Starch-Rich Microalgae as an Active Ingredient in Beer Brewing
Abstract
:1. Introduction
2. Materials and Methods
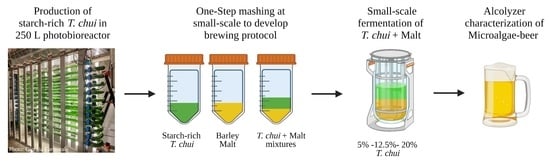
2.1. Biomass Production
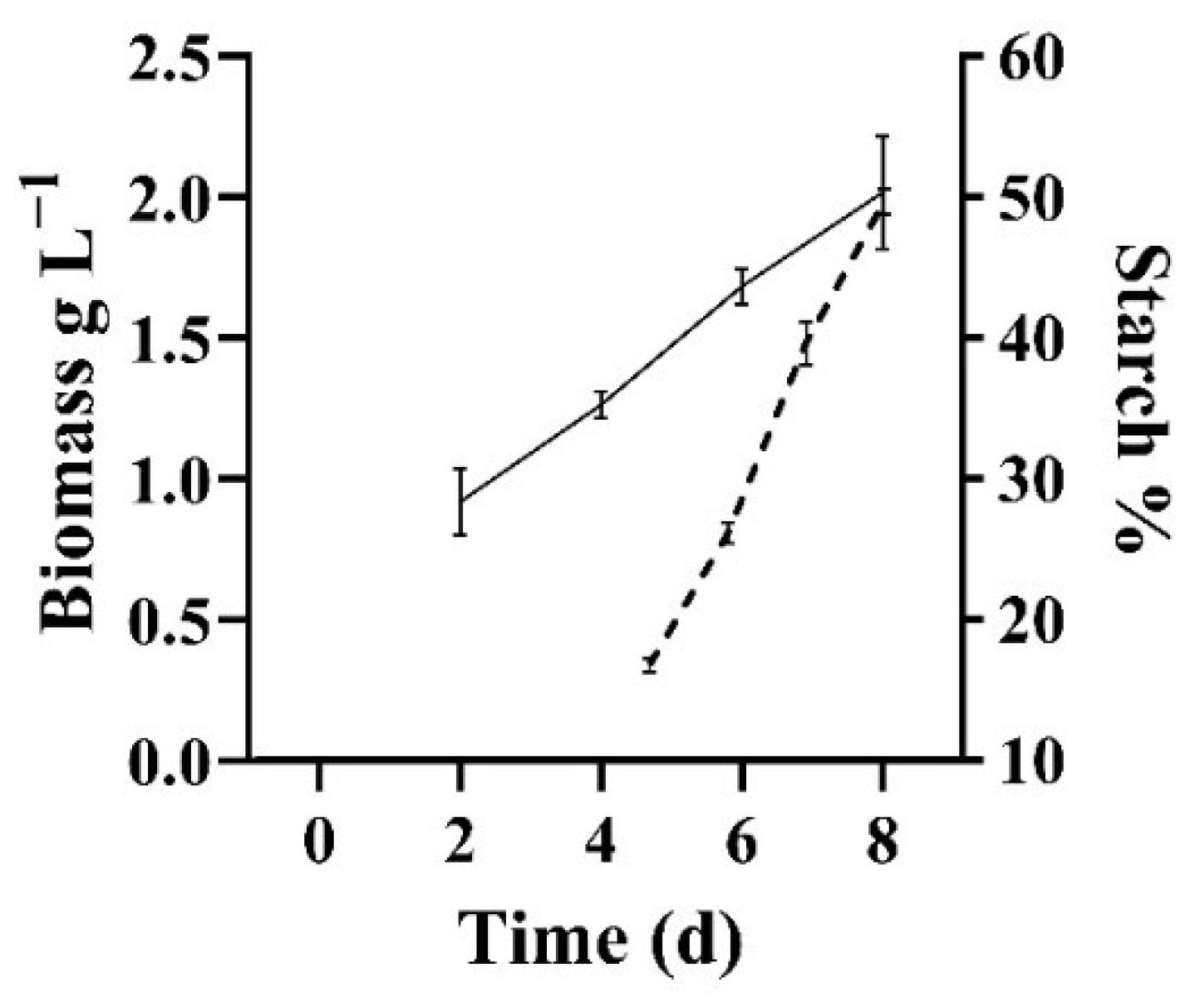
2.1.1. Microalgal Cultivation
2.1.2. Microalgal Biomass Harvesting and Milling
2.1.3. Microalgal Biomass Analysis
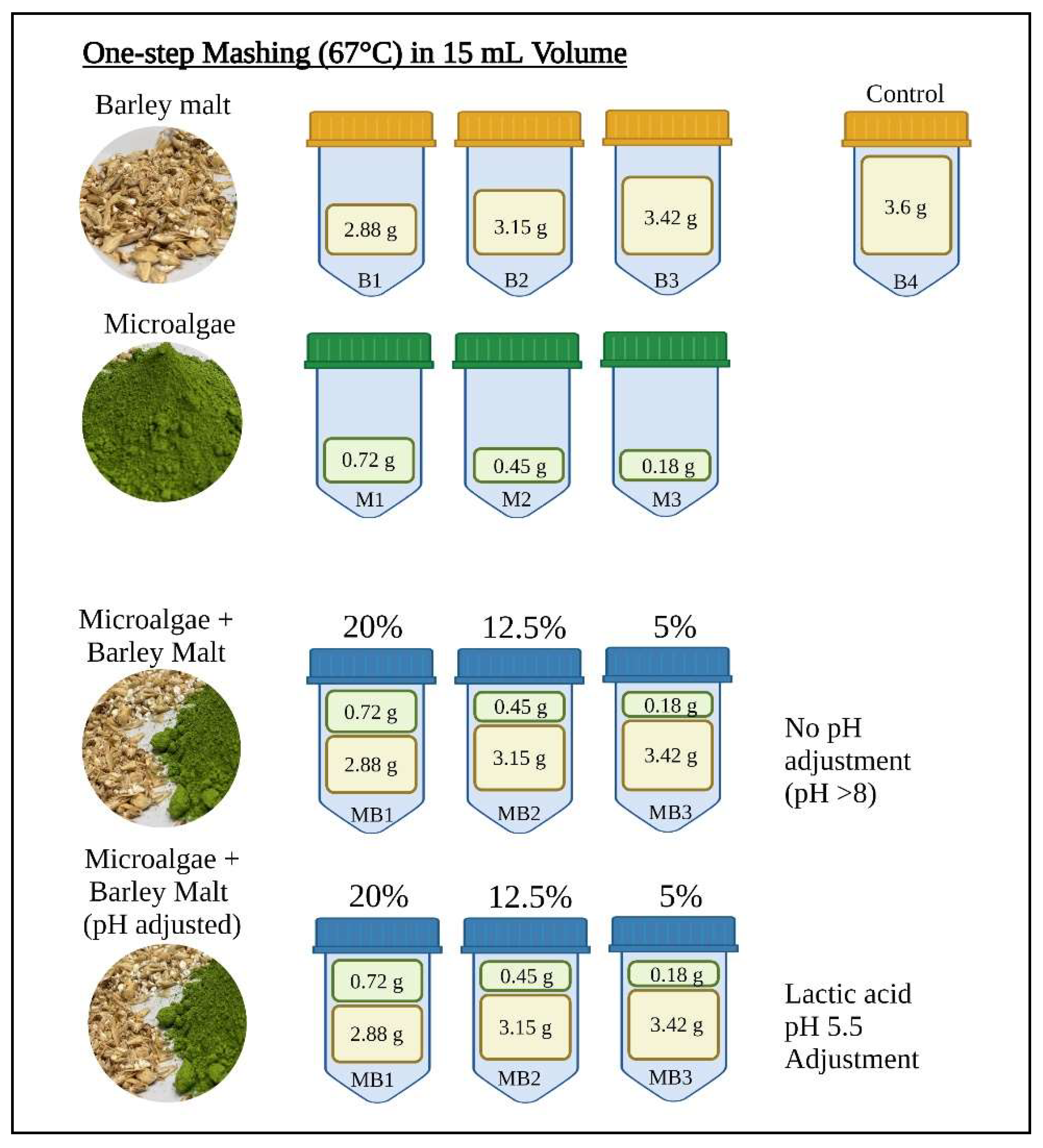
2.2. Mashing Experiments with ALGAL Biomass
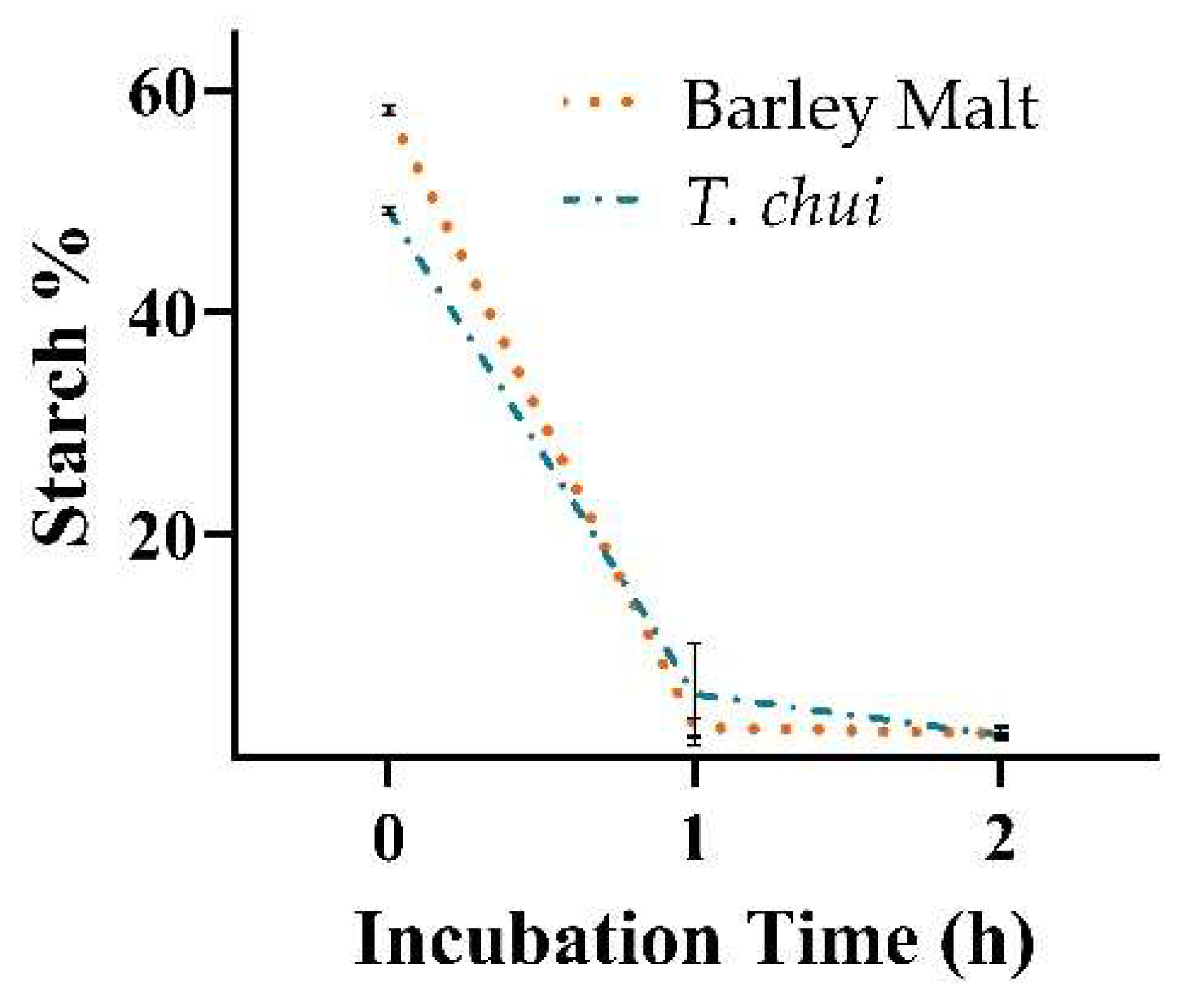
2.2.1. Microalgal Starch Degradation by Barley Enzymes
2.2.2. Mashing with Malt and Microalgae
2.3. Brewing Experiment
2.4. Ethanol and Apparent Degree of Fermentation (ADF)
2.5. HPLC Analysis of Glucose and Maltose
2.6. Data Analysis
3. Results and Discussion
3.1. Microalgal Biomass Production and Characteristics
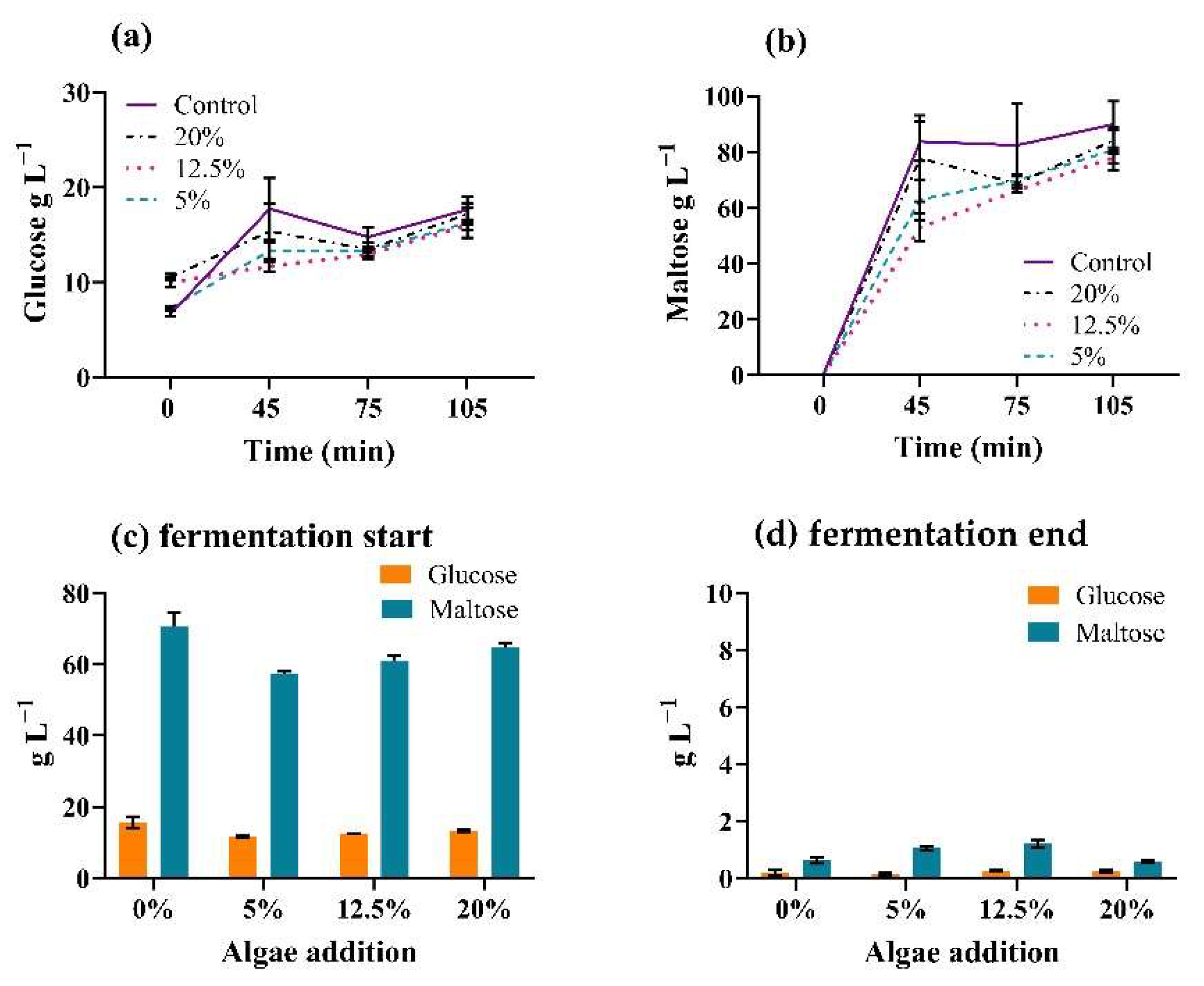
3.2. Mashing Trials
3.3. Brewing Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Raja, R.; Ravi Kumar, R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Sedjati, S.; Pringgenies, D.; Fajri, M. Determination of the Pigment Content and Antioxidant Activity of the Marine Microalga Tetraselmis seucica. Jordan J. Biol. Sci. 2020, 13, 55–58. [Google Scholar]
- Lee, S.-H.; Chang, D.-U.; Lee, B.-J.; Jeon, Y.-J. Antioxidant Activity of Solubilized Tetraselmis suecica and Chlorella ellipsoidea by Enzymatic Digests. Prev. Nutr. Food Sci. 2009, 14, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Reitan, K.I.; Øie, G.; Jørgensen, H.; Wang, X. Chemical composition of selected marine microalgae, with emphasis on lipid and carbohydrate production for potential use as feed resources. J. Appl. Phycol. 2021, 33, 3831–3842. [Google Scholar] [CrossRef]
- Mantecón, L.; Moyano, R.; Cameán, A.M.; Jos, A. Safety assessment of a lyophilized biomass of Tetraselmis chui (TetraSOD®) in a 90 day feeding study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Uhlen, A.K.; Kousoulaki, K.; Haugen, J.E.; Rieder, A. Protein enrichment of wheat bread with the marine green microalgae Tetraselmis chuii—Impact on dough rheology and bread quality. LWT 2021, 143, 111115. [Google Scholar] [CrossRef]
- Lafarga, T.; Mayre, E.; Echeverria, G.; Viñas, I.; Villaró, S.; Acién-Fernández, F.G.; Castellari, M.; Aguiló-Aguayo, I. Potential of the microalgae Nannochloropsis and Tetraselmis for being used as innovative ingredients in baked goods. LWT 2019, 115, 108439. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.I.; Sousa, I.; Raymundo, A. Tetraselmis chui as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A.; et al. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. Biofuels Bioprod. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Nunes, M.C.; Graça, C.; Vlaisavljević, S.; Tenreiro, A.M.; Sousa, I.; Raymundo, A. Microalgal cell disruption: Effect on the bioactivity and rheology of wheat bread. Algal Res. Biofuels Bioprod. 2020, 45, 101749. [Google Scholar] [CrossRef]
- Song, C.; Hu, X.; Liu, Z.; Li, S.; Kitamura, Y. Combination of brewery wastewater purification and CO2 fixation with potential value-added ingredients production via different microalgae strains cultivation. J. Clean. Prod. 2020, 268, 122332. [Google Scholar] [CrossRef]
- Amenorfenyo, D.K.; Huang, X.; Zhang, Y.; Zeng, Q.; Zhang, N.; Ren, J.; Huang, Q. Microalgae Brewery Wastewater Treatment: Potentials, Benefits and the Challenges. Int. J. Environ. Res. Public Health 2019, 16, 1910. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Han, T.; Yarish, C.; Kim, J.K. Microalgae and Alcohol. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 227–234. ISBN 9780128114056. [Google Scholar]
- Dantas, D.M.M.; Cahú, T.B.; Oliveira, C.Y.B.; Abadie-Guedes, R.; Roberto, N.A.; Santana, W.M.; Gálvez, A.O.; Guedes, R.C.A.; Bezerra, R.S. Chlorella vulgaris functional alcoholic beverage: Effect on propagation of cortical spreading depression and functional properties. PLoS ONE 2021, 16, e0255996. [Google Scholar] [CrossRef]
- Brányiková, I.; Maršálková, B.; Doucha, J.; Brányik, T.; Bišová, K.; Zachleder, V.; Vítová, M. Microalgae-novel highly efficient starch producers. Biotechnol. Bioeng. 2011, 108, 766–776. [Google Scholar] [CrossRef]
- Yao, C.; Ai, J.; Cao, X.; Xue, S.; Zhang, W. Enhancing starch production of a marine green microalga Tetraselmis subcordiformis through nutrient limitation. Bioresour. Technol. 2012, 118, 438–444. [Google Scholar] [CrossRef]
- Carnovale, G.; Lama, C.; Torres, S.; Rosa, F.; Mantecón, L.; Horn, S.J.; Skjånes, K.; Infante, C. Metabolic pathways for biosynthesis and degradation of starch in Tetraselmis chui during nitrogen deprivation and recovery. Bioresour. Technol. 2022, 354, 127222. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Springer: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar]
- Carnovale, G.; Rosa, F.; Shapaval, V.; Dzurendova, S.; Kohler, A.; Wicklund, T.; Horn, S.J.; Barbosa, M.J.; Skjånes, K. Starch Rich Chlorella vulgaris: High-Throughput Screening and Up-Scale for Tailored Biomass Production. Appl. Sci. 2021, 11, 9025. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Bio. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Pereira, H.; Silva, J.; Santos, T.; Gangadhar, K.N.; Raposo, A.; Nunes, C.; Coimbra, M.A.; Gouveia, L.; Barreira, L.; Varela, J. Nutritional Potential and Toxicological Evaluation of Tetraselmis sp. CTP4 Microalgal Biomass Produced in Industrial Photobioreactors. Molecules 2019, 24, 3192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahmen-Ben Moussa, I.; Chtourou, H.; Karray, F.; Sayadi, S.; Dhouib, A. Nitrogen or phosphorus repletion strategies for enhancing lipid or carotenoid production from Tetraselmis marina. Bioresour. Technol. 2017, 238, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Kaneda, H.; Kuroda, H.; Watari, J.; Kurihara, T.; Shinotsuka, K. Behavior of Mono-, Di-, and trihydroxyoctadecenoic acids during mashing and methods of controlling their production. J. Biosci. Bioeng. 2000, 90, 69–73. [Google Scholar] [CrossRef]
- Cozzolino, D.; Degner, S. An overview on the role of lipids and fatty acids in barley grain and their products during beer brewing. Food Res. Int. 2016, 81, 114–121. [Google Scholar] [CrossRef]
- Ran, W.; Wang, H.; Liu, Y.; Qi, M.; Xiang, Q.; Yao, C.; Zhang, Y.; Lan, X. Storage of starch and lipids in microalgae: Biosynthesis and manipulation by nutrients. Bioresour. Technol. 2019, 291, 121894. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.; Jha, A.; Rasane, P.; Gautam, A.K. Optimization of a process for high fibre and high protein biscuit. J. Food Sci. Technol. 2015, 52, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Graça, C.; Fradinho, P.; Sousa, I.; Raymundo, A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT 2018, 89, 466–474. [Google Scholar] [CrossRef]

| Beer 1 | Beer 2 | Beer 3 | Beer 4 | |
|---|---|---|---|---|
| Malt % | 100 | 95 | 87.5 | 80 |
| Algae % | 0 | 5 | 12.5 | 20 |
| Pilsner Malt (g) | 182.4 | 173.28 | 159.6 | 145.92 |
| Cara malt (g) | 9.6 | 9.12 | 8.4 | 7.68 |
| Tetraselmis chui (g) | 0 | 9.6 | 24 | 38.4 |
| Northern Brewer 60 min (g) | 0.4 | 0.4 | 0.4 | 0.4 |
| Cascade 0 min (g) | 1.6 | 1.6 | 1.6 | 1.6 |
| Saaz 0 min (g) | 1.6 | 1.6 | 1.6 | 1.6 |
| % Dry Weight | SD | |
|---|---|---|
| Starch | 49.42 | 0.82 |
| Proteins | 22.41 | 1.57 |
| Fatty Acids | 5.20 | 0.56 |
| SFA | 1.13 | |
| MUFA | 1.35 | |
| PUFA | 2.71 |
| Microalgae Content | Alcohol | Haze | Er | Ea | Colour | Calories | pH | CO2 |
|---|---|---|---|---|---|---|---|---|
| %v/v | EBC | %w/w | %w/w | EBC | kJ 100 mL−1 | - | vol | |
| Control, 0% | 5.62 ± 0.14 | 4.1 ± 4.07 | 4.13 ± 0.04 | 2.1 ± 0.03 | 7.55 ± 1.36 | 191.09 ± 3.71 | 4.15 ± 0.02 | 1.88 ± 0.31 |
| 5% | 5.61 ± 0.06 | 3.23 ± 0.17 | 4.41 ± 0.13 | 2.38 ± 0.12 | 8.22 ± 0.11 | 195.08 ± 3.2 | 4.4 ± 0 | 1.88 ± 0.08 |
| 12.5% | 5.46 ± 0.09 | 3.47 ± 1.63 | 5.04 ± 0.09 | 3.09 ± 0.13 | 9.3 ± 0.36 | 201.41 ± 0.56 | 4.6 ± 0.02 | 1.8 ± 0.21 |
| 20% | 5.11 ± 0.07 | 4.38 ± 0.24 | 5.45 ± 0.06 | 3.62 ± 0.04 | 9.69 ± 0.81 | 199.83 ± 2.26 | 4.87 ± 0.03 | 1.73 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnovale, G.; Leivers, S.; Rosa, F.; Norli, H.-R.; Hortemo, E.; Wicklund, T.; Horn, S.J.; Skjånes, K. Starch-Rich Microalgae as an Active Ingredient in Beer Brewing. Foods 2022, 11, 1449. https://doi.org/10.3390/foods11101449
Carnovale G, Leivers S, Rosa F, Norli H-R, Hortemo E, Wicklund T, Horn SJ, Skjånes K. Starch-Rich Microalgae as an Active Ingredient in Beer Brewing. Foods. 2022; 11(10):1449. https://doi.org/10.3390/foods11101449
Chicago/Turabian StyleCarnovale, Giorgia, Shaun Leivers, Filipa Rosa, Hans-Ragnar Norli, Edvard Hortemo, Trude Wicklund, Svein Jarle Horn, and Kari Skjånes. 2022. "Starch-Rich Microalgae as an Active Ingredient in Beer Brewing" Foods 11, no. 10: 1449. https://doi.org/10.3390/foods11101449
APA StyleCarnovale, G., Leivers, S., Rosa, F., Norli, H.-R., Hortemo, E., Wicklund, T., Horn, S. J., & Skjånes, K. (2022). Starch-Rich Microalgae as an Active Ingredient in Beer Brewing. Foods, 11(10), 1449. https://doi.org/10.3390/foods11101449