum, Triticum Turgidum and Triticum Aestivum: A Two-Years Evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.; Weng, Y.; Ma, Y.; Gu, Z.; Yang, R. Comparison of Phenolic Profiles, Antioxidant Capacity and Relevant Enzyme Activity of Different Chinese Wheat Varieties during Germination. Food Biosci. 2017, 20, 159–167. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Nowak, R.; Świeca, M.; Olech, M.; Pietrzak, W. Influence of Sprouting and Elicitation on Phenolic Acids Profile and Antioxidant Activity of Wheat Seedlings. J. Cereal Sci. 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Van Hung, P.; Hatcher, D.W.; Barker, W. Phenolic Acid Composition of Sprouted Wheats by Ultra-Performance Liquid Chromatography (UPLC) and Their Antioxidant Activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Ehmke, L.; Miller, R.; Li, Y. Changes in Bread Quality, Antioxidant Activity, and Phenolic Acid Composition of Wheats During Early-Stage Germination. J. Food Sci. 2019, 84, 457–465. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol Composition and in Vitro Antioxidant Activity of Amaranth, Quinoa Buckwheat and Wheat as Affected by Sprouting and Baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Huang, X.; Cai, W.; Xu, B. Kinetic Changes of Nutrients and Antioxidant Capacities of Germinated Soybean (Glycine Max l.) and Mung Bean (Vigna Radiata L.) with Germination Time. Food Chem. 2014, 143, 268–276. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic Profile and Antioxidant Activity in Selected Seeds and Sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, K.; Ahmed, N.; Chauhan, D.; Eain Hyder Rizvi, Q.U.; Jan, S.; Singh, T.P.; Dhaliwal, H.S. Effect of Soaking and Germination Treatments on Nutritional, Anti-Nutritional, and Bioactive Properties of Amaranth (Amaranthus Hypochondriacus L.), Quinoa (Chenopodium Quinoa L.), and Buckwheat (Fagopyrum Esculentum L.). Curr. Res. Food Sci. 2021, 4, 917–925. [Google Scholar] [CrossRef]
- Wunthunyarat, W.; Seo, H.S.; Wang, Y.J. Effects of Germination Conditions on Enzyme Activities and Starch Hydrolysis of Long-Grain Brown Rice in Relation to Flour Properties and Bread Qualities. J. Food Sci. 2020, 85, 349–357. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Z.; Gao, Y.; Huang, X.; Zou, Y.; Yang, T. Effects of Germination on the Nutritional Properties, Phenolic Profiles, and Antioxidant Activities of Buckwheat. J. Food Sci. 2015, 80, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Zincă, G.; Vizireanu, C. Impact of Germination on Phenolic Compounds Content and Antioxidant Activity of Alfalfa Seeds (Medicago Sativa L.). J. Agroaliment. Process. Technol. 2013, 19, 105–110. [Google Scholar]
- Mattioli, S.; Dal Bosco, A.; Castellini, C.; Falcinelli, B.; Sileoni, V.; Marconi, O.; Mancinelli, A.C.; Cotozzolo, E.; Benincasa, P. Effect of Heat- and Freeze-Drying Treatments on Phytochemical Content and Fatty Acid Profile of Alfalfa and Flax Sprouts. J. Sci. Food Agric. 2019, 99, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Gómez-Jodar, I.; Moreno, D.A.; García-Viguera, C.; Periago, P.M. Broccoli and Radish Sprouts Are Safe and Rich in Bioactive Phytochemicals. Postharvest Biol. Technol. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Sun, Y.-J.; Wang, M.-T.; Li, X.-Y.; Guo, X.; Hu, R.; Ma, J. Effects of Seed Priming on Germination and Seedling Growth Under Water Stress in Rice. Acta Agron. Sin. 2010, 36, 1931–1940. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Peñas, E.; Ciska, E.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C.; Frias, J. Time Dependence of Bioactive Compounds and Antioxidant Capacity during Germination of Different Cultivars of Broccoli and Radish Seeds. Food Chem. 2010, 120, 710–716. [Google Scholar] [CrossRef]
- Singkhornart, S.; Ryu, G.-H. Effect of Soaking Time and Steeping Temperature on Biochemical Properties and γ-Aminobutyric Acid (GABA) Content of Germinated Wheat and Barley. J. Food Sci. Nutr. 2011, 16, 67–73. [Google Scholar] [CrossRef]
- Jakobsen, J. Optimization of the Determination of Thiamin, 2-(1-Hydroxyethyl)Thiamin, and Riboflavin in Food Samples by Use of HPLC. Food Chem. 2008, 106, 1209–1217. [Google Scholar] [CrossRef]
- Plaza, L.; de Ancos, B.; Cano, P.M. Nutritional and Health-Related Compounds in Sprouts and Seeds of Soybean (Glycine Max), Wheat (Triticum Aestivum L.) and Alfalfa (Medicago Sativa) Treated by a New Drying Method. Eur. Food Res. Technol. 2003, 216, 138–144. [Google Scholar] [CrossRef]
- Ranga, R.; Sarada, A.R.; Baskaran, V.; Ravishankar, G.A. Identification of Carotenoids from Green Alga Haematococcus Pluvialis by HPLC and LC-MS (APCI) and Their Antioxidant Properties. J. Microbiol. Biotechnol. 2009, 19, 1333–1341. [Google Scholar] [CrossRef]
- Millan-Sango, D.; Sammut, E.; Van Impe, J.F.; Valdramidis, V.P. Decontamination of Alfalfa and Mung Bean Sprouts by Ultrasound and Aqueous Chlorine Dioxide. LWT—Food Sci. Technol. 2017, 78, 90–96. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Impact of Germination on Phenolic Content and Antioxidant Activity of 13 Edible Seed Species. Food Chem. 2010, 119, 1485–1490. [Google Scholar] [CrossRef]
- Grassi, S.; Cardone, G.; Bigagnoli, D.; Marti, A. Monitoring the Sprouting Process of Wheat by Non-Conventional Approaches. J. Cereal Sci. 2018, 83, 180–187. [Google Scholar] [CrossRef]
- Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z. The Role of Sprouts in Human Nutrition. A Review. Aliment. Hung. Univ. Transylvania 2010, 3, 81–117. [Google Scholar]
- Marton, M.; Mandoki, Z.; Csapo, J. Evaluation of Biological Value of Sprouts I. Fat Content, Fatty Acid Composition. Acta Univ. Sapientiae Aliment. 2010, 3, 53–65. [Google Scholar]
- Świeca, M.; Dziki, D. Improvement in Sprouted Wheat Flour Functionality: Effect of Time, Temperature and Elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Xiao, Z.; Nou, X.; Luo, Y.; Wang, Q. Comparison of the Growth of Escherichia Coli O157: H7 and O104: H4 during Sprouting and Microgreen Production from Contaminated Radish Seeds. Food Microbiol. 2014, 44, 60–63. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Y. The Primary Active Components, Antioxidant Properties, and Differential Metabolite Profiles of Radish Sprouts (Raphanus Sativus L.) upon Domestic Storage: Analysis of Nutritional Quality. J. Sci. Food Agric. 2018, 98, 5853–5860. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Villaluenga, C.; Frías, J.; Gulewicz, P.; Gulewicz, K.; Vidal-Valverde, C. Food Safety Evaluation of Broccoli and Radish Sprouts. Food Chem. Toxicol. 2008, 46, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Donkor, O.N.; Stojanovska, L.; Ginn, P.; Ashton, J.; Vasiljevic, T. Germinated Grains–Sources of Bioactive Compounds. Food Chem. 2012, 135, 950–959. [Google Scholar] [CrossRef]
- Van Hung, P.; Maeda, T.; Yamamoto, S.; Morita, N. Effects of Germination on Nutritional Composition of Waxy Wheat. J. Sci. Food Agric. 2011, 92, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.S.; Grunwald, C. Lipid and Fatty Acid Changes during Germination of Alfalfa Seeds. Phytochemistry 1990, 29, 1441–1445. [Google Scholar] [CrossRef]
- Kaymak, H.C. Seed Fatty Acid Profiles: Potential Relations between Seed Germination under Temperature Stress in Selected Vegetable Species. Acta Sci. Pol. Hortorum Cultus 2014, 13, 119–133. [Google Scholar]
- Gulewicz, P.; Martínez-Villaluenga, C.; Frias, J.; Ciesiołka, D.; Gulewicz, K.; Vidal-Valverde, C. Effect of Germination on the Protein Fraction Composition of Different Lupin Seeds. Food Chem. 2008, 107, 830–844. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Viršilė, A.; Danilčenko, H.; Duchovskis, P.; Paulauskienė, A.; Gajewski, M. Effects of Germination Time on the Antioxidant Properties of Edible Seeds. CyTA- J. Food 2019, 17, 447–454. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a Process to Increase the Polyphenol Content and Antioxidant Activity of Lupin Seeds (Lupinus Angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic Compounds in Grains, Sprouts and Wheatgrass of Hulled and Non-Hulled Wheat Species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, Extraction and Encapsulation: A Review; Institution of Chemical Engineers: London, UK, 2015; Volume 93, ISBN 9182125139. [Google Scholar]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Magdalena, G.-P. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. Hindawi BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Yang, F.; Basu, T.K.; Ooraikul, B. Studies on Germination Conditions and Antioxidant Contents of Wheat Grain. Int. J. Food Sci. Nutr. 2001, 52, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, H.; Frias, J.; Piskuła, M.K.; Kozłowska, H.; Vidal-Valverde, C. Vitamin B1 and B2, Dietary Fiber and Minerals Content of Cruciferae Sprouts. Eur. Food Res. Technol. 2005, 221, 78–83. [Google Scholar] [CrossRef]
- Žilić, S.; Basić, Z.; Hadži-Tašković Šukalović, V.; Maksimović, V.; Janković, M.; Filipović, M. Can the Sprouting Process Applied to Wheat Improve the Contents of Vitamins and Phenolic Compounds and Antioxidant Capacity of the Flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Žilić, S.; Hadži-Tašković Šukalović, V.; Dodig, D.; Maksimović, V.; Maksimović, M.; Basić, Z. Antioxidant Activity of Small Grain Cereals Caused by Phenolics and Lipid Soluble Antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Mrad, R.; Debs, E.; Maroun, R.G.; Louka, N. Multiple Optimization of Chemical Components and Texture of Purple Maize Expanded by IVDV Treatment Using the Response Surface Methodology. Food Chem. 2014, 165, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Nader, J.; Afif, C.; Louka, N. Expansion of Partially Defatted Peanuts by a New Texturizing Process Called “Intensification of Vaporization by Decompression to the Vacuum” (IVDV). Innov. Food Sci. Emerg. Technol. 2017, 41, 179–187. [Google Scholar] [CrossRef]
- Abi-Khattar, A.M.; Rajha, H.N.; Abdel-Massih, R.M.; Habchi, R.; Maroun, R.G.; Debs, E.; Louka, N. “Intensification of Vaporization by Decompression to the Vacuum” (IVDV), a Novel Technology Applied as a Pretreatment to Improve Polyphenols Extraction from Olive Leaves. Food Chem. 2021, 342, 128236. [Google Scholar] [CrossRef]


| Wheat | Alfalfa | Radish | ||||
|---|---|---|---|---|---|---|
| Days of Germination | Crude Fat | Crude Proteins | Crude Fat | Crude Proteins | Crude Fat | Crude Proteins |
| 0 | 2.55 ± 0.01 a | 10.42 ± 0.17 a | 10.18 ± 0.05 a | 36.59 ± 0.20 a | 35.11 ± 0.43 a | 31.12 ± 0.08 a |
| 1 | 2.52 ± 0.05 a | 10.29 ± 0.17 a | 10.22 ± 0.25 a | 36.55 ± 0.20 a | 35.30 ± 0.43 a | 31.06 ± 0.03 a |
| 2 | 2.54 ± 0.07 a | 10.23 ± 0.17 a | 10.35 ± 0.21 a | 38.36 ± 0.32 b | 35.16 ± 0.50 a | 31.00 ± 0.04 a |
| 3 | 2.75 ± 0.03 a | 10.67 ± 0.06 a | 8.62 ± 0.33 b | 39.94 ± 0.37 c | 34.67 ± 0.25 a | 31.69 ± 0.18 b |
| 4 | 2.86 ± 0.02 b | 10.76 ± 0.12 a | 8.49 ± 0.05 b | 41.46 ± 0.09 d | 35.61 ± 0.46 a | 32.04 ± 0.09 b |
| 5 | 3.01 ± 0.03 c | 11.29 ± 0.08 b | 7.71 ± 0.13 c | 42.86 ± 0.24 e | 33.95 ± 0.48 b | 33.07 ± 0.02 c |
| 6 | 3.39 ± 0.03 d | 12.20 ± 0.05 c | 6.63 ± 0.20 d | 43.90 ± 0.21 f | 27.49 ± 0.37 c | 33.98 ± 0.15 d |
| 7 | 3.89 ± 0.02 e | 13.24 ± 0.13 d | 6.07 ± 0.36 d | 44.77 ± 0.16 g | 25.43 ± 0.18 d | 35.07 ± 0.10 e |
| RDAs per Day | Content per Cup (33 g) | |||||
|---|---|---|---|---|---|---|
| Women | Men | Wheat | Alfalfa | Radish | ||
| Optimal day of germination | Day 7 | Day 6 | Day 4 | |||
| Vitamin B2 | (mg) | 1.3 | 1.1 | 0.04 | 0.08 | 0.09 |
| Vitamin B6 | (mg) | 1.3 | 1.3 | 0.05 | 0.56 | 0.80 |
| Vitamin E | (mg) | 15 | 15 | 0.49 | 8.12 | 5.31 |
| Vitamin A | (µg RAE) | 700 | 900 | 12.73 | 50.53 | 72.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, H.; Debs, E.; Koubaa, M.; Alrayess, Z.; Maroun, R.G.; Louka, N. Sprouts Use as Functional Foods. Optimization of Germination of Wheat (Triticum aestivum L.), Alfalfa (Medicago sativa L.), and Radish (Raphanus sativus L.) Seeds Based on Their Nutritional Content Evolution. Foods 2022, 11, 1460. https://doi.org/10.3390/foods11101460
Francis H, Debs E, Koubaa M, Alrayess Z, Maroun RG, Louka N. Sprouts Use as Functional Foods. Optimization of Germination of Wheat (Triticum aestivum L.), Alfalfa (Medicago sativa L.), and Radish (Raphanus sativus L.) Seeds Based on Their Nutritional Content Evolution. Foods. 2022; 11(10):1460. https://doi.org/10.3390/foods11101460
Chicago/Turabian StyleFrancis, Helga, Espérance Debs, Mohamed Koubaa, Zeina Alrayess, Richard G. Maroun, and Nicolas Louka. 2022. "Sprouts Use as Functional Foods. Optimization of Germination of Wheat (Triticum aestivum L.), Alfalfa (Medicago sativa L.), and Radish (Raphanus sativus L.) Seeds Based on Their Nutritional Content Evolution" Foods 11, no. 10: 1460. https://doi.org/10.3390/foods11101460
APA StyleFrancis, H., Debs, E., Koubaa, M., Alrayess, Z., Maroun, R. G., & Louka, N. (2022). Sprouts Use as Functional Foods. Optimization of Germination of Wheat (Triticum aestivum L.), Alfalfa (Medicago sativa L.), and Radish (Raphanus sativus L.) Seeds Based on Their Nutritional Content Evolution. Foods, 11(10), 1460. https://doi.org/10.3390/foods11101460











