Development of “Quadrello di Ovino”, a Novel Fresh Ewe’s Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk and Starter Culture
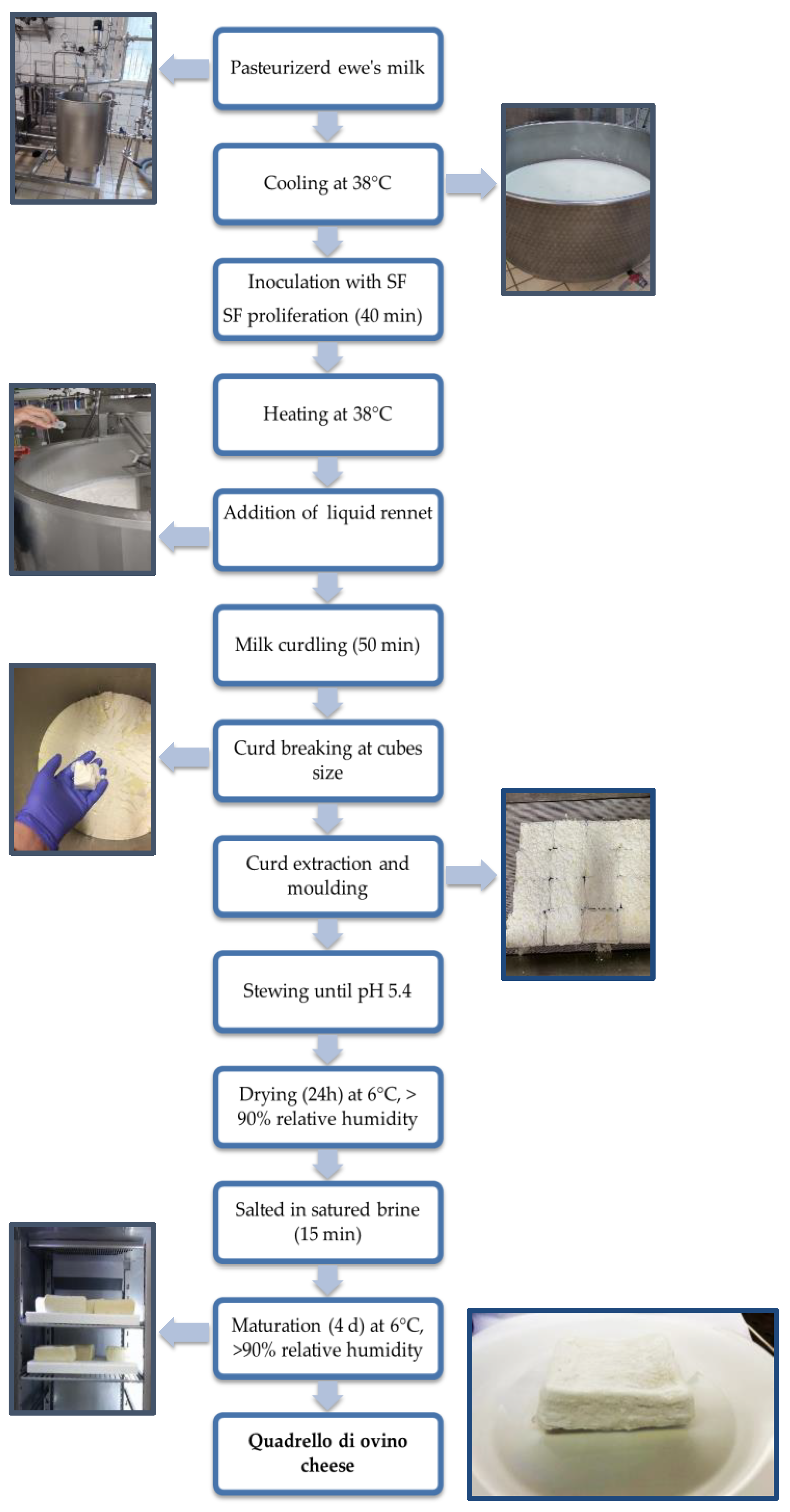
2.2. “Quadrello di Ovino” Cheese Making
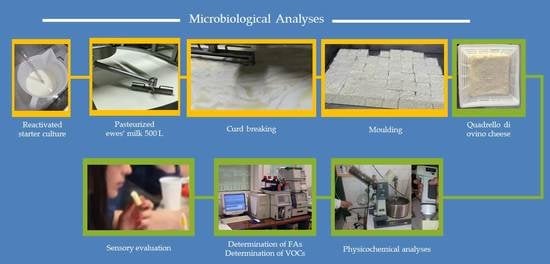
2.3. Microbiological Analyses
2.4. Physicochemical Analyses
2.4.1. Physical Determination
2.4.2. Content of Basic Ingredients and Antioxidant Activity
2.5. Determination of Cheese Fatty Acids
2.6. Volatile Organic Compound Determination
2.7. Sensory Evaluations
2.8. Statistical Analyses
3. Results
3.1. Microbiological Evolution during Cheese Productions
3.2. Physicochemical Characterisation of Cheeses
3.2.1. Cheese Color and Hardness
3.2.2. Basic Chemical Composition and Antioxidant Capacity of Cheese
3.3. Cheese Fatty Acids Composition
3.4. Chemical Composition of Volatile Organic Compounds
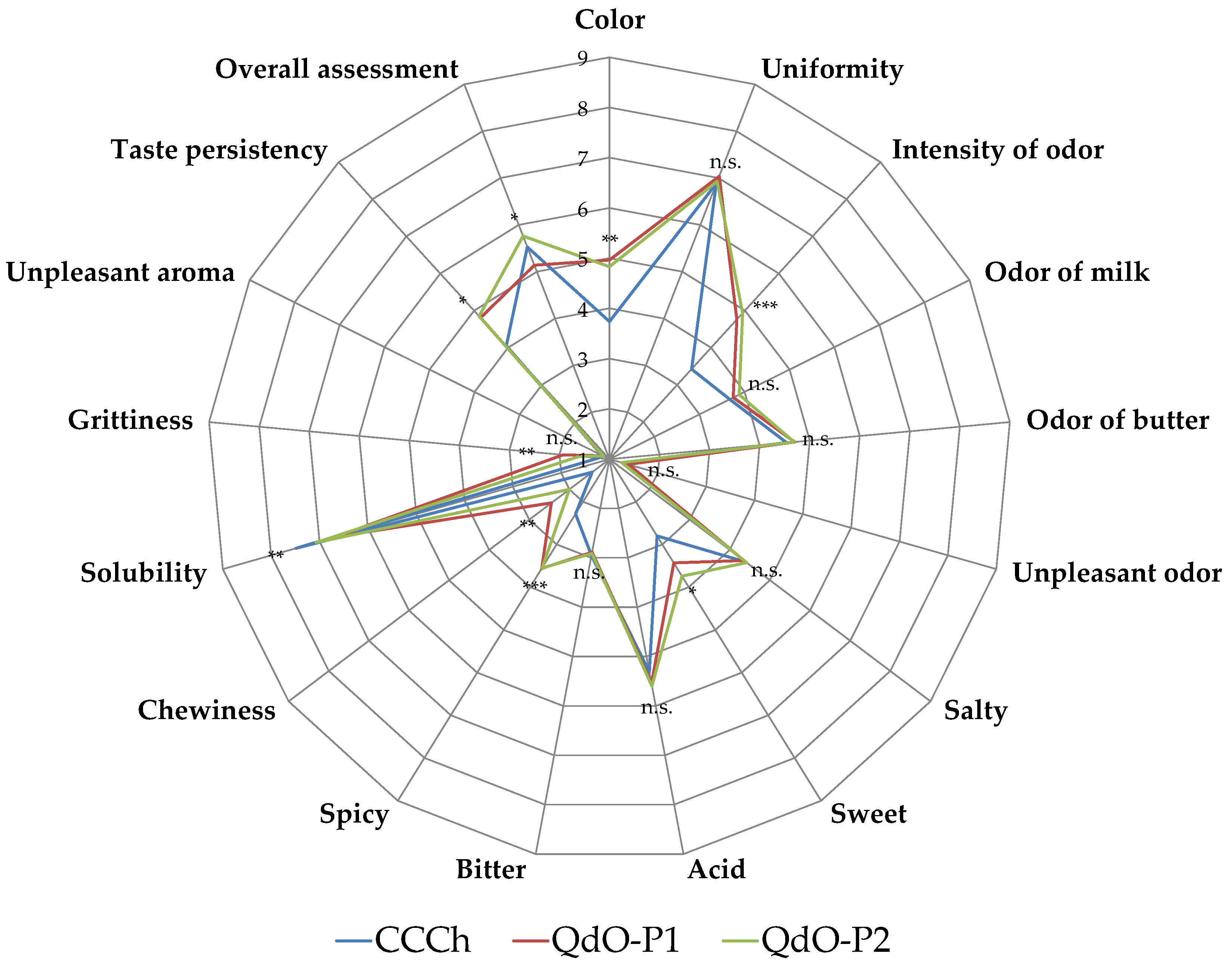
3.5. Sensory Aspects of Cheeses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiné, R.; Correia, P.; Ferrão, A.C. Cheeses around the World: Types, Production, Properties and Cultural and Nutritional Relevance; Nova Science Publishers: New York, NY, USA, 2019. [Google Scholar]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Monfredini, L.; Settanni, L.; Poznanski, E.; Cavazza, A.; Franciosi, E. The spatial distribution of bacteria in Grana-cheese during ripening. Syst. Appl. Microbiol. 2012, 35, 54–63. [Google Scholar] [CrossRef] [PubMed]
- ISMEA, XVIII Rapporto Ismea-Qualivita, L’indagine Socio-Economica del Comparto Italiano Agroalimentare e Vitivinicolo DOP, IGP e STG. 2020. Available online: https://www.qualivita.it/rapporto-ismea-qualivita-2020/ (accessed on 13 October 2020).
- Johnson, M.E.; Kapoor, R.; McMahon, D.J.; McCoy, D.R.; Narasimmon, R.G. Reduction of sodium and fat levels in natural and processed cheeses: Scientific and technological aspects. Compr. Rev. Food Sci. Food Saf. 2009, 8, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, J.A.; Gonzalo, C.; Carriedo, J.A.; San Primitivo, F. Parameters of test day milk yield and milk components for dairy ewes. J. Dairy Sci. 1998, 81, 1300–1307. [Google Scholar] [CrossRef]
- Wei, F.; Yano, H. Development of “New” Bread and Cheese. Processes 2020, 8, 1541. [Google Scholar] [CrossRef]
- Gaglio, R.; Barbaccia, P.; Barbera, M.; Restivo, I.; Attanzio, A.; Maniaci, G.; Di Grigoli, A.; Francesca, N.; Tesoriere, L.; Bonanno, A.; et al. The Use of Winery by-Products to Enhance the Functional Aspects of the Fresh Ovine “Primosale” Cheese. Foods 2021, 10, 461. [Google Scholar] [CrossRef]
- García-Gómez, B.; Vázquez-Odériz, M.L.; Muñoz-Ferreiro, N.; Romero-Rodríguez, M.Á.; Vázquez, M. Novel cheese with vegetal rennet and microbial transglutaminase: Effect of storage on consumer acceptability, sensory and instrumental properties. Int. J. Dairy Technol. 2021, 74, 202–214. [Google Scholar] [CrossRef]
- Barbaccia, P.; Busetta, G.; Barbera, M.; Alfonzo, A.; Garofalo, G.; Francesca, N.; Moscarelli, A.; Moschetti, G.; Settanni, L.; Gaglio, R. Effect of grape pomace from red cultivar “Nero d’Avola” on the microbiological, physicochemical, phenolic profile and sensory aspects of ovine Vastedda-like stretched cheese. J. Appl. Microbiol. 2021; in press. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Marinelli, V.; Saccotelli, M.A.; Del Nobile, M.A.; Conte, A. Fruit and vegetable by-products to fortify spreadable cheese. Antioxidants 2018, 7, 61. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, Z.; Zheng, Y.; Liu, J. A novel application of Monascus purpureus in semi-soft cheese making. J. Food Process. Preserv. 2021, 45, e15209. [Google Scholar] [CrossRef]
- Plessas, S.; Ganatsios, V.; Mantzourani, I.; Bosnea, L. White brined cheese production by incorporation of a traditional milk-cereal prebiotic matrix with a candidate probiotic bacterial strain. Appl. Sci. 2021, 11, 6182. [Google Scholar] [CrossRef]
- Palyvou-Gianna, E.; Vilela, T.P.; Gomes, A.M.; Ferreira, J.P. A starch-milk paste enables the incorporation of ripened cheese in novel fresh cheeses. Food Technol. Biotechnol. 2021, 59, 1–30. [Google Scholar] [CrossRef]
- Storelli, M.M.; Scarano, C.; Spanu, C.; De Santis, E.P.L.; Busco, V.P.; Storelli, A.; Marcotrigiano, G.O. Levels and congener profiles of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) in sheep milk from an industrialised area of Sardinia, Italy. Food Chem. Toxicol. 2012, 50, 1413–1417. [Google Scholar] [CrossRef]
- Gaglio, R.; Restivo, I.; Barbera, M.; Barbaccia, P.; Ponte, M.; Tesoriere, L.; Bonanno, A.; Attanzio, A.; Di Grigoli, A.; Francesca, N.; et al. Effect on the antioxidant, lipoperoxyl radical scavenger capacity, nutritional, sensory and microbiological traits of an ovine stretched cheese produced with grape pomace powder addition. Antioxidants 2021, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Todaro, M.; Settanni, L. Improvement of raw milk cheese hygiene through the selection of starter and non-starter lactic acid bacteria: The successful case of PDO Pecorino Siciliano cheese. Int. J. Environ. 2021, 18, 1834. [Google Scholar] [CrossRef]
- McSweeney, P.L.H.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheese during ripening. Lait 2000, 89, 29–324. [Google Scholar] [CrossRef]
- Mucchetti, G.; Neviani, E. Microbiologia e Tecnologia Lattiero—Casearia—Qualità e Sicurezza, 1st ed.; Tecniche Nuove: Milano, Italy, 2006. [Google Scholar]
- Di Cairano, M.; Pacelli, C.; Bragaglio, A.; Napolitano, F.; Braghieri, A. Sensory Properties and Consumer Liking of Buffalo Stracchino Cheese. J. Buffalo Sci. 2021, 10, 78–84. [Google Scholar] [CrossRef]
- CIE (Commission International de l’Eclairage). Colorimetry. Volume CIE 15.2; Commission International de l’Eclairage: Vienna, Austria, 1986. [Google Scholar]
- Bonanno, A.; Di Grigoli, A.; Vitale, F.; Di Miceli, G.; Todaro, M.; Alabiso, M.; Gargano, M.L.; Venturella, G.; Anike, F.N.; Isikhuemhenal, O.S. Effects of feeding diets supplemented with medicinal mushrooms myceliated grains on some production, health and oxidation traits of dairy ewes. Int. J. Med. Mushrooms 2019, 21, 89–103. [Google Scholar] [CrossRef]
- Maniaci, G.; Alabiso, M.; Francesca, N.; Giosuè, C.; Di Grigoli, A.; Corona, O.; Cardamone, C.; Graci, G.; Portolano, B.; Bonanno, A. Bresaola made from Cinisara cattle: Effect of muscle type and animal category on physicochemical and sensory traits. CyTA-J. Food 2020, 18, 383–391. [Google Scholar] [CrossRef]
- IDF (International Dairy Federation). Determination of the Peroxide Value. Standard FIL-IDF 74A; International Dairy Federation: Brussels, Belgium, 1991. [Google Scholar]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Alabiso, M.; Maniaci, G.; Giosuè, C.; Gaglio, R.; Francesca, N.; Di Grigoli, A.; Portolano, B.; Bonanno, A. Effect of muscle type and animal category on fatty acid composition of bresaola made from meat of Cinisara cattle: Preliminary investigation. CYTA -J. Food. 2020, 18, 734–741. [Google Scholar] [CrossRef]
- Ashkezary, M.R.; Bonanno, A.; Todaro, M.; Settanni, L.; Gaglio, R.; Todaro, A.; Alabiso, M.; Maniaci, G.; Mazza, F.; Di Grigoli, A. Effects of adding solid and molten chocolate on the physicochemical, antioxidant, microbiological, and sensory properties of ewe’s milk cheese. J. Food Sci. 2020, 85, 556–566. [Google Scholar] [CrossRef]
- Tunick, M.; Iandola, S.; Van Hekken, D. Comparison of SPME Methods for Determining Volatile Compounds in Milk, Cheese, and Whey Powder. Foods 2013, 2, 534–543. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- ISO (International Organization for Standardization). Sensory Analysis e General Guidance for the Design of Test Rooms; International Standardization Organization (ISO): Geneva, Switzerland, 2007; Volume 8589. [Google Scholar]
- Tidona, F.; Francolino, S.; Ghiglietti, R.; Locci, F.; Carminati, D.; Laforce, P.; Giraffa, G. Characterization and pre-industrial validation of Streptococcus thermophilus strains to be used as starter cultures for Crescenza, an Italian soft cheese. Food Microbiol. 2020, 92, 103599. [Google Scholar] [CrossRef]
- Ballarini, G. Allevamento Degli Animali e Trasformazione Degli Alimenti di Origine Animale nel Mondo Omerico. In Demografia, Sistemi Agrari, Regimi Alimentari nel Mondo Antico, 1st ed.; Vera, D., Ed.; EdiPuglia: Bari, Italy, 1999; pp. 37–51. [Google Scholar]
- Mucchetti, G.; Bonvini, B.; Remagni, M.C.; Ghiglietti, R.; Locci, F.; Barzaghi, S.; Francolino, S.; Perrone, A.; Rubiloni, A.; Campo, P.; et al. Influence of cheese-making technology on composition and microbiological characteristics of Vastedda cheese. Food Control 2008, 19, 119–125. [Google Scholar] [CrossRef]
- Parente, E.; Guidone, A.; Matera, A.; De Filippis, F.; Mauriello, G.; Ricciardi, A. Microbial community dynamics in thermophilic undefined milk starter cultures. Int. J. Food Microbiol. 2016, 217, 59–67. [Google Scholar] [CrossRef]
- Tidona, F.; Francolino, S.; Ghiglietti, R.; Locci, F.; Brusa, G.; Alinovi, M.; Mucchetti, G.; Giraffa, G. Application of recombined milk to produce crescenza-type cheese in laboratory-scale cheesemaking: Implications on technology and sensory properties. Foods 2020, 9, 928. [Google Scholar] [CrossRef]
- Alinovi, M.; Cordioli, M.; Francolino, S.; Locci, F.; Ghiglietti, R.; Monti, L.; Tidona, F.; Mucchetti, G.; Giraffa, G. Effect of fermentation-produced camel chymosin on quality of Crescenza cheese. Int. Dairy J. 2018, 84, 72–78. [Google Scholar] [CrossRef]
- Burns, P.; Patrignani, F.; Serrazanetti, D.; Vinderola, G.C.; Reinheimer, J.A.; Lanciotti, R.; Guerzoni, M.E. Probiotic Crescenza cheese containing Lactobacillus casei and Lactobacillus acidophilus manufactured with high-pressure homogenized milk. J. Dairy Sci. 2008, 91, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Salvadori del Prato. Trattato di Tecnologia Casearia, 1st ed.; Edagricole: Bologna, Italy, 1998. [Google Scholar]
- Saccenti, E.; Nieuwenhuijse, D.; Koehorst, J.J.; Martins dos Santos, V.A.; Schaap, P.J. Assessing the metabolic diversity of Streptococcus from a protein domain point of view. PLoS ONE 2015, 10, e0137908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food 2021, 61, 1448–1469. [Google Scholar] [CrossRef]
- Rynne, N.M.; Beresford, T.P.; Kelly, A.L.; Guinee, T.P. Effect of milk pasteurisation temperature on age-related changes in lactose metabolism, pH and the growth of non-starter lactic acid bacteria in half-fat Cheddar cheese. Food Chem. 2007, 100, 375–382. [Google Scholar] [CrossRef]
- Gaglio, R.; Gentile, C.; Bonanno, A.; Vintaloro, L.; Perrone, A.; Mazza, F.; Barbaccia, P.; Settanni, L.; Di Grigoli, A. Effect of saffron addition on the microbiological, physicochemical, antioxidant and sensory characteristics of yoghurt. Int. J. Dairy Technol. 2019, 72, 208–217. [Google Scholar] [CrossRef]
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Mancini, L.; Fox, P.F. Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci. Technol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Barbaccia, P.; Busetta, G.; Matraxia, M.; Sutera, A.M.; Craparo, V.; Moschetti, G.; Francesca, N.; Settanni, L.; Gaglio, R. Monitoring commercial starter culture development in presence of red grape pomace powder to produce polyphenol-enriched fresh ovine cheeses at industrial scale level. Fermentation 2021, 7, 35. [Google Scholar] [CrossRef]
- Settanni, L.; Gaglio, R.; Guarcello, R.; Francesca, N.; Carpino, S.; Sannino, C.; Todaro, M. Selected lactic acid bacteria as a hurdle to the microbial spoilage of cheese: Application on a traditional raw ewes’ milk cheese. Int. Dairy J. 2013, 32, 126–132. [Google Scholar] [CrossRef]
- Guarcello, R.; Carpino, S.; Gaglio, R.; Pino, A.; Rapisarda, T.; Caggia, C.; Marino, G.; Randazzo, C.L.; Settanni, L.; Todaro, M. A large factory-scale application of selected autochthonous lactic acid bacteria for PDO Pecorino Siciliano cheese production. Food Microbiol. 2016, 59, 66–75. [Google Scholar] [CrossRef]
- Comi, G.; Cocolin, L.; Collovati, S.; Croattini, I.; Surmelj, A. Studio e prevenzione di un’alterazionecromatica superficiale dello stracchino. Ind. Aliment. 2001, 40, 729–737. [Google Scholar]
- Gaglio, R.; Cruciata, M.; Scatassa, M.L.; Tolone, M.; Mancuso, I.; Cardamone, C.; Corona, O.; Todaro, M.; Settanni, L. Influence of the early bacterial biofilms developed on vats made with seven wood types on PDO Vastedda della valle del Belìce cheese characteristics. Int. J. Food Microbiol. 2019, 291, 91–103. [Google Scholar] [CrossRef]
- Ramírez-Navas, J.S. Espectrocolorimetría: Caracterización de leche y quesos. Tecnol. Láct Latinoam. 2010, 61, 52–58. [Google Scholar]
- Marcuzzo, E.; Peressini, D.; Sensidoni, A. Shelf life of short ripened soft cheese stored under various packaging conditions. J. Food Process. Preserv. 2013, 37, 1094–1102. [Google Scholar] [CrossRef]
- Ricciardi, A.; Guidone, A.; Zotta, T.; Matera, A.; Claps, S.; Parente, E. Evolution of microbial counts and chemical and physico-chemical parameters in high-moisture Mozzarella cheese during refrigerated storage. LWT-Food Sci. Technol. 2015, 63, 821–827. [Google Scholar] [CrossRef]
- Lucisano, M.; Pompei, C.; Casiraghi, E. Texture evaluation of some Italian cheeses by instrumental texture profile analysis. J. Food Qual. 1987, 10, 73–89. [Google Scholar] [CrossRef]
- Filipczak-Fiutak, M.; Pluta-Kubica, A.; Domagała, J.; Duda, I.; Migdał, W. Nutritional value and organoleptic assessment of traditionally smoked cheeses made from goat, sheep and cow’s milk. PLoS ONE 2021, 16, e0254431. [Google Scholar] [CrossRef]
- Branciari, R.; Galarini, R.; Miraglia, D.; Ranucci, D.; Valiani, A.; Giusepponi, D.; Servili, M.; Acuti, G.; Pauselli, M.; Trabalza-Marinucci, M. Dietary supplementation with olive mill wastewater in dairy sheep: Evaluation of cheese characteristics and presence of bioactive molecules. Animals 2020, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Nakagawa, H.; Miyazaki, T. Beneficial effects of antioxidative lactic acid bacteria. AIMS Microbiol. 2017, 3, 1–7. [Google Scholar]
- Gaglio, R.; Franciosi, E.; Todaro, A.; Guarcello, R.; Alfeo, V.; Randazzo, C.L.; Settanni, L.; Todaro, M. Addition of selected starter/non-starter lactic acid bacterial inoculums to stabilise PDO Pecorino Siciliano cheese production. Int. Food Res. J. 2020, 136, 109335. [Google Scholar] [CrossRef] [PubMed]
- Felicio, T.L.; Esmerino, E.A.; Vidal, V.A.S.; Cappato, L.P.; Garcia, R.K.A.; Cavalcanti, R.N.; Freitas, M.Q.; Conte Junior, C.A.; Padilha, M.C.; Silva, M.C.; et al. Physico-chemical changes during storage and sensory acceptance of low sodium probiotic Minas cheese added with arginine. Food Chem. 2016, 196, 628–637. [Google Scholar] [CrossRef]
- Gursoy, O.; Seckin, A.K.; Kinik, O.; Karaman, A.D. The effect of using different probiotic cultures on conjugated linoleic acid (CLA) concentration and fatty acid composition of white pickle cheese. Int. J. Food Sci. Nutr. 2012, 63, 610–615. [Google Scholar] [CrossRef]
- Branciari, R.; Mughetti, L.; Ranucci, D.; Miraglia, D.; Valiani, A.; Acuti, G.; Selvaggini, R.; Trabalza-Marinucci, M. Influence of manufacturing procedure on the compositional and sensory properties of n-3 fatty acid-enriched pecorino cheese. J. Dairy Res. 2014, 81, 455–461. [Google Scholar] [CrossRef]
- Reyrolle, M.; Ghislain, M.; Bru, N.; Vallverdu, G.; Pigot, T.; Desauziers, V.; Le Bechec, M. Volatile fingerprint of food products with untargeted SIFT-MS data coupled with mixOmics methods for profile discrimination: Application case on cheese. Food Chem. 2022, 369, 130801. [Google Scholar] [CrossRef]
- Torri, L.; Aprea, E.; Piochi, M.; Cabrino, G.; Endrizzi, I.; Colaianni, A.; Gasperi, F. Relationship between Sensory Attributes, (Dis) Liking and Volatile Organic Composition of Gorgonzola PDO Cheese. Foods 2021, 10, 2791. [Google Scholar] [CrossRef]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Formation of the aroma of a raw goat milk cheese during maturation analysed by SPME-GC-MS. Food Chem. 2011, 1156, 1153–1163. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Martino, G. Volatile flavor compounds in cheese as affected by ruminant diet. Molecules 2020, 25, 461. [Google Scholar] [CrossRef]
- Bontinis, T.G.; Mallatou, H.; Pappa, E.C.; Massouras, T.; Alichanidis, E. Study of proteolysis, lipolysis and volatile profile of a traditional Greekgoat cheese (Xinotyri) during ripening. Small Rumin. Res. 2012, 105, 193–201. [Google Scholar] [CrossRef]
- Le Quéré, J.-L.; Molimard, P. Cheese Flavour. In Encyclopedia of Dairy Sciences, 1st ed.; Roginski, H., Fuquay, J.W., Fox, P.F., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 330–340. [Google Scholar]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; García-Parra, J.; Ramírez, R. Characterisation by SPME–GC–MS of the volatile profile of a Spanish soft cheese P.D.O. Torta del Casar during ripening. Food Chem. 2010, 118, 182–189. [Google Scholar] [CrossRef]
- Wolf, I.V.; Perotti, M.C.; Bernal, S.M.; Zalazar, C.A. Study of the chemical composition, proteolysis, lipolysis and volatile compounds profile of commercial Reggianito Argentino cheese: Characterization of Reggianito Argentino cheese. Int. Food Res. J. 2010, 43, 1204–1211. [Google Scholar] [CrossRef]
- Guarcello, R.; De Angelis, M.; Settanni, L.; Formiglio, S.; Gaglio, R.; Minervini, F.; Moschetti, G.; Gobbetti, M. Selection of Amine-Oxidizing Dairy Lactic Acid Bacteria and Identification of the Enzyme and Gene Involved in the Decrease of Biogenic Amines. Appl. Environ. Microbiol. 2016, 82, 6870–6880. [Google Scholar] [PubMed]
- Le Quéré, J.-L. Cheese|Cheese Flavor. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., McSweeney, P.L., Fox, P.F., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 675–684. [Google Scholar]
- Guarcello, R.; Diviccaro, A.; Barbera, M.; Giancippoli, E.; Settanni, L.; Minervini, F.; Moschetti, G.; Gobbetti, M. A survey of the main technology, biochemical and microbiological features influencing the concentration of biogenic amines of twenty Apulian and Sicilian (southern Italy) cheeses. Int. Dairy J. 2015, 43, 61–69. [Google Scholar]
- Vivek, K.; Subbarao, K.V.; Routray, W.; Kamini, N.R.; Dash, K.K. Application of fuzzy logic in sensory evaluation of food products: A comprehensive study. Food Bioproc. Tech. 2020, 13, 1–29. [Google Scholar] [CrossRef]
- Ryffel, S.; Piccinali, P.; Bütikofer, U. Sensory descriptive analysis and consumer acceptability of selected Swiss goat and sheep cheeses. Small Rumin. Res. 2008, 79, 80–86. [Google Scholar] [CrossRef]
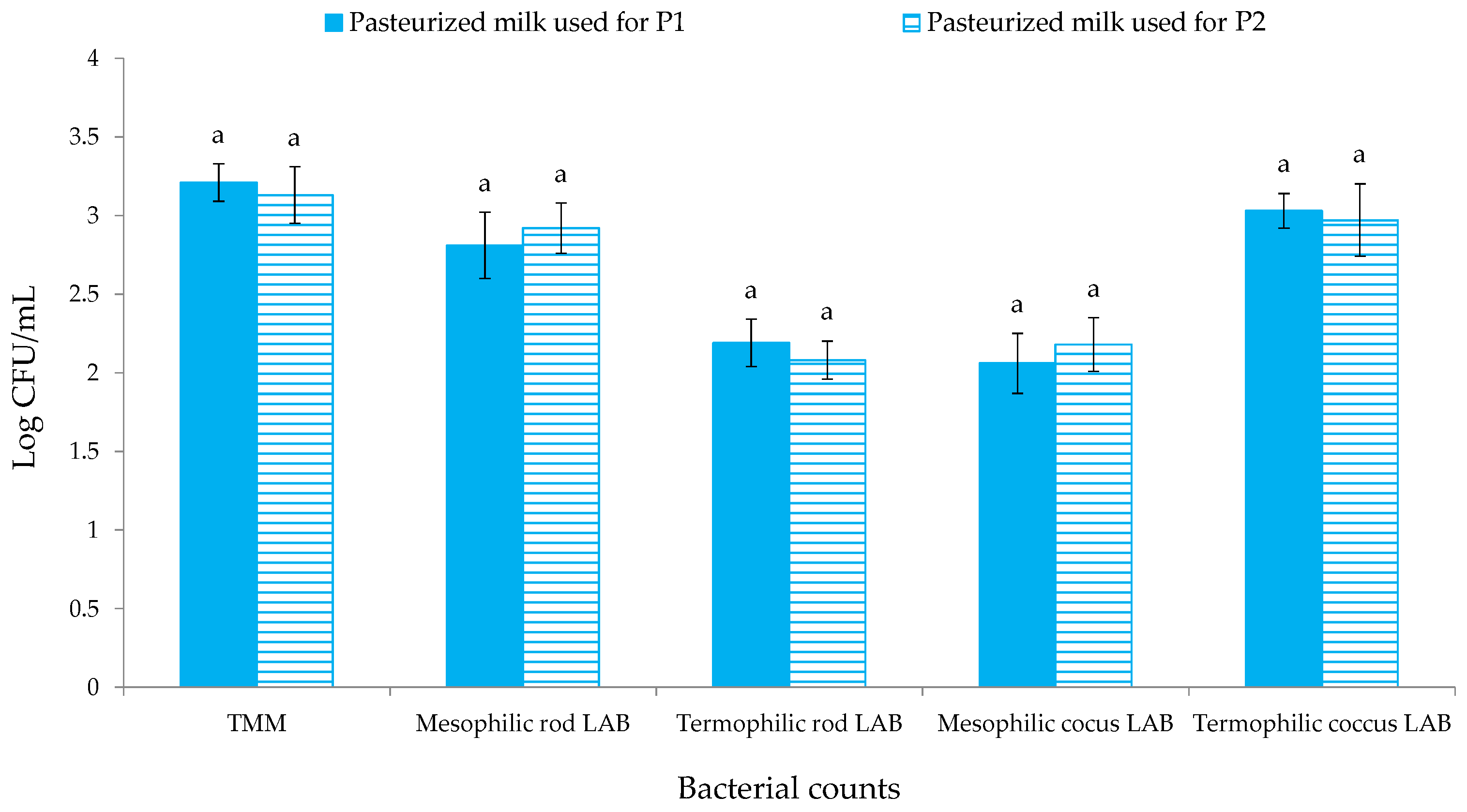
| Samples | Bacterial Counts | |
|---|---|---|
| TMM | S. thermophilus | |
| Inoculated milk | ||
| P1 | 6.84 ± 0.21 a | 7.09 ± 0.12 a |
| P2 | 7.03 ± 0.13 a | 7.26 ± 0.16 a |
| Curd | ||
| P1 | 7.77 ± 0.23 a | 7.98 ± 0.18 a |
| P2 | 7.92 ± 0.20 a | 8.02 ± 0.22 a |
| Cheese | ||
| QdO-P1 | 8.82 ± 0.11 a | 8.99 ± 0.29 a |
| QdO-P2 | 8.73 ± 0.14 a | 8.89 ± 0.11 a |
| Parameters | Samples | |
|---|---|---|
| QdO-P1 | QdO-P2 | |
| Lightness (L*) | 88.32 ± 0.30 a | 86.30 ± 0.63 b |
| Redness (a*) | −3.81 ± 0.28 a | −4.76 ± 0.24 b |
| Yellowness (b*) | 15.18 ± 0.69 b | 16.88 ± 0.08 a |
| Hardness, N/mm2 | 0.18 ± 0.02 b | 0.26 ± 0.03 a |
| Parameters | Samples | |
|---|---|---|
| QdO-P1 | QdO-P2 | |
| Dry matter (DM), % | 63.17 ± 1.47 a | 64.99 ± 0.13 a |
| Ash, % | 5.57 ± 0.08 a | 5.53 ± 0.05 a |
| Protein, % | 35.52 ± 0.89 a | 37.26 ± 0.91 a |
| Fat, % | 54.99 ± 1.01 a | 54.98 ± 0.88 a |
| NaCl, % | 1.68 ± 0.08 a | 1.54 ± 0.01 a |
| PI, % | 1.02 ± 0.04 a | 1.01 ± 0.08 a |
| POV, meq peroxide/kg fat | 0.42 ± 0.01 a | 0.44 ± 0.03 a |
| TBARs, MDA mg/kg DM | 42.71 ± 0.74 a | 36.69 ± 0.37 b |
| Fatty Acids | Samples | |
|---|---|---|
| QdO-P1 | QdO-P2 | |
| Total FAs, % DM | 48.38 ± 0.05 a | 49.80 ± 1.24 a |
| C4 | 1.67 ± 0.04 a | 1.45 ± 0.00 b |
| C6 | 1.88 ± 0.02 a | 1.44 ± 0.03 b |
| C8 | 2.31 ± 0.01 a | 1.71 ± 0.03 b |
| C10:0 | 7.25 ±0.02 a | 5.50 ± 0.06 b |
| C10:1Δc9 | 0.28 ± 0.00 a | 0.22 ± 0.00 b |
| C12:0 | 4.11 ± 0.02 a | 3.43 ± 0.02 b |
| C12:1Δc5 | 0.13 ± 0.00 a | 0.11 ± 0.00 b |
| C14:0 | 11.01 ± 0.05 a | 10.67 ± 0.10 b |
| C14:1Δc9 | 0.23 ± 0.00 a | 0.23 ± 0.00 a |
| C15:0 | 1.89 ± 0.01 a | 1.77 ± 0.02 b |
| C15:1Δc14 | 0.51 ± 0.00 a | 0.48 ± 0.00 b |
| C16:0 | 24.84 ± 0.05 b | 25.73 ± 0.09 a |
| C16:1Δc9 | 0.23 ± 0.00 a | 0.22 ± 0.00 a |
| C17:0 | 1.47 ± 0.00 a | 1.46 ± 0.01 a |
| C17:1Δc9 | 0.29 ± 0.00 a | 0.24 ± 0.00 b |
| C18:0 | 9.81 ± 0.09 b | 10.5 ± 0.04 a |
| C18:1Δc9 (OA) | 17.11 ± 0.04 b | 20.14 ± 0.07 a |
| C18:1Δc11 | 0.64 ± 0.00 a | 0.59 ± 0.01 b |
| C18:1Δt11 | 3.68 ± 0.02 b | 3.91 ± 0.04 a |
| C18:1Δc13 | 3.28 ± 0.02 b | 3.82 ± 0.02 a |
| C18:2Δt9,c12 | 0.88 ± 0.01 a | 0.62 ± 0.01 b |
| C18:2Δc9,c12 (LA) | 1.91 ± 0.01 a | 1.86 ± 0.01 b |
| C18:2Δc9,t11 (RA) | 1.20 ± 0.03 a | 1.30 ± 0.04 a |
| C18:2Δt10,c12 | 0.46 ± 0.00 a | 0.39 ± 0.01 b |
| C18:3Δc9,c12,c15 (ALA) | 1.54 ± 0.00 a | 1.15 ± 0.00 b |
| C18:3Δc6,c9,c12 (GLA) | 0.34 ± 0.00 b | 0.37 ± 0.00 a |
| C20:0 | 0.10 ± 0.01 a | 0.09 ± 0.01 a |
| C20:1Δc11 | 0.07 ± 0.00 b | 0.09 ± 0.00 a |
| C20:2Δc11,c14 | 0.10 ± 0.00 a | 0.08 ± 0.00 b |
| C20:3Δc11,c14,c17 | 0.02 ± 0.00 a | 0.01 ± 0.00 a |
| C20:3Δc8,c11,c14 | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| C20:4Δc5,c8,c11,c14 (AA) | 0.11 ± 0.01 a | 0.11 ± 0.00 a |
| C20:5Δc5,c8,c11,c14,c17 (EPA) | 0.15 ± 0.02 a | 0.15 ± 0.00 a |
| C22:0 | 0.12 ± 0.00 a | 0.13 ± 0.00 a |
| C22:2Δc13,c16 | 0.15 ± 0.00 a | 0.15 ± 0.00 a |
| C22:4Δc8,c11,c14,c17 | 0.03 ± 0.00 a | 0.04 ± 0.00 a |
| C22:5Δc7,c10,c13,c16,c19 (DPA) | 0.12 ± 0.00 a | 0.10 ± 0.00 b |
| C22:6Δc4,c7,c10,c13,c16,c19 | 0.05 ± 0.00 a | 0.03 ± 0.00 b |
| C24:0 | 0.02 ± 0.01 a | 0.02 ± 0.00 a |
| SFA | 66.59 ± 0.04 a | 63.82 ± 0.09 b |
| MUFA | 26.51 ± 0.03 b | 29.92 ± 0.10 a |
| PUFA | 6.94 ± 0.07 a | 6.25 ± 0.01 b |
| n6 | 2.53 ± 0.00 a | 2.49 ± 0.01 b |
| n3 | 1.86 ± 0.03 a | 1.45 ± 0.01 b |
| n6/n3 | 1.36 ± 0.02 b | 1.71 ± 0.00 a |
| HPI | 0.42 ± 0.00 b | 0.47 ± 0.00 a |
| Retention Time (min.sec) | Compounds a (Common Names) | Samples | Identification | |
|---|---|---|---|---|
| QdO-P1 | QdO-P2 | |||
| Σ Alcohols | 4.30 ± 0.34 a | 1.49 ± 0.03 b | ||
| 6.30 | 3-Methyl-1-butanol | 4.30 ± 0.34 a | 1.49 ± 0.03 b | b |
| Σ Ethers | 9.03 ± 0.21 b | 10.33 ± 0.24 a | ||
| 2.75 | Ethyl ether | 9.03 ± 0.21 b | 10.33 ± 0.24 a | b, c |
| Σ Aldehydes | 0.33 ± 0.01 a | n.d. b | ||
| 19.45 | Benzaldehyde | 0.33 ± 0.01 a | n.d. b | b, c |
| Σ Ketones | 41.21 ± 0.96 a | 1.49 ± 0.04 b | ||
| 4.10 | 2-Pentanone | 5.93 ± 0.14 a | n.d. b | b |
| 5.40 | Butan-2-one-3-hydroxy (Acetoin) | 31.33 ± 0.73 a | n.d. b | b |
| 8.50 | 2-Hexanone | 1.22 ± 0.03 a | n.d. b | b |
| 15.35 | 2-Heptanone | n.d. b | 1.09 ± 0.03 a | b |
| 29.45 | 2-Nonanone | 2.73 ± 0.06 a | 0.40 ± 0.01 b | b |
| Σ Carboxylic Acids | 1.29 ± 0.10 b | 21.25 ± 0.49 a | ||
| 4.40 | Acetic acid | 1.29 ± 0.10 b | 15.35 ± 0.35 a | b, c |
| 9.45 | Butanoic acid | n.d. b | 5.90 ± 0.14 a | b |
| Σ Esters | 19.32 ± 0.56 b | 25.52 ± 0.60 a | ||
| 4.27 | Ethyl acetate | n.d. b | 8.43 ± 0.20 a | b, c |
| 9.56 | Ethyl butanoate | n.d. b | 9.83 ± 0.23 a | b |
| 14.45 | Phenethyl hexanoate (Phenethyl caproate) | 17.25 ± 0.40 a | n.d. b | b |
| 22.55 | Ethyl hexanoate (Ethyl caproate) | 0.23 ± 0.02 b | 3.40 ± 0.08 a | b |
| 37.34 | Ethyl octanoate (Ethyl caprylate) | 1.14 ± 0.09 b | 2.33 ± 0.05 a | b, c |
| 50.94 | Ethyl decanoate (Ethyl caprate) | 0.70 ± 0.05 b | 1.53 ± 0.04 a | b |
| Σ Amines | 4.94 ± 0.39 a | n.d. | ||
| 2.55 | Dimethylamine | 4.94 ± 0.39 a | n.d. | b |
| Σ Monoterpenes | n.d. b | 0.20 ± 0.00 a | ||
| 24.60 | Sylvestrene | n.d. b | 0.20 ± 0.00 a | b |
| Σ Others | 1.11 ± 0.03 b | 1.45 ± 0.04 a | ||
| 12.40 | Ethyl benzene | 0.28 ± 0.01 b | 0.67 ± 0.02 a | b |
| 13.00 | 1,3-Dimethyl benzene | 0.83 ± 0.02 a | 0.39 ± 0.01 b | b |
| 30.01 | 1-Butenyl benzene | n.d. b | 0.39 ± 0.01 a | b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garofalo, G.; Busetta, G.; Maniaci, G.; Sardina, M.T.; Portolano, B.; Badalamenti, N.; Maggio, A.; Bruno, M.; Gaglio, R.; Settanni, L. Development of “Quadrello di Ovino”, a Novel Fresh Ewe’s Cheese. Foods 2022, 11, 25. https://doi.org/10.3390/foods11010025
Garofalo G, Busetta G, Maniaci G, Sardina MT, Portolano B, Badalamenti N, Maggio A, Bruno M, Gaglio R, Settanni L. Development of “Quadrello di Ovino”, a Novel Fresh Ewe’s Cheese. Foods. 2022; 11(1):25. https://doi.org/10.3390/foods11010025
Chicago/Turabian StyleGarofalo, Giuliana, Gabriele Busetta, Giuseppe Maniaci, Maria Teresa Sardina, Baldassare Portolano, Natale Badalamenti, Antonella Maggio, Maurizio Bruno, Raimondo Gaglio, and Luca Settanni. 2022. "Development of “Quadrello di Ovino”, a Novel Fresh Ewe’s Cheese" Foods 11, no. 1: 25. https://doi.org/10.3390/foods11010025
APA StyleGarofalo, G., Busetta, G., Maniaci, G., Sardina, M. T., Portolano, B., Badalamenti, N., Maggio, A., Bruno, M., Gaglio, R., & Settanni, L. (2022). Development of “Quadrello di Ovino”, a Novel Fresh Ewe’s Cheese. Foods, 11(1), 25. https://doi.org/10.3390/foods11010025