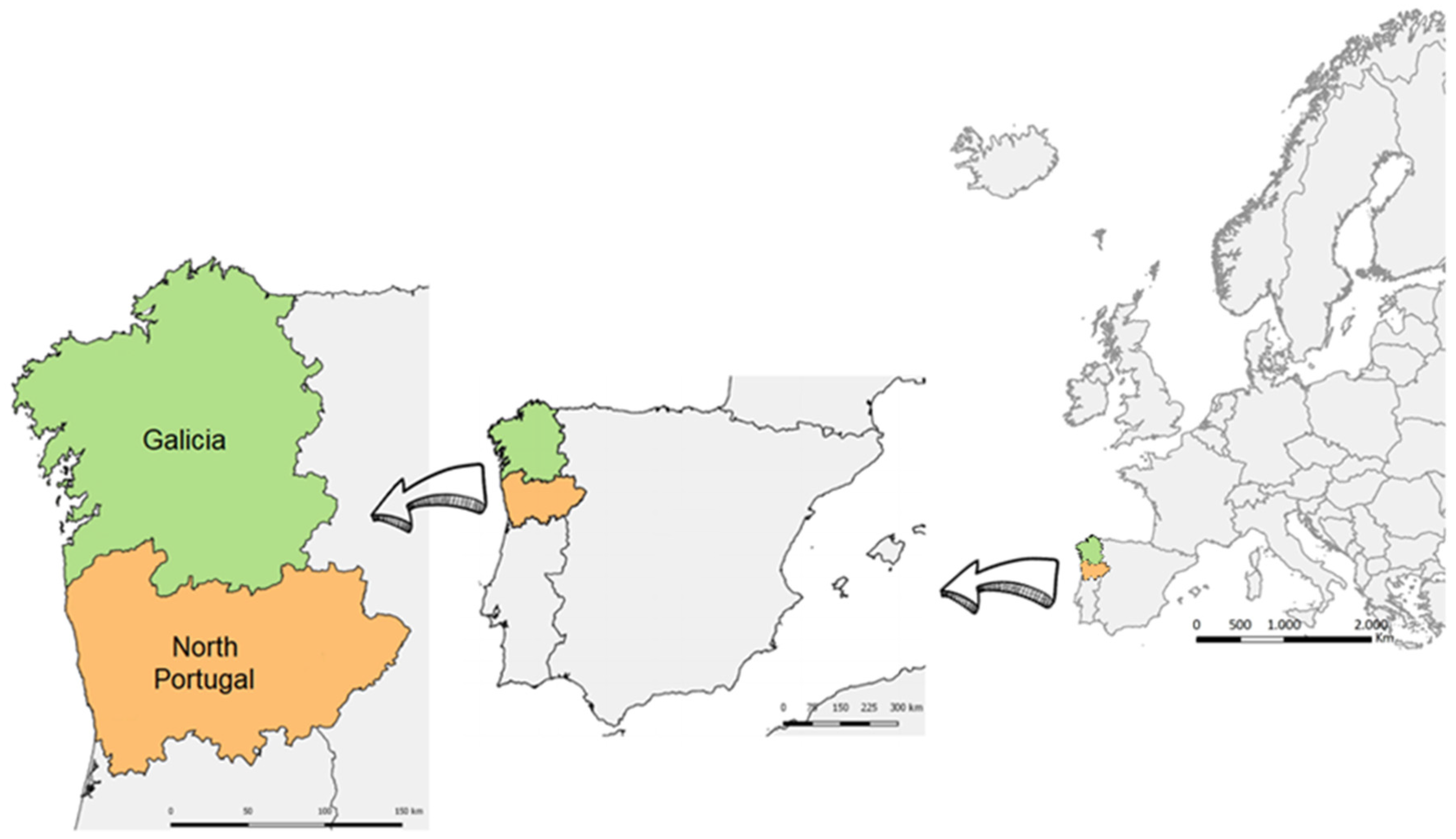
Methodological Approaches for Monitoring Five Major Food Safety Hazards Affecting Food Production in the Galicia–Northern Portugal Euroregion
Abstract
:1. Introduction
2. Toxic Phytoplankton
2.1. Characterization of the Hazard
2.2. Methodologies of Monitoring
2.3. Market, Current State and Prospective
3. Listeria monocytogenes-Containing Biofilms
3.1. Characterization of the Hazard
3.2. Methodologies for Detection
3.3. Market Opportunity
4. Mycotoxins
4.1. Characterization of the Hazard
4.2. Methodologies of Monitoring
4.3. Market, Current State and Prospective
5. Food Allergens
5.1. Characterization of the Hazard
5.2. Methodologies of Monitoring
5.3. Market, Current State and Prospective
6. Polycyclic Aromatic Hydrocarbons (PAHs)
6.1. Characterization of the Hazard
6.2. Methodologies of Monitoring
6.3. Current and Prospective Market State
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IGE. Instituto Galego de Estatística. Available online: https://www.ige.eu/web/index.jsp?idioma=es (accessed on 9 June 2021).
- Norte 2020. Estratégia Regional de Especialização Inteligente. Available online: https://www.norte2020.pt/sites/default/files/public/uploads/documentos/norte2020_ris3.pdf (accessed on 6 July 2021).
- INE. Instituto Nacional de Estadística. Available online: https://www.ine.es/ (accessed on 9 June 2021).
- Cross-Border Smart Specialisation Strategy of Galicia-Northern Portugal. Available online: http://gain.xunta.gal/artigos/74/investigacion+referencia+competitiva?locale=es_ES (accessed on 21 December 2021).
- FoodSens. Available online: https://qa.ff.up.pt/foodsens/indexe.php (accessed on 9 June 2021).
- Sousa, C.A.M.; Cunha, M.E.; Ribeiro, L. Tracking 130 Years of Coastal Wetland Reclamation in Ria Formosa, Portugal: Opportunities for Conservation and Aquaculture. Land Use Policy 2020, 94, 104544. [Google Scholar] [CrossRef]
- Harmful Algal Information System. Available online: http://haedat.iode.org/browseEvents.php (accessed on 8 June 2021).
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Micotoxinas em Portugal: Ocorrência e Toxicidade. Acta Farm. Port. 2012, 1, 61–73. [Google Scholar]
- Abrunhosa, L.; Morales, H.; Soares, C.; Calado, T.; Vila-Chã, A.S.; Pereira, M.; Venâncio, A. A Review of Mycotoxins in Food and Feed Products in Portugal and Estimation of Probable Daily Intakes. Crit. Rev. Food Sci. Nutr. 2016, 56, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Scientific Opinion on the Evaluation of Allergenic Foods and Food Ingredients for Labelling Purposes. Available online: https://www.efsa.europa.eu/es/efsajournal/pub/3894 (accessed on 9 June 2021).
- SEAIC. 2017. Available online: https://www.congresoseaic.org/SEAIC2017 (accessed on 6 July 2021).
- Ingenbleek, L.; Veyrand, B.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.J.; Dembélé, Y.K.; Eyangoh, S.; Verger, P.; et al. Polycyclic Aromatic Hydrocarbons in Foods from the First Regional Total Diet Study in Sub-Saharan Africa: Contamination Profile and Occurrence Data. Food Control. 2019, 103, 133–144. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commission Recommendation of 4 February 2005 on the Further Investigation into the Levels of Polycyclic Aromatic Hydrocarbons in Certain Foods [notified under document number C(2005) 256 ] (Text with EEA relevance). Off. J. Eur. Union 2005, 34, 43–45.
- The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [CrossRef]
- Listeriose en Humanos e Alimentos en Galicia, 2009–2015. Available online: https://www.sergas.es/Saude-publica/Documents/3965/BEG%20XXVIII-3.pdf (accessed on 8 July 2021).
- Chen, J.-Q.; Regan, P.; Laksanalamai, P.; Healey, S.; Hu, Z. Prevalence and Methodologies for Detection, Characterization and Subtyping of Listeria monocytogenes and L. ivanovii in Foods and Environmental Sources. Food Sci. Hum. Wellness 2017, 6, 97–120. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Bonaventura, G.D.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- EFSA’s Activities on Emerging Risks in 2014. EFSA Supporting Publ. 2015, 12, 838E. [CrossRef]
- Carpenter, A.C.; Paulsen, I.T.; Williams, T.C. Blueprints for Biosensors: Design, Limitations, and Applications. Genes 2018, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- Vale, P. Shellfish Contamination with Marine Biotoxins in Portugal and Spring Tides: A Dangerous Health Coincidence. Environ. Sci. Pollut. Res. Int. 2020, 27, 41143–41156. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing Risks and Mitigating Impacts of Harmful Algal Blooms on Mariculture and Marine Fisheries. Rev. Aquac. 2020, 12, 1663–1688. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.D.; Lewis, A.M.; Bradley, K.; Maskrey, B.H. Marine Invertebrate Interactions with Harmful Algal Blooms—Implications for One Health. J. Invertebr. Pathol. 2021, 107555. [Google Scholar] [CrossRef]
- Zohdi, E.; Abbaspour, M. Harmful Algal Blooms (Red Tide): A Review of Causes, Impacts and Approaches to Monitoring and Prediction. Int. J. Environ. Sci. Technol. 2019, 16, 1789–1806. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine Harmful Algal Blooms, Human Health and Wellbeing: Challenges and Opportunities in the 21st Century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef] [Green Version]
- Parsaeimehr, A.; Lutzu, G.A.; Rahman Shah, M.; Parra Saldivar, R. Detection to Treatment and Global Impacts of Algal Toxins. Front. Biosci. Sch. Ed. 2019, 11, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Bresnan, E.; Arévalo, F.; Belin, C.; Branco, M.A.C.; Cembella, A.D.; Clarke, D.; Correa, J.; Davidson, K.; Dhanji-Rapkova, M.; Lozano, R.F.; et al. Diversity and Regional Distribution of Harmful Algal Events along the Atlantic Margin of Europe. Harmful Algae 2021, 102, 101976. [Google Scholar] [CrossRef]
- Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin. Off. J. Eur. Union 2004, 139, 55–205.
- Regulation (EC) No. 854/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Rules for the Organisation of Official Controls on Products of Animal Origin Intended for Human Consumption. Off. J. Eur. Union 2004, 139, 206–320.
- Commission Regulation (EU) No. 15/2011 of 10 January 2011 Amending Regulation (EC) No. 2074/2005 as Regards Recognised Testing Methods for Detecting Marine Biotoxins in Live Bivalve Molluscs (Text with EEA Relevance). Off. J. Eur. Union 2011, 6, 3–6.
- Commission Regulation (EU) No 786/2013 of 16 August 2013 Amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as Regards the Permitted Limits of Yessotoxins in Live Bivalve Molluscs Text with EEA Relevance. Off. J. Eur. Union 2013, 220, 14.
- Commission Implementing Regulation (EU) 2019/627of 15 March 2019 Laying Down Uniform Practical Arrangements for the Performance of Official Controls on Products of Animal Origin Intended for Human Consumption in Accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as Regards Official Controls (Text with EEA Relevance). Off. J. Eur. Union 2019, 131, 51–100.
- Sistema Nacional de Monitorização de Moluscos Bivalves. Valores de Referência Utilizados Pelo Laboratório de Fitoplâncton (IPMA) para Avaliação da Concentração de Risco de Espécies de Fitoplâncton Tóxico na Água; Sistema Nacional de Monitorização de Moluscos Bivalves: Lisbon, Portugal, 2021; p. 1. Available online: https://www.ipma.pt/bin/docs/publicacoes/pescas.mar/tabela-112017-fitoplancton_zdp.pdf (accessed on 15 June 2021).
- Girault, M.; Beneyton, T.; del Amo, Y.; Baret, J.-C. Microfluidic Technology for Plankton Research. Curr. Opin. Biotechnol. 2019, 55, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Basti, L.; Hégaret, H.; Shumway, S.E. Harmful Algal Blooms and Shellfish. In Harmful Algal Blooms: A Compendium Desk Reference; Shumway, S.E., Burkholder, J.M., Morton, S.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 135–190. ISBN 978-1-118-99467-2. [Google Scholar]
- FAO—Organización de Las Naciones Unidas Para La Alimentación y La Agricultura. El Estado Mundial de La Pesca y La Acuicultura. 2016. Available online: http://www.fao.org/publications/sofia/2016/es/ (accessed on 8 June 2021).
- FAO. (Ed.) The State of World Fisheries and Aquaculture: Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; ISBN 978-92-5-109185-2. [Google Scholar]
- Carpenter, G.; Owen, H. Fish Dependence Day 2018: The Reliance of EU on Fish from Elsewhere. New Economics Foundation, 3 May 2018. [Google Scholar]
- Glibert, P.M.; Pitcher, G.C.; Bernard, S.; Li, M. Advancements and Continuing Challenges of Emerging Technologies and Tools for Detecting Harmful Algal Blooms, Their Antecedent Conditions and Toxins, and Applications in Predictive Models. In Global Ecology and Oceanography of Harmful Algal Blooms; Ecological Studies; Glibert, P.M., Berdalet, E., Burford, M.A., Pitcher, G.C., Zhou, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 339–357. ISBN 978-3-319-70069-4. [Google Scholar]
- Moscetta, P.; Sanfilippo, L.; Savino, E.; Moscetta, P.; Allabashi, R.; Gunatilaka, A. Instrumentation for Continuous Monitoring in Marine Environments. In Proceedings of the MTS/IEEE Biloxi - Marine Technology for Our Future: Global and Local Challenges, OCEANS 2009, Biloxi, MS, USA, 26–29 October 2009; pp. 1–10. [Google Scholar]
- Edler, L.; Elbrächter, M. The Utermöhl Method for Quantitative Phytoplankton Analysis. Microsc. Mol. Methods Quant. Phytoplankton Anal. 2010, 110, 13–20. [Google Scholar]
- Zieger, S.E.; Mistlberger, G.; Troi, L.; Lang, A.; Confalonieri, F.; Klimant, I. Compact and Low-Cost Fluorescence Based Flow-Through Analyzer for Early-Stage Classification of Potentially Toxic Algae and in Situ Semiquantification. Environ. Sci. Technol. 2018, 52, 7399–7408. [Google Scholar] [CrossRef]
- Dunker, S. Hidden Secrets Behind Dots: Improved Phytoplankton Taxonomic Resolution Using High-Throughput Imaging Flow Cytometry. Cytom. Part A 2019, 95, 854–868. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.-H.; Gutierrez-Wing, M.T.; Choi, J.-W. Review—Recent Progress in Portable Fluorescence Sensors. J. Electrochem. Soc. 2021, 168, 017502. [Google Scholar] [CrossRef]
- Otálora, P.; Guzmán, J.L.; Acién, F.G.; Berenguel, M.; Reul, A. Microalgae Classification Based on Machine Learning Techniques. Algal Res. 2021, 55, 102256. [Google Scholar] [CrossRef]
- oxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; Chorus, I.; Welker, M. (Eds.) CRC Press: London, UK, 2021; ISBN 978-1-00-308144-9. [Google Scholar]
- Bracher, A.; Bouman, H.A.; Brewin, R.J.W.; Bricaud, A.; Brotas, V.; Ciotti, A.M.; Clementson, L.; Devred, E.; Di Cicco, A.; Dutkiewicz, S.; et al. Obtaining Phytoplankton Diversity from Ocean Color: A Scientific Roadmap for Future Development. Front. Mar. Sci. 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.-H.; Barnett, J.Z.; Gutierrez-Wing, M.T.; Rusch, K.A.; Choi, J.-W. A Hand-Held Fluorescent Sensor Platform for Selectively Estimating Green Algae and Cyanobacteria Biomass. Sens. Actuators B Chem. 2018, 262, 938–946. [Google Scholar] [CrossRef]
- Cruz, R.C.; Reis Costa, P.; Vinga, S.; Krippahl, L.; Lopes, M.B. A Review of Recent Machine Learning Advances for Forecasting Harmful Algal Blooms and Shellfish Contamination. J. Mar. Sci. Eng. 2021, 9, 283. [Google Scholar] [CrossRef]
- Charlier, C.; Perrodeau, É.; Leclercq, A.; Cazenave, B.; Pilmis, B.; Henry, B.; Lopes, A.; Maury, M.M.; Moura, A.; Goffinet, F.; et al. Clinical Features and Prognostic Factors of Listeriosis: The MONALISA National Prospective Cohort Study. Lancet Infect. Dis. 2017, 17, 510–519. [Google Scholar] [CrossRef]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes Lineages: Genomics, Evolution, Ecology, and Phenotypic Characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- EFSA. Emerging Risks Identification on Food and Feed. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5359 (accessed on 5 August 2020).
- Analysis of the Baseline Survey on the Prevalence of Listeria monocytogenes in Certain Ready-to-Eat Foods in the EU, 2010–2011 Part A: Listeria monocytogenes Prevalence Estimates. EFSA J. 2013, 11, 3241. [CrossRef]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union 2005, 338, 1–26.
- Food Hygiene and Toxicology in Ready-to-Eat. Foods, 1st ed.; Kotzekidou, P. (Ed.) Academic Press: Cambridge, MA, USA, 2016; Available online: https://www.elsevier.com/books/food-hygiene-and-toxicology-in-ready-to-eat-foods/kotzekidou/978-0-12-801916-0 (accessed on 5 July 2021).
- Statista Market Forecast. Ready-to-Eat Meals—Spain. Available online: https://www.statista.com/outlook/cmo/food/convenience-food/ready-to-eat-meals/spain (accessed on 5 July 2021).
- Rodríguez-López, P.; Bernárdez, M.; Rodríguez-Herrera, J.J.; Comesaña, Á.S.; Cabo, M.L. Identification and Metagenetic Characterisation of Listeria monocytogenes -Harbouring Communities Present in Food-Related Industrial Environments. Food Control. 2019, 95, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, B.; Cerf, O. Review—Persistence of Listeria monocytogenes in Food Industry Equipment and Premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 1: Detection Method. UNE-EN ISO 11290-1:2018. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0059546 (accessed on 2 July 2021).
- Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp.—Part 2: Enumeration Method. UNE-EN ISO 11290-2:2018. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0059595 (accessed on 2 July 2021).
- Carpentier, B.; Barre, L. Guidelines on Sampling the Food Processing Area and Equipment for the Detection of Listeria monocytogenes; French Agency for Food, Environmental and Occupational Health Safety: Maisons-Alfort Cedex, France, 2012; pp. 1–15. [Google Scholar]
- González-Rivas, F.; Ripolles-Avila, C.; Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Rodríguez-Jerez, J.J. Biofilms in the Spotlight: Detection, Quantification, and Removal Methods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1261–1276. [Google Scholar] [CrossRef] [Green Version]
- Thouvenot, P.; Vales, G.; Bracq-Dieye, H.; Tessaud-Rita, N.; Maury, M.M.; Moura, A.; Lecuit, M.; Leclercq, A. MALDI-TOF Mass Spectrometry-Based Identification of Listeria Species in Surveillance: A Prospective Study. J. Microbiol. Methods 2018, 144, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-Q.; Healey, S.; Regan, P.; Laksanalamai, P.; Hu, Z. PCR-Based Methodologies for Detection and Characterization of Listeria monocytogenes and Listeria ivanovii in Foods and Environmental Sources. Food Sci. Hum. Wellness 2017, 6, 39–59. [Google Scholar] [CrossRef]
- O’Grady, J.; Ruttledge, M.; Sedano-Balbás, S.; Smith, T.J.; Barry, T.; Maher, M. Rapid Detection of Listeria monocytogenes in Food Using Culture Enrichment Combined with Real-Time PCR. Food Microbiol. 2009, 26, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.J.; Bentham, R.H.; Ross, K.E. Limitations of Using Propidium Monoazide with QPCR to Discriminate between Live and Dead Legionella in Biofilm Samples. Microbiol. Insights 2014, 7, MBI.S17723. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Cousineau, A.; Liu, X.; Luo, Y.; Sun, D.; Li, S.; Gu, T.; Sun, L.; Dillow, H.; Lepine, J.; et al. Direct Metatranscriptome RNA-Seq and Multiplex RT-PCR Amplicon Sequencing on Nanopore MinION—Promising Strategies for Multiplex Identification of Viable Pathogens in Food. Front. Microbiol. 2020, 11, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-T.; Lee, Y.-C.; Lai, Y.-H.; Lim, J.-C.; Huang, N.-T.; Lin, C.-T.; Huang, J.-J. Review of Integrated Optical Biosensors for Point-of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Soni, D.K.; Ahmad, R.; Dubey, S.K. Biosensor for the Detection of Listeria monocytogenes: Emerging Trends. Crit. Rev. Microbiol. 2018, 44, 590–608. [Google Scholar] [CrossRef]
- Vizzini, P.; Braidot, M.; Vidic, J.; Manzano, M. Electrochemical and Optical Biosensors for the Detection of Campylobacter and Listeria: An Update Look. Micromachines 2019, 10, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbinot, S.; Srivastav, A.M.; Vidic, J.; Abdulhalim, I.; Manzano, M. Plasmonic Biosensors for Food Control. Trends Food Sci. Technol. 2021, 111, 128–140. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Tian, S.; Hong, T. Novel Surface-Enhanced Raman Spectroscopy Techniques for DNA, Protein and Drug Detection. Sensors 2019, 19, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, R.; Poltronieri, P. Fluorescence-Free Biosensor Methods in Detection of Food Pathogens with a Special Focus on Listeria monocytogenes. Biosensors 2017, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tsuji, S.; Kitaoka, H.; Kobayashi, H.; Tamai, M.; Honjoh, K.-I.; Miyamoto, T. Simultaneous Detection of Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes at a Very Low Level Using Simultaneous Enrichment Broth and Multichannel SPR Biosensor. J. Food Sci. 2017, 82, 2357–2363. [Google Scholar] [CrossRef]
- Liu, Y.; Guang, J.; Liu, C.; Bi, S.; Liu, Q.; Li, P.; Zhang, N.; Chen, S.; Yuan, H.; Zhou, D.; et al. Simple and Low-Cost Plasmonic Fiber-Optic Probe as SERS and Biosensing Platform. Adv. Opt. Mater. 2019, 7, 1900337. [Google Scholar] [CrossRef]
- Witkowska, E.; Korsak, D.; Kowalska, A.; Janeczek, A.; Kamińska, A. Strain-Level Typing and Identification of Bacteria—A Novel Approach for SERS Active Plasmonic Nanostructures. Anal. Bioanal. Chem. 2018, 410, 5019–5031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeğenoğlu Akçinar, H.; Aslim, B.; Torul, H.; Güven, B.; Zengin, A.; Suludere, Z.; Boyaci, I.H.; Tamer, U. Immunomagnetic Separation and Listeria monocytogenes Detection with Surface-Enhanced Raman Scattering. Turk. J. Med. Sci. 2020, 50, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Yáez-Sedeño, P.; Pingarrón, J.M. Electrochemical Affinity Biosensors in Food Safety. Chemosensors 2017, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Chiriacò, M.S.; Parlangeli, I.; Sirsi, F.; Poltronieri, P.; Primiceri, E. Impedance Sensing Platform for Detection of the Food Pathogen Listeria monocytogenes. Electronics 2018, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Otero, F.; Magner, E. Biosensors—Recent Advances and Future Challenges in Electrode Materials. Sensors 2020, 20, 3561. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6, 1–25. Available online: http://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf (accessed on 5 July 2021).
- Mortensen, B.K. Formation and Detection of Biofilms. Report from Bactoforce International A/S, 1-5. Available online: https://businessdocbox.com/Green_Solutions/89354360-Formation-and-detection-of-biofilms.html (accessed on 5 July 2021).
- Van den Driessche, F.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of Resazurin-Based Viability Staining for Quantification of Microbial Biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef]
- Cattò, C.; Cappitelli, F. Testing Anti-Biofilm Polymeric Surfaces: Where to Start? Int. J. Mol. Sci. 2019, 20, 3794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, M.; Horn, H. Optical Coherence Tomography in Biofilm Research: A Comprehensive Review. Biotechnol. Bioeng. 2017, 114, 1386–1402. [Google Scholar] [CrossRef]
- Wang, K.; Pu, H.; Sun, D.-W. Emerging Spectroscopic and Spectral Imaging Techniques for the Rapid Detection of Microorganisms: An Overview. Compr. Rev. Food Sci. Food Saf. 2018, 17, 256–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartsch, S.M.; Asti, L.; Nyathi, S.; Spiker, M.L.; Lee, B.Y. Estimated Cost to a Restaurant of a Foodborne Illness Outbreak. Public Health Rep. 2018, 133, 274–286. [Google Scholar] [CrossRef] [Green Version]
- USDA ERS—Economic Cost of Major Foodborne Illnesses Increased $2 Billion from 2013 to 2018. Available online: https://www.ers.usda.gov/amber-waves/2021/april/economic-cost-of-major-foodborne-illnesses-increased-2-billion-from-2013-to-2018/ (accessed on 5 July 2021).
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes Persistence in Food-Associated Environments: Epidemiology, Strain Characteristics, and Implications for Public Health. J. Food Prot. 2014, 77, 150–170. [Google Scholar] [CrossRef]
- Vettorazzi, A.; López de Cerain, A. Chapter 17—Mycotoxins as Food Carcinogens. In Environmental Mycology in Public Health; Viegas, C., Pinheiro, A.C., Sabino, R., Viegas, S., Brandão, J., Veríssimo, C., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 261–298. ISBN 978-0-12-411471-5. [Google Scholar]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Commission Directive 2003/100/EC of 31 October 2003 Amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council on Undesirable Substances in Animal Feed (Text with EEA Relevance). Off. J. Eur. Union 2003, 285, 33–37.
- 2013/165/EU: Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products Text with EEA Relevance. Off. J. Eur. Union 2013, 91, 12–15.
- Lerda, D. Mycotoxins Factsheet—4th Edition—EURL-Mycotoxins. Available online: https://ec.europa.eu/jrc/en/eurl/mycotoxins (accessed on 9 June 2021).
- Santos Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent Mycotoxins in Animal Feed: Occurrence and Analytical Methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [Green Version]
- Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs (Text with EEA relevance). Off. J. Eur. Union 2006, 70, 12–34.
- Commission Regulation (EU) No 691/2013 of 19 July 2013 Amending Regulation (EC) No 152/2009 as Regards Methods of Sampling and Analysis (Text with EEA Relevance). Off. J. Eur. Union 2013, 197, 1–12.
- Cunha, S.C.; Sá, S.V.M.; Fernandes, J.O. Multiple Mycotoxin Analysis in Nut Products: Occurrence and Risk Characterization. Food Chem. Toxicol. 2018, 114, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Fernandes, J.O. Development and Validation of a Method Based on a QuEChERS Procedure and Heart-Cutting GC-MS for Determination of Five Mycotoxins in Cereal Products. J. Sep. Sci. 2010, 33, 600–609. [Google Scholar] [CrossRef]
- Ferreira, I.; Fernandes, J.O.; Cunha, S.C. Optimization and Validation of a Method Based in a QuEChERS Procedure and Gas Chromatography–Mass Spectrometry for the Determination of Multi-Mycotoxins in Popcorn. Food Control. 2012, 27, 188–193. [Google Scholar] [CrossRef]
- Xie, L.; Chen, M.; Ying, Y. Development of Methods for Determination of Aflatoxins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2642–2664. [Google Scholar] [CrossRef]
- Nolan, P.; Auer, S.; Spehar, A.; Elliott, C.T.; Campbell, K. Current Trends in Rapid Tests for Mycotoxins. Food Addit. Contam. Part. A 2019, 36, 800–814. [Google Scholar] [CrossRef] [Green Version]
- AHDB. Mycotoxin Contamination in Animal Feed and Forages—BRP+. Available online: https://ahdb.org.uk/knowledge-library/brp-mycotoxin-contamination-in-animal-feed-and-forages (accessed on 5 July 2021).
- Porricelli, A.C.R.; Lippolis, V.; Valenzano, S.; Cortese, M.; Suman, M.; Zanardi, S.; Pascale, M. Optimization and Validation of a Fluorescence Polarization Immunoassay for Rapid Detection of T-2 and HT-2 Toxins in Cereals and Cereal-Based Products. Food Anal. Methods 2016, 9, 3310–3318. [Google Scholar] [CrossRef]
- Castro, R.C.; Ribeiro, D.S.M.; Santos, J.L.M. Visual Detection Using Quantum Dots Sensing Platforms. Coord. Chem. Rev. 2020, 429, 213637. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food Allergy. J. Allergy Clin. Immunol. 2010, 125, S116–S125. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food Allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Planque, M.; Arnould, T.; Gillard, N. Food Allergen Analysis: Detection, Quantification and Validation by Mass Spectrometry; IntechOpen: London, UK, 2017; ISBN 978-953-51-3568-5. [Google Scholar]
- Lomer, M.C.E. Review Article: The Aetiology, Diagnosis, Mechanisms and Clinical Evidence for Food Intolerance. Aliment. Pharm. 2015, 41, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo Definitions for Coeliac Disease and Related Terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA Relevance. Off. J. Eur. Union 2011, 304, 18–63.
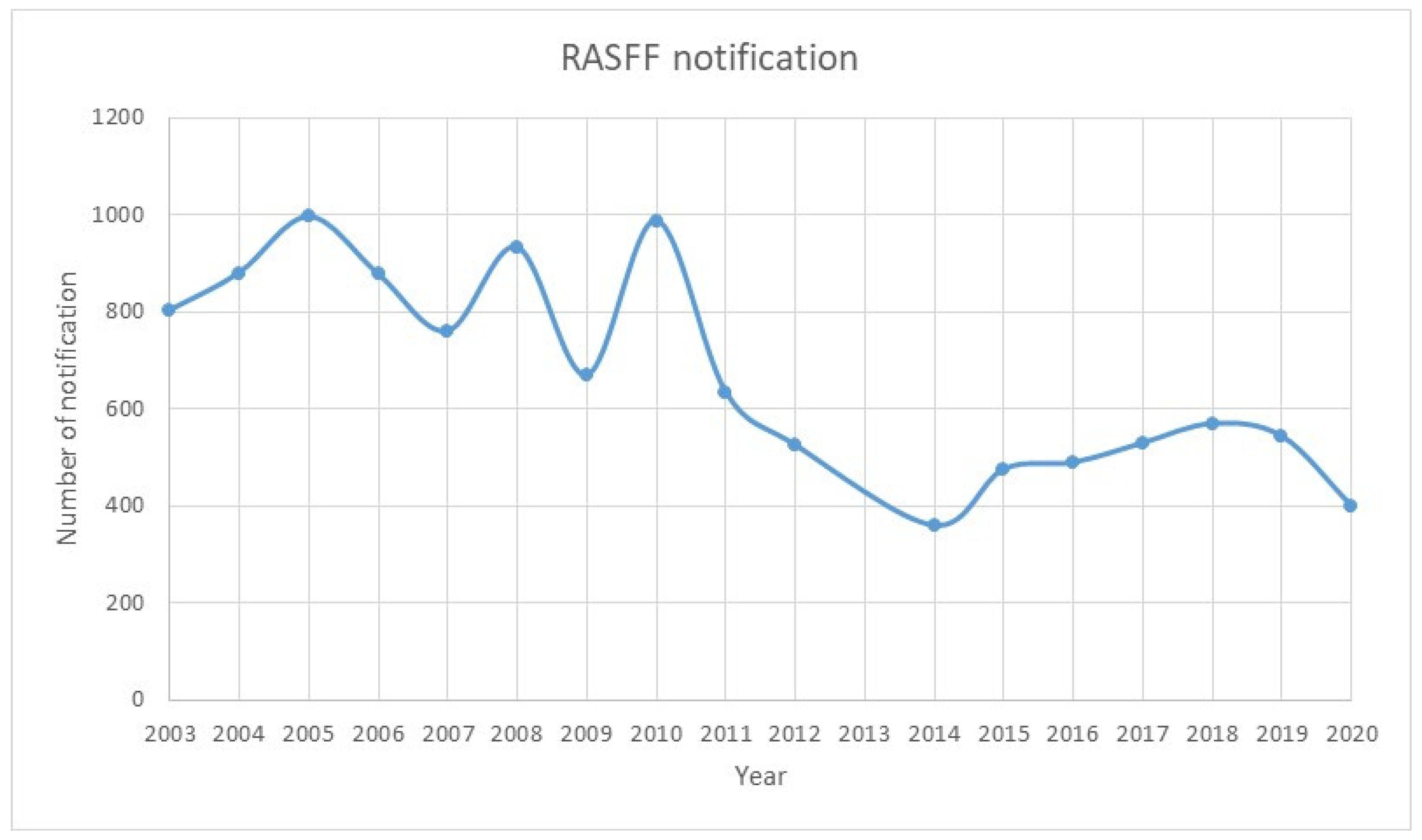
- RASFF WINDOW. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 7 July 2021).
- Comisión Europea, Dirección General de Salud y Seguridad Alimentaria. RASFF Annual Report 2019; Publications Office, 2020; Available online: https://data.europa.eu/doi/10.2875/233333117 (accessed on 9 June 2021).
- Taylor, S.L.; Baumert, J.L. Cross-Contamination of Foods and Implications for Food Allergic Patients. Curr. Allergy Asthma Rep. 2010, 10, 265–270. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible Insects: Cross-Recognition of IgE from Crustacean- and House Dust Mite Allergic Patients, and Reduction of Allergenicity by Food Processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [Green Version]
- De Gier, S.; Verhoeckx, K. Insect (Food) Allergy and Allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef]
- Abbott, M.; Hayward, S.; Ross, W.; Godefroy, S.B.; Ulberth, F.; Van Hengel, A.J.; Roberts, J.; Akiyama, H.; Popping, B.; Yeung, J.M.; et al. Validation Procedures for Quantitative Food Allergen ELISA Methods: Community Guidance and Best Practices. J. AOAC Int. 2010, 93, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Sena-Torralba, A.; Pallás-Tamarit, Y.; Morais, S.; Maquieira, Á. Recent Advances and Challenges in Food-Borne Allergen Detection. TrAC Trends Anal. Chem. 2020, 132, 116050. [Google Scholar] [CrossRef]
- Bräcker, J.; Brockmeyer, J. Characterization and Detection of Food Allergens Using High-Resolution Mass Spectrometry: Current Status and Future Perspective. J. Agric. Food Chem. 2018, 66, 8935–8940. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Lorenzo, J.M.; Gagaoua, M.; Franco, D. Current Trends in Proteomic Advances for Food Allergen Analysis. Biology 2020, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- 14:00-17:00 ISO 21571:2005/Amd 1:2013. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/61/56166.html (accessed on 9 June 2021).
- EN 15633-1:2019—Foodstuffs—Detection of Food Allergens by Immunological Methods—Part 1: General Considerations. Available online: https://standards.iteh.ai/catalog/standards/cen/08c0833c-62f3-4c37-934d-01666c875ea8/en-15633-1-2019 (accessed on 9 June 2021).
- 14:00-17:00 ISO 21571:2005. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/46/34616.html (accessed on 9 June 2021).
- Villa, C.; Costa, J.; Mafra, I. Lupine Allergens: Clinical Relevance, Molecular Characterization, Cross-Reactivity, and Detection Strategies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3886–3915. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Qian, Y.; Zhang, T.; Zheng, L.; Fu, L. Quantification of Shellfish Major Allergen Tropomyosin by SPR Biosensor with Gold Patterned Biochips. Food Control. 2020, 107, 106547. [Google Scholar] [CrossRef]
- Benedé, S.; Lozano-Ojalvo, D.; Cristobal, S.; Costa, J.; D’Auria, E.; Velickovic, T.C.; Garrido-Arandia, M.; Karakaya, S.; Mafra, I.; Mazzucchelli, G.; et al. New Applications of Advanced Instrumental Techniques for the Characterization of Food Allergenic Proteins. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Cutting-Edge Advances in Electrochemical Affinity Biosensing at Different Molecular Level of Emerging Food Allergens and Adulterants. Biosensors 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Aurelio, R.; Ashley, J.; Rodgers, T.L.; Trinh, L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Development of a NanoMIPs-SPR-Based Sensor for β-Lactoglobulin Detection. Chemosensors 2020, 8, 94. [Google Scholar] [CrossRef]
- Aquino, A.; Conte-Junior, C.A. A Systematic Review of Food Allergy: Nanobiosensor and Food Allergen Detection. Biosensors 2020, 10, 194. [Google Scholar] [CrossRef]
- Fu, L.; Qian, Y.; Zhou, J.; Zheng, L.; Wang, Y. Fluorescence-Based Quantitative Platform for Ultrasensitive Food Allergen Detection: From Immunoassays to DNA Sensors. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3343–3364. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.L.; Vipperman-Cohen, A.; Green, P.H.R.; Lee, A.R.; Reilly, N.R.; Zybert, P.; Lebwohl, B. Portable Gluten Sensors: Qualitative Assessments by Adults and Adolescents with Coeliac Disease. J. Hum. Nutr. Diet. 2020, 33, 876–880. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, C.-H.; Park, J.; Pathania, D.; Castro, C.M.; Fasano, A.; Weissleder, R.; Lee, H. Integrated Magneto-Chemical Sensor For On-Site Food Allergen Detection. ACS Nano 2017, 11, 10062–10069. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The Occurrence of 16 EPA PAHs in Food—A Review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Bansal, V.; Kim, K.-H. Review of PAH Contamination in Food Products and Their Health Hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Amirdivani, S.; Khorshidian, N.; Dana, M.G.; Mohammadi, R.; Mortazavian, A.M.; de Souza, S.L.Q.; Rocha, H.B.; Raices, R. Polycyclic Aromatic Hydrocarbons in Milk and Dairy Products. Int. J. Dairy Technol. 2019, 72, 120–131. [Google Scholar] [CrossRef] [Green Version]
- Barreal, J.; Jannes, G. Spatial and Temporal Wildfire Decomposition as a Tool for Assessment and Planning of an Efficient Forest Policy in Galicia (Spain). Forests 2020, 11, 811. [Google Scholar] [CrossRef]
- Ré, A.; Campos, I.; Keizer, J.J.; Gonçalves, F.J.M.; Pereira, J.L.; Abrantes, N. Effects of Post-Fire Contamination in Sediment-Dwelling Species of Riverine Systems. Sci. Total Environ. 2021, 771, 144813. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Silva, V.; Campos, I.; Pereira, J.L.; Pereira, P.; Keizer, J.J.; Gonçalves, F.; Abrantes, N. Off-Site Impacts of Wildfires on Aquatic Systems - Biomarker Responses of the Mosquitofish Gambusia Holbrooki. Sci. Total Environ. 2017, 581–582, 305–313. [Google Scholar] [CrossRef]
- Labarta, U.; Fernández-Reiriz, M.J. The Galician Mussel Industry: Innovation and Changes in the Last Forty Years. Ocean. Coast. Manag. 2019, 167, 208–218. [Google Scholar] [CrossRef]
- López Cabo, M.; Romalde, J.L.; Simal-Gandara, J.; Gago Martínez, A.; Giráldez Fernández, J.; Bernárdez Costas, M.; Pascual del Hierro, S.; Pousa Ortega, Á.; Manaia, C.M.; Abreu Silva, J.; et al. Identification of Emerging Hazards in Mussels by the Galician Emerging Food Safety Risks Network (RISEGAL). A First Approach. Foods 2020, 9, 1641. [Google Scholar] [CrossRef]
- Rodil, R.; Villaverde-de-Sáa, E.; Cobas, J.; Quintana, J.B.; Cela, R.; Carro, N. Legacy and Emerging Pollutants in Marine Bivalves from the Galician Coast (NW Spain). Environ. Int. 2019, 129, 364–375. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic Aromatic Hydrocarbons’ Formation and Occurrence in Processed Food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Purriños, L.; Bermudez, R.; Cobas, N.; Figueiredo, M.; García Fontán, M.C. Polycyclic Aromatic Hydrocarbons (PAHs) in Two Spanish Traditional Smoked Sausage Varieties: “Chorizo Gallego” and “Chorizo de Cebolla. ” Meat Sci. 2011, 89, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Gomes, A.; Roseiro, L.C. Polycyclic Aromatic Hydrocarbons Incidence in Portuguese Traditional Smoked Meat Products. Food Chem. Toxicol. 2011, 49, 2343–2347. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, L.C.; Gomes, A.; Patarata, L.; Santos, C. Comparative Survey of PAHs Incidence in Portuguese Traditional Meat and Blood Sausages. Food Chem. Toxicol. 2012, 50, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, M.J.; Laranjo, M.; Alves, S.; Fernandes, M.H.; Agulheiro-Santos, A.C.; Fernandes, M.J.; Potes, M.E.; Elias, M. Dry-Cured Meat Products According to the Smoking Regime: Process Optimization to Control Polycyclic Aromatic Hydrocarbons. Foods 2020, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Purriños, L.; Fontán, M.C.G.; Franco, D. Polycyclic Aromatic Hydrocarbons (PAHs) in Two Spanish Traditional Smoked Sausage Varieties: “Androlla” and “Botillo”. Meat Sci. 2010, 86, 660–664. [Google Scholar] [CrossRef]
- Comnea-Stancu, I.R.; Staden, J.F.; Staden, R.-I.S. Review—Trends in Recent Developments in Electrochemical Sensors for the Determination of Polycyclic Aromatic Hydrocarbons from Water Resources and Catchment Areas. J. Electrochem. Soc. 2021, 168, 047504. [Google Scholar] [CrossRef]
- Muñoz, J.; Campos-Lendinez, Á.; Crivillers, N.; Mas-Torrent, M. Selective Discrimination of Toxic Polycyclic Aromatic Hydrocarbons in Water by Targeting π-Stacking Interactions. ACS Appl. Mater. Interfaces 2020, 12, 26688–26693. [Google Scholar] [CrossRef]
- Felemban, S.; Vazquez, P.; Moore, E. Future Trends for In Situ Monitoring of Polycyclic Aromatic Hydrocarbons in Water Sources: The Role of Immunosensing Techniques. Biosensors 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Munawar, H.; Mankar, J.S.; Sharma, M.D.; Garcia-Cruz, A.; Fernandes, L.A.L.; Peacock, M.; Krupadam, R.J. Highly Selective Electrochemical Nanofilm Sensor for Detection of Carcinogenic PAHs in Environmental Samples. Talanta 2020, 219, 121273. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Bodelón, G.; Pastoriza-Santos, I. Recent Progress in Surface-Enhanced Raman Scattering for the Detection of Chemical Contaminants in Water. Front. Chem. 2020, 8, 478. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. One-Step Fabrication of Dopamine-Inspired Au for SERS Sensing of Cd2+ and Polycyclic Aromatic Hydrocarbons. Anal. Chim. Acta 2019, 1062, 131–139. [Google Scholar] [CrossRef]
- Castro-Grijalba, A.; Montes-García, V.; Cordero-Ferradás, M.J.; Coronado, E.; Pérez-Juste, J.; Pastoriza-Santos, I. SERS-Based Molecularly Imprinted Plasmonic Sensor for Highly Sensitive PAH Detection. ACS Sens. 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, X.; Meng, H.; Selvaraj, K.K.; Li, H.; He, H.; Du, W.; Yang, S.; Li, S.; Zhang, L. Past, Present, and Future Perspectives on the Assessment of Bioavailability/Bioaccessibility of Polycyclic Aromatic Hydrocarbons: A 20-Year Systemic Review Based on Scientific Econometrics. Sci. Total Environ. 2021, 774, 145585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, J.; Wang, J.; Zou, Y.; Liu, T.; Zhang, Y.; Liu, G.; Tian, Z. Trace Detection of Polycyclic Aromatic Hydrocarbons in Environmental Waters by SERS. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 234, 118250. [Google Scholar] [CrossRef] [PubMed]
| Type of Poisonings | Toxins Produced | Causative Organisms | Symptoms |
|---|---|---|---|
| PSP (Paralytic Shellfish Poisoning) | Saxitoxins | Gymnodinium catenatum, Pyrodinium bahamense | Potentially fatal (8.5–14%), death occurs within 24 h. In nonlethal cases: tingling, numbness, ataxia, giddiness, drowsiness, fever, rash, and staggering. |
| Alexandrium spp. (e.g., A. minutum, A. tamarense, A. ostenfeldii) | |||
| Marine cyanobacteria (e.g., Anabaena, Aphanizomenon, Planktothrix, Lyngbya, Cylindrospermopsis) | |||
| ASP (Amnesic Shellfish Poisoning) | Domoic Acid | Pseudo-nitzschia spp. Seriata group (cell width >3 µm) | Within 24 h: nausea, vomiting, abdominal cramp and diarrhea. Within 48 h: neurological symptoms, such as dizziness, headache, seizures, disorientation, short-term memory loss, respiratory difficulty, and coma. |
| Pseudo-nitzschia spp. Delicatissima group (cell width <3 µm) | |||
| DSP (Diarrhetic Shellfish Poisoning) | Okadaic Acid | Dinophysis spp. (e.g., D. acuta, D. acuminata, D. fortii, D. ovum) Prorocentrum spp. (e.g., P. lima, P. cordatum) | Diarrhea, nausea, vomiting, abdominal cramps, and chills. |
| AZP (Azaspiracid Shellfish Poisoning) | Azaspiracid | Azadinium spinosum | Diarrhea, vomiting, and abdominal cramps. |
| CFP (Ciguatera Fish Poisoning) | Ciguatoxin/Maitotoxin | Gambierdiscus toxicus, Prorocentrum spp., Ostreopsis spp., Coolia monotis, Thecadinium sp., Amphidinium carterae | Initially diarrhea, vomiting and abdominal pain, followed by neurological dysfunction or temperature sensation, muscle aches, dizziness, anxiety, sweating, numbness, and tingling of the mouth and digits. |
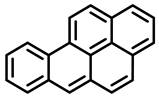 |  | 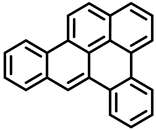 |
| Benzo[a]pyrene | Dibenz[a,h]anthracene | Dibenzo[a,e]pyrene |
| Group 1 | Group 2A | Group 3 |
 |  | 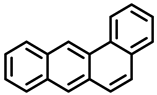 |
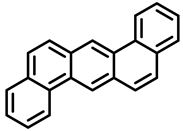
| Dibenzo[a,h]pyrene | Dibenzo[a,i]pyrene | Benzo[a]anthracene |
| Group 2B | Group 2B | Group 2B |
 | 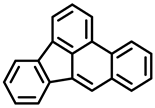 |  |
| Dibenzo[a,l]pyrene | Benzo[b]fluoranthene | Benzo[j]fluoranthene |
| Group 2A | Group 2B | Group 2B |
 | 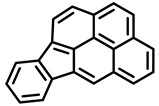 | 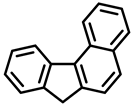 |
| Benzo[k]fluoranthene | Indeno[1,2,3-cd]pyrene | Benzo[c]fluorene |
| Group 2B | Group 2B | Group 3 |
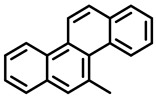 | 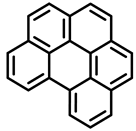 |  |
| 5-Methylchrysene | Benzo[ghi]perylene | Chrysene |
| Group 2B | Group 1 | Group 2B |
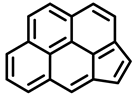 | ||
| Cyclopenta[cd]pyrene | ||
| Group 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Herrera, J.; Cabado, A.G.; Bodelón, G.; Cunha, S.C.; Pinto, V.; Fernandes, J.O.; Lago, J.; Muñoz, S.; Pastoriza-Santos, I.; Sousa, P.; et al. Methodological Approaches for Monitoring Five Major Food Safety Hazards Affecting Food Production in the Galicia–Northern Portugal Euroregion. Foods 2022, 11, 84. https://doi.org/10.3390/foods11010084
Rodríguez-Herrera J, Cabado AG, Bodelón G, Cunha SC, Pinto V, Fernandes JO, Lago J, Muñoz S, Pastoriza-Santos I, Sousa P, et al. Methodological Approaches for Monitoring Five Major Food Safety Hazards Affecting Food Production in the Galicia–Northern Portugal Euroregion. Foods. 2022; 11(1):84. https://doi.org/10.3390/foods11010084
Chicago/Turabian StyleRodríguez-Herrera, Juan, Ana G. Cabado, Gustavo Bodelón, Sara C. Cunha, Vânia Pinto, José O. Fernandes, Jorge Lago, Silvia Muñoz, Isabel Pastoriza-Santos, Paulo Sousa, and et al. 2022. "Methodological Approaches for Monitoring Five Major Food Safety Hazards Affecting Food Production in the Galicia–Northern Portugal Euroregion" Foods 11, no. 1: 84. https://doi.org/10.3390/foods11010084
APA StyleRodríguez-Herrera, J., Cabado, A. G., Bodelón, G., Cunha, S. C., Pinto, V., Fernandes, J. O., Lago, J., Muñoz, S., Pastoriza-Santos, I., Sousa, P., Gonçalves, L., López-Cabo, M., Pérez-Juste, J., Santos, J., & Minas, G. (2022). Methodological Approaches for Monitoring Five Major Food Safety Hazards Affecting Food Production in the Galicia–Northern Portugal Euroregion. Foods, 11(1), 84. https://doi.org/10.3390/foods11010084











