A Review on Chromatography–Mass Spectrometry Applications on Anthocyanin and Ellagitannin Metabolites of Blackberries and Raspberries
Abstract
1. Introduction
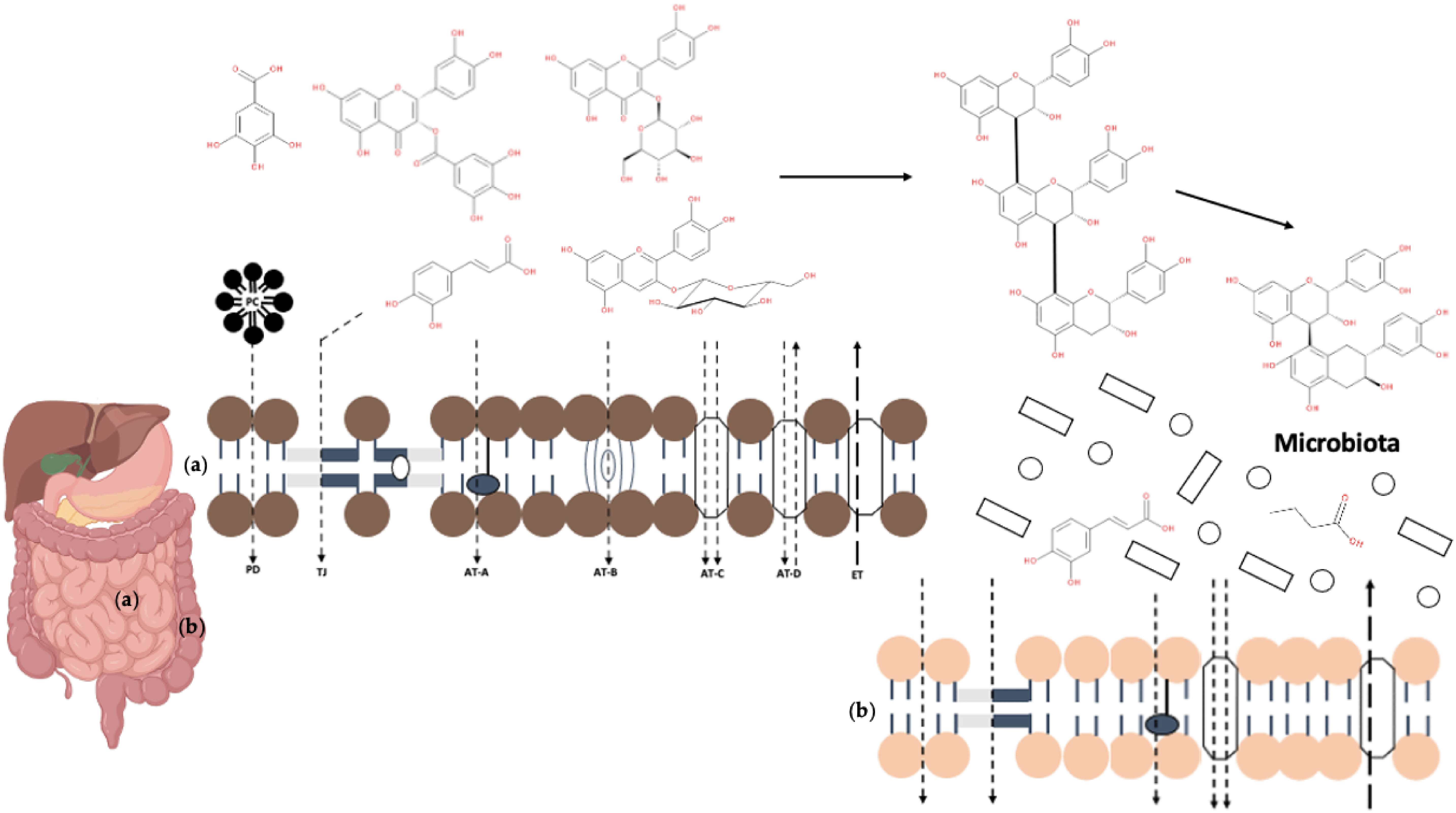
2. Absorption and Metabolism of (Poly)Phenols
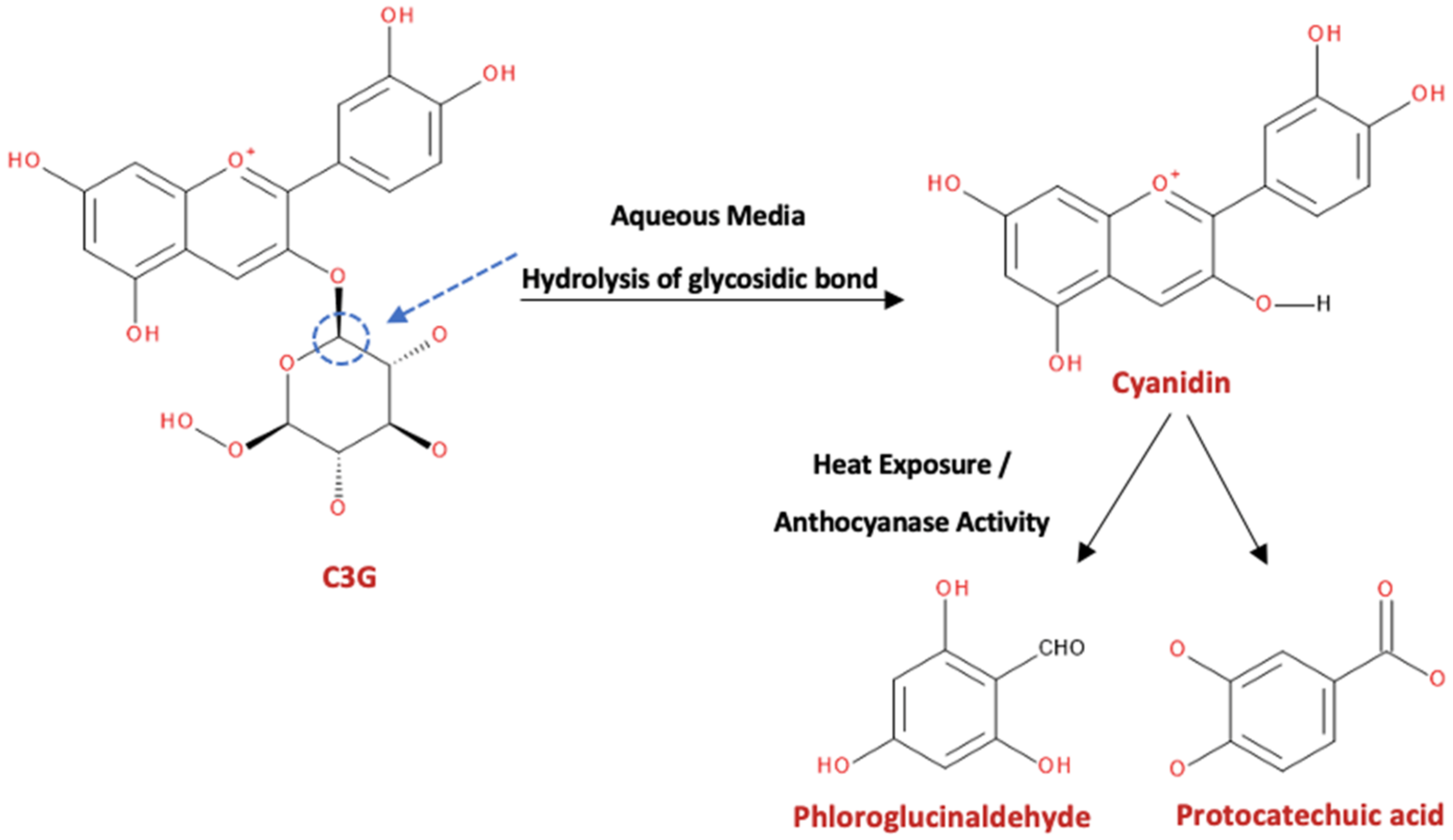
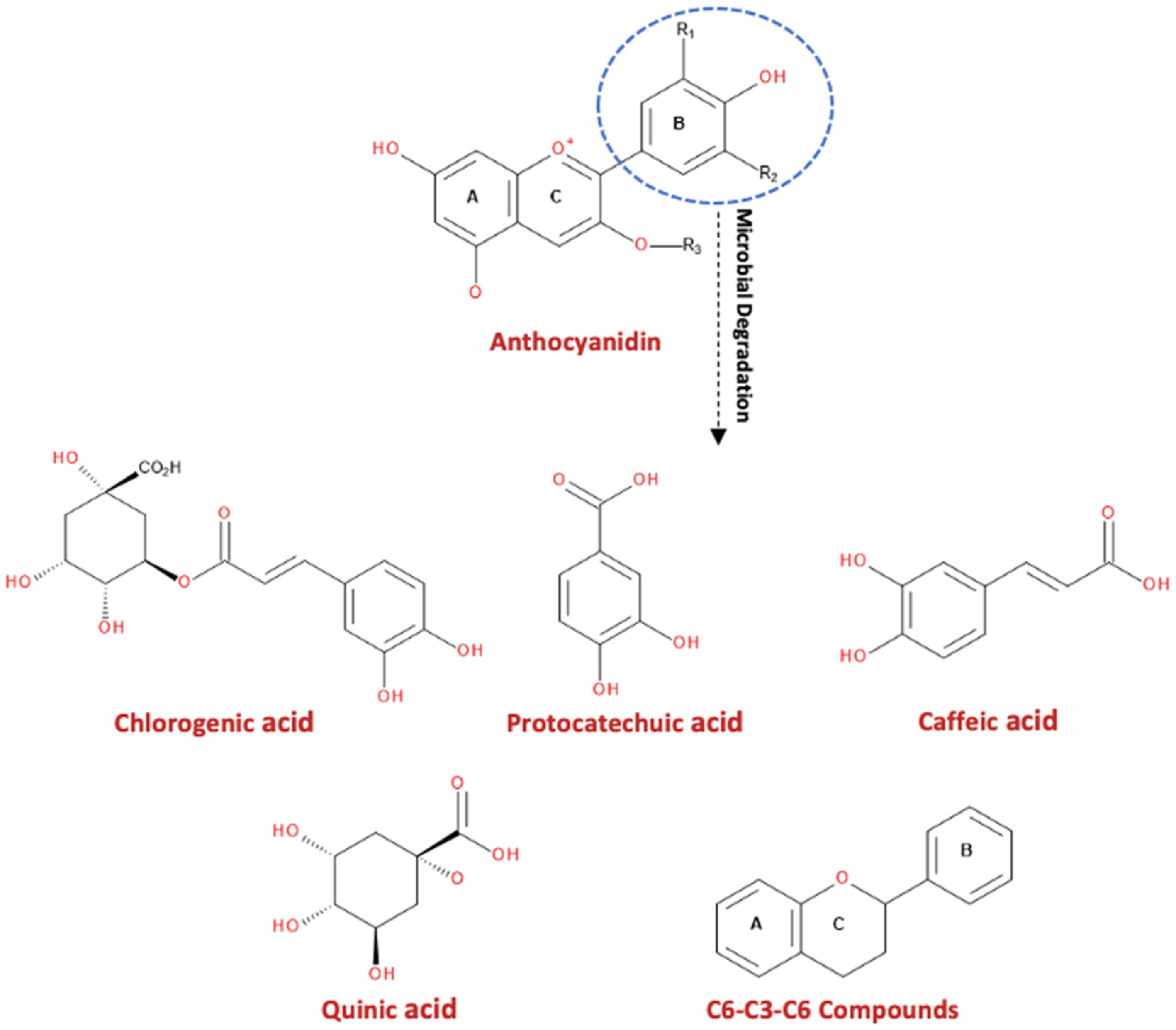
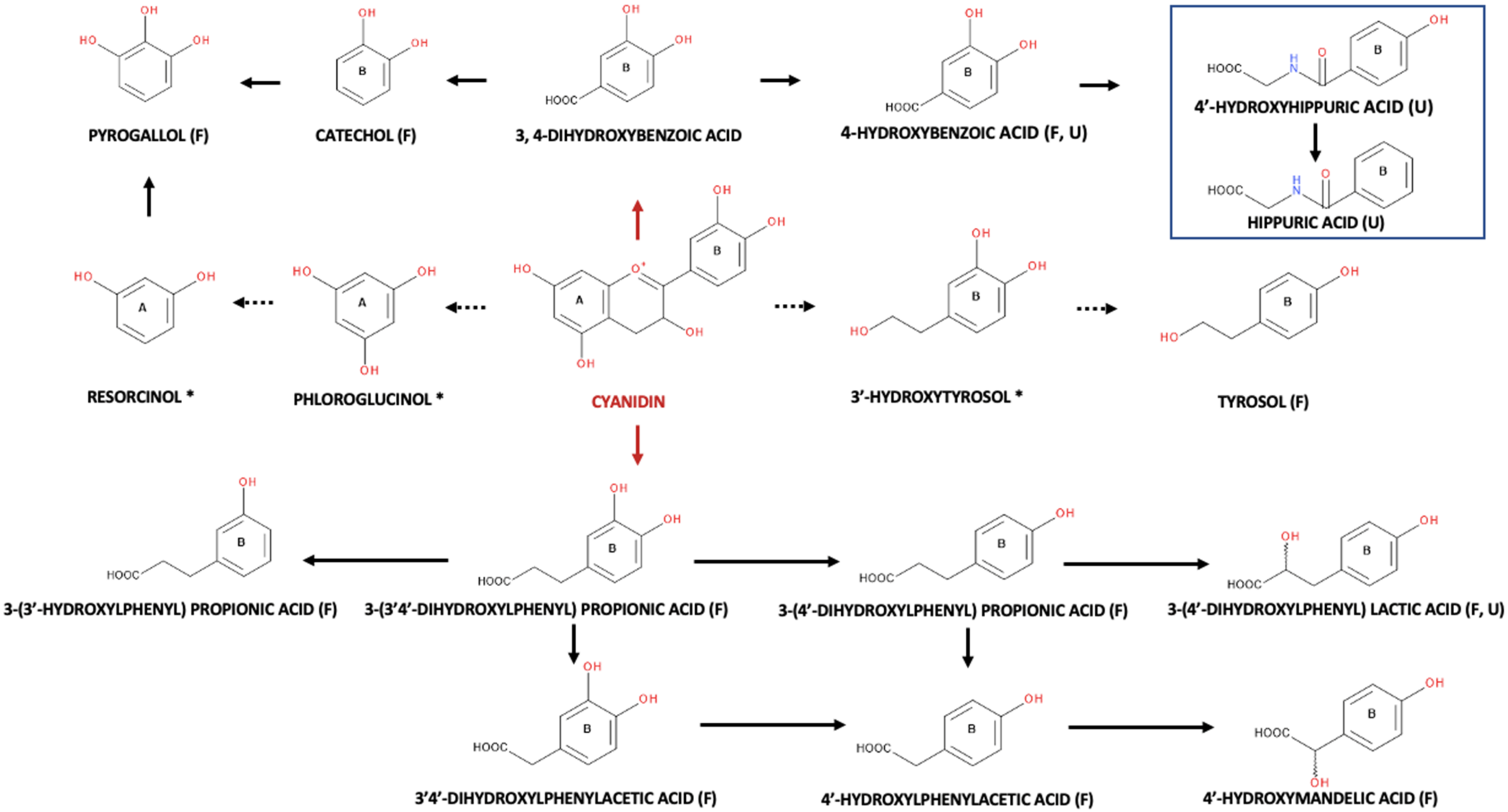
3. Anthocyanins and Their Metabolites in Blackberries and Raspberries
3.1. In Vitro/Ex Vivo Digestion and Fermentation Models
3.2. In Vivo Animal Models (Rat)
3.3. In Vivo Human Models
| Compounds/Samples | Chromatography | Detection System | Chromatographic Conditions | Reference(s) |
|---|---|---|---|---|
| Identification/Quantification | ||||
| Anthocyanins in Blackberries/Extracts of 0.12 mol/L hydrochloric acid (HCl) in MeOH | HPLC | DAD-UV/Vis | Column: Hypersil C18 (4.6 × 150 mm2, 5.0 µm) Guard Column: Hypersil C18 (10 × 4 mm2, 5 µm) Eluents: (A) 1.0% Phosphoric acid (H3PO4) in H2O; (B) Acetonitrile (ACN) Gradient: 100–90% A for 10 min; 90–75% A for 30 min Run (t): 40 min Flow rate: 1.0 mL/min Detection wavelength: 524 nm | [2] |
| Identification/Quantification | ||||
| Anthocyanins in Human urine after Blackberries ingestion/Acidified samples with 0.2 mol/L HCl | HPLC | ESI+-MS/MS | Column: Hypersil BDS C18 (2.1 × 150 mm2, 5.0 µm) Eluents: (A) (5:2:93) ACN: Formic acid (HCOOH): H2O; (B) (40:2:58) ACN: HCOOH: Water (H2O) Run (t): 40 min Flow rate: 0.2 mL/min Internal Standard: Cyanidin 3,5-diglucoside | |
| Identification | ||||
| Sugar-free Blackberry/Extracts of 80% MeOH | HPLC | ESI−/+-Q-TOF-MS | Column: Zorbax Eclipse Plus C18 (2.1 × 50 mm2, 1.8 µm) Guard Column: Same packing Eluents: (A) 0.1% HCOOH in H2O; (B) ACN Run (t): 12.5 min Flow rate: 0.4 mL/min MS scan: 100–1000 m/z | [7] |
| Untargeted Identification/Semi-Quantification | ||||
| First-pass metabolism of phenolic compounds of Blackberries/Ex vivo first-pass metabolism biosystem | HPLC | ESI−/+-Q-TOF-MS | As for identification, under the recommendations of Koistinen et al. [45] | |
| The subsequent considerations were based on the real-time oxidation/reduction phenomena in the employed ex vivo system: m/z ± 0.3 (mass accuracy), [M-H]+ (Anthocyanins; rutin) [M-H]− (All other polyphenols) | Semi-Quantification: Ion abundance at t0 and t120 of parent polyphenols and their metabolites | |||
| Identification/Quantification | ||||
| Phenolic compounds in Blackberries/After in vitro digestion of Blackberries; After colonic fermentation of Blackberries | HPLC | DAD | Column: Promosil C18 (4.6 × 250 mm2, 5.0 μm) Eluents: (A) 1.5% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 5–13% B (0–15 min); 13–15% B (15–20 min); 15–30% B (20–25 min); 30% B (25–28 min); 30–45% B (28–32 min); 45% B (32–35 min); 45–90% B (35–40 min); 90% B (40–45 min); 90–5% B (45–55 min); 5% B (5 min) Run (t): 60 min Flow rate: 0.8 mL/min Injection volume: 10 μL Detection wavelength: 280 and 520 nm | [35] |
| Identification and Quantification | ||||
| Derivatized phenolic acids in urine and faecal suspensions | GC | Dual-Stage Quadrupole (DSQ)+-MS | Injection volume: 1 μL in split mode (25:1). Inlet T: 220 °C Oven T: 40–160 °C at 20 °C/min; 160–200 °C at 1.5 °C/min; 200–250 °C at 10 °C/min; 250–300 °C at 40 °C/min, held for 5 min. Transferred line T: 310 °C Gas: Helium Flow rate: 1.2 mL/min | [40] |
| Identification and Quantification | ||||
| Anthocyanins, ellagic acid, punicalagin, and urolithins in urine and purified faecal suspensions | HPLC | PDA-ESI-MS/MS [M-H]+ (Anthocyanins); [M-H]− (Ellagitannins, ellagic acid and urolithins) | Column: Phenomenex Synergi RP-POLAR 80Å (4.6 × 250 mm2, 4.0 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) MeOH Gradient: 10–40% B (60 min) for anthocyanins; 10–65% B (50 min) for punicalagin, ellagic acid and urolithins Run (t): 60 min for anthocyanins; 50 min for punicalagin, ellagic acid and urolithins Flow rate: 1.0 mL/min T: 40 °C MS scan: 150–1500 m/z Wavelength for urolithins: 305 nm | |
| Identification | ||||
| Phenolic Compounds of Blackberries/Supernatant of fresh fruits acidified with 1% HCOOH (v/v) | HPLC | ESI−/+-MS/MS [M-H]+ (Anthocyanins); [M-H]− (All other polyphenols | Column: Atlantis dC18 (2.1 × 150 mm2, 3 μm) Eluents: (A) 1% HCOOH in H2O; (B) Acetonitrile (ACN) Gradient: 5–40% B for 40 min for all the phenolic compounds except for anthocyanins (5–35% B for 30 min) Run time (t): 40 min for all the phenolic compounds except for anthocyanins (30 min) Flow rate: 0.17 mL/min | [1] |
| Identification | ||||
| Metabolites after Faecal Fermentation of Blackberries/Samples after 5 and 24 h | HPLC | ESI−/+-MS/MS [M-H]+ (Anthocyanins); [M-H]− (All other polyphenols) | Column: Atlantis dC18 (2.1 × 150 mm2, 3 μm) Eluents: (A) 1% HCOOH in H2O; (B) Acetonitrile (ACN) Gradient: 0–40% B (Initial rough identification); 0–35% B (Multiple reaction monitoring (MRM) method) Run (t): 35 min (Initial rough identification); 10 min (MRM method) Flow rate: 0.17 mL/min | |
| Identification/Quantification | ||||
| Protocatechuic acid in extracts of mouse plasma, liver, prostate, colon tissue, and luminal content after consumption of black raspberries | UPLC | TQD-MS/MS | Column: Zorbax Eclipse Plus HD C18 (2.1 × 50 mm2, 1.8 μm) Eluents: (A) 0.01% acetic acid in H2O; (B) 0.01% acetic acid in MeOH Gradient: 0% B (0.5 min); linear increase to 95% B (5 min); 0% B (6.5 min) Run (t): 6.5 min Flow rate: 0.4 mL/min; T: 40 °C | [79] |
| Identification | ||||
| In vitro digested blackberry juice samples | HPLC | DAD-ESI−/+- Ion Trap (TRAP)- MS/MS | Column: Lichrospher ODS-2 (4.6 × 250 mm2, 5.0 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) ACN: H2O: HCOOH (80:18:2 v/v/v) Gradient: 5-25% B (50 min); 25–100% B (10 min); wash (5 min); equilibration (15 min); 0% B (0.5 min); linear increase to 95% B (5 min); 0% B (6.5 min) Run (t): 92 min Flow rate: 0.5 mL/min Detection Wavelength: 200–600 nm MS scan: 100–2000 m/z | [77] |
| Quantification | ||||
| HPLC | DAD-ESI−/+-TRAP- MS/MS | Column: Lichrospher ODS-2 (4.6 × 250 mm2, 5.0 μm) Eluents: (A) 0.1% orthophosphoric acid in H2O; (B) 0.1% orthophosphoric acid in MeOH Gradient: 0–30% B (3 min); 30–50% B (8 min); 50–70% B (12 min); 70–80% B (17 min); wash with 100% of B (2 min); equilibration for 15 min prior to each analysis Run (t): 34 min Flow rate: 1.0 mL/min Quantification Wavelength: 254 nm | ||
| Identification/Quantification | ||||
| Anthocyanins after Blackberries ingestion in Rat urine, plasma, bladder, prostate, heart, testes and adipose tissue | HPLC | DAD-UV/Vis | Column: Uptisphere 3 ODB C18 (4.6 × 150 mm2, 3.0 μm) Guard Column: Uptisphere 3 ODB C18 (10.0 × 4.0 mm2, 5 μm) Eluents: (A) 1.0 % H3PO4 in H2O; (B) ACN Gradient: 100–90% A for 10 min; 90–75% A for 30 min Run (t): 40 min Flow rate: 1.0 mL/min Detection wavelength: 524 nm Internal Standard: Cyanidin 3,5-diglucoside | [84] |
| Identification | ||||
| Polyphenols in Red Raspberry and their metabolites in human biological samples (plasma, urine, breast milk) | UPLC for raspberry polyphenols/metabolites; not including phenolic acids | Q-TOF-ESI−/+-MS/MS [M-H]+ (Anthocyanins); [M-H]− (Ellagitannins, flavon-3-ols, urolithins and other analytes) | Column: RP Poroshell C18 StableBond (2.1 × 150 mm2, 2.7 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 5% B; 5–15% B (10 min); 15–20% B (12 min); 20–50% B (20 min); 50–90% B (23 min); 5% B (25–30 min) Temperature (T): 35 °C Run (t): 30 min Flow rate: 0.3 mL/min Injection volume: 1.0 μL for raspberry and 5.0 μL for biological samples | [85] |
| Identification | ||||
| UPLC for phenolic acids and derivatives in biological samples | Q-TOF-ESI--MS/MS | Column: Pursuit 3 PFP (2.0 × 150 mm2, 3.0 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 5% B; 5–10% B (3 min); 10–15% B (7 min); 15 B (9 min); 15–20% B (10 min); 20% B (10–11 min); 20–25% B (12 min); 25–30% (13 min); 30% (13–14 min); 30–95% (15 min); 5% (16–20 min) T: 40 °C Run (t): 20 min Flow rate: 0.4 mL/min Injection volume: 5.0 μL | ||
| Quantification | ||||
| UPLC | TQD Same ESI conditions with Q-TOF | Same Separation Conditions with Q-TOF and dynamic multiple re-action monitoring mode | ||
| Targeted Identification | ||||
| Anthocyanins in in vitro digested blackberry purée | HPLC | PDA | Column: Supelcosil C18 (4.6 × 250 mm2, 5 μm) Eluents: (A) 0.1% trifluoroacetic acid (TFA) in H2O; (B) 0.1% TFA in ACN Gradient: 5% B (0 min); 35% B (0–45 min); 75% B (45–47 min); 5% B (47–54 min) Run (t): 54 min | [89] |
| Untargeted Identification/Semi-Quantification | ||||
| Free and bound polyphenols in undigested matrices (black-berry purée)In vitro fermented samples af-ter the addition of in vitro digested blackberry purée | UPLC | ESI+-Q-TOF-MS | Column: Agilent Zorbax Eclipse-plus (2.1 × 75 mm2, 1.8 μm) Eluents: (A) 1.5% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 6–94% B Run (t): 33 min Flow rate: 0.2 mL/min MS scan: 50–1200 m/z | |
| Identification | ||||
| Blackberry in vitro digested (poly) phenols and its enzymatically hydrolysed major aglycones | HPLC | Linear ion trap (LTQ) Orbitrap-MSn | Column: Phenomenex Synergi RP18 (2.0 × 150 mm2, 4.0 μm) Guard Column: Phenomenex Security GuardTM guard (2.0 × 4.0 mm2) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 98% A to reach 5% B (5 min); 35% B (25 min); 100% B (26–29 min); 2% B (30 min) Run (t): 30 minFlow rate: 0.2 mL/min | [95] |
| Identification/Quantification | ||||
| In vitro digested wild raspberry extracts | UPLC | UV-ESI−-TOF-MS/MS | Column: Promosil C18 (4.6 × 250 mm2, 5.0 μm) Eluents: (A) MeOH; (B) 0.1% HCOOH in H2O Gradient: 5–14.2% A (20 min); 14.2–60% A (50 min); 60–5% (10 min); isocratic elution (5 min) Run (t): 92 min Flow rate: 0.8 mL/min Detection Wavelength: 260 nm MS scan: 100–2000 m/z | [16] |
| Identification/Quantification | ||||
| Anthocyanins from blackberry at simulated GI and colonic levels | HPLC | UV-PDA | Column: Phenomenex Gemini (4.6 × 250 mm2, 5.0 μm) Eluents: (A) 5.0% HCOOH in H2O; (B) MeOH Gradient: 10% B (0 min); 15% B (5 min);20% B (15 min); 25% B (20 min); 30% B (25 min); 60% B (40 min), 10% B (42 min); 10% B (45 min) Run (t): 45 min Flow rate: 1.0 mL/min Detection Wavelength: 520 nm T: 30 °C Quantification of anthocyanins: C3G | [97] |
| Identification/Quantification | ||||
| Digested and non-digested raspberry extracts | HPLC | ESI+/−-MS | Column: Synergi Hydro C18 (2.0 × 150 mm2,.0 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 5% B (0 min) to 40% B (30 min) Run (t): 30 min Flow rate: 0.2 mL/min | [100] |
| Targeted Analysis | ||||
| Raspberry phenolics in ileal fluids | HPLC | PDA-ESI+/--MS/MS [M-H]+ (Anthocyanins); [M-H]− (Ellagitannins, ellagic acid and urolithins) | Column: Synergi RP-POLAR 80 Å (4.6 × 250 mm2, 4.0 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in MeOH Gradient: 10–40% B (60 min) Run (t): 60 min Flow rate: 1.0 mL/min, split to 0.3 mL/min after passing DAD | [105] |
| Quantification Anthocyanins (520 nm) as C3G equivalents; Ellagitannins (260 nm) as punicalagin equivalents; Ellagic acid and its conjugates (365 nm) as ellagic acid equivalents | ||||
| Untargeted Analysis | ||||
| HPLC | PDA-ESI−-MS/MS | Column: Synergi RP-POLAR 80 Å (4.6 × 250 mm2, 4.0 μm) Guard Column: Phenomenex (4.0 × 2.0 mm2) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in ACN: H2O (1:1 v/v) Gradient: 5% B (0–4 min); 5–50% B (4–22 min); 50–100% B (22–32 min) Run (t): 60 min Flow rate: 0.3 mL/min Detection Wavelength: 280, 325, 365, and 520 nm T: 30 °C MS scan: 80–2000 m/z |
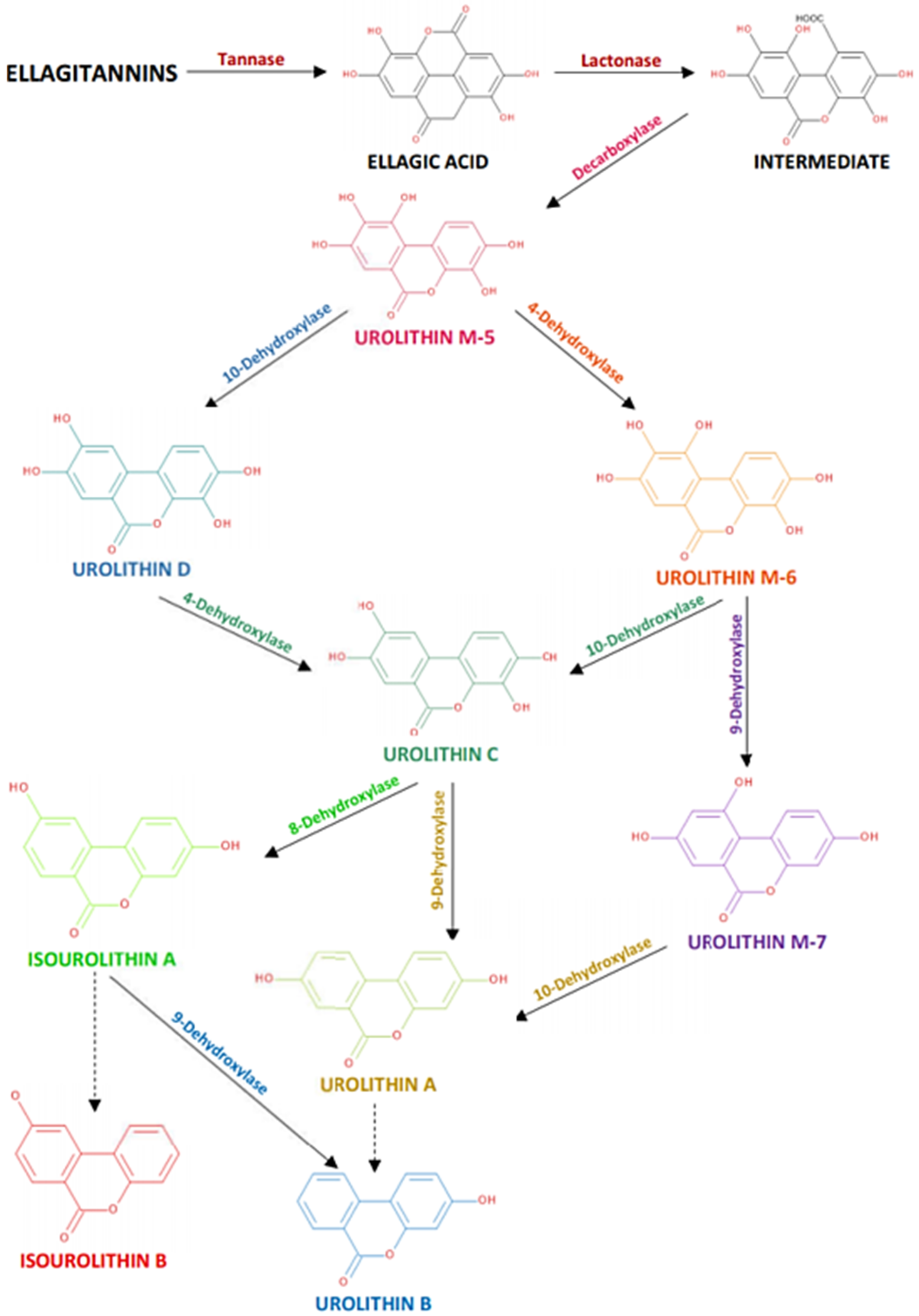
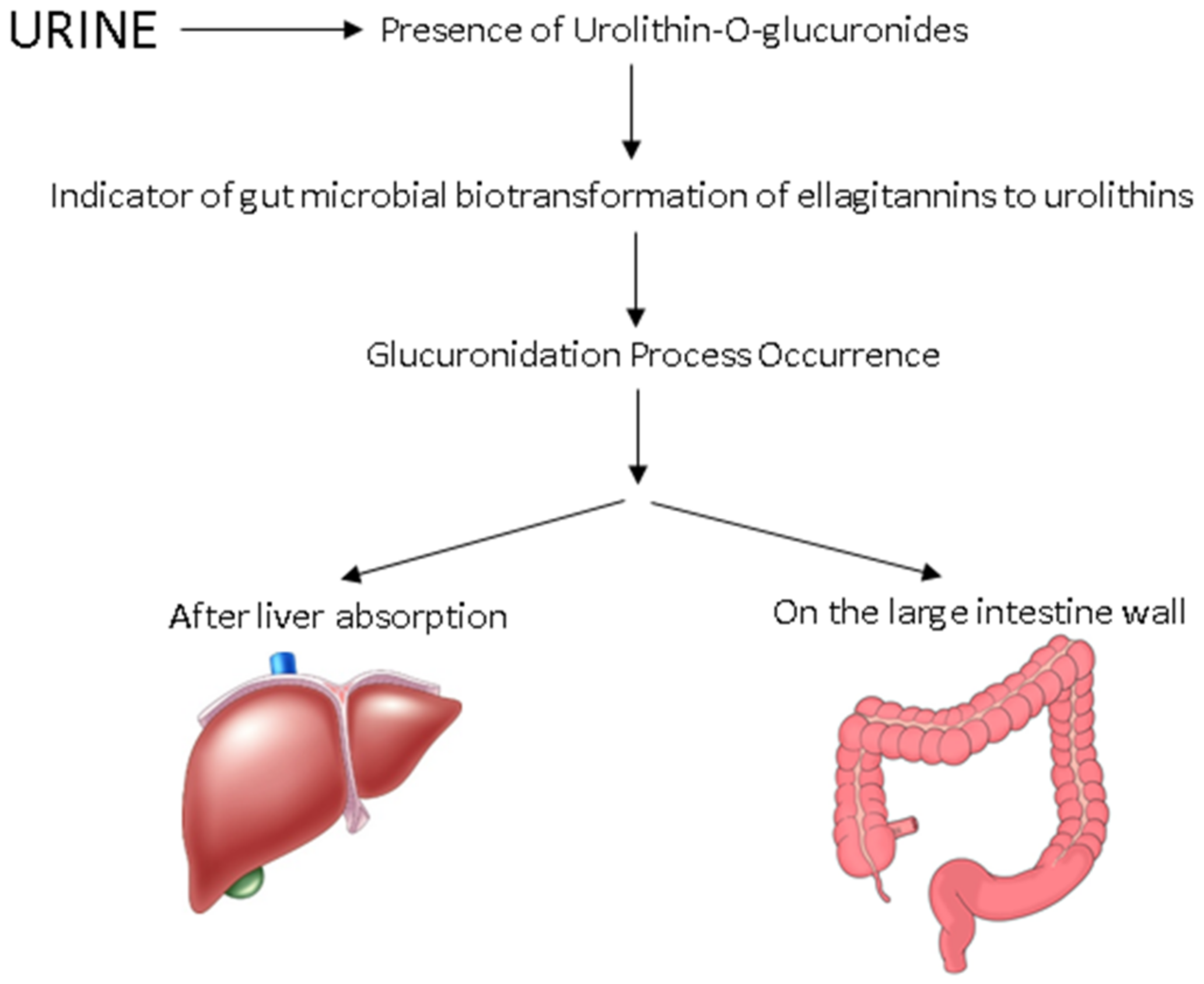
4. Ellagitannins and Their Metabolites in Blackberries and Raspberries
4.1. In Vitro Models
4.2. In Vivo Animal Models
4.3. In Vivo Human Models
| Compounds/Samples | Chromatography | Detection System | Chromatographic Conditions | Reference(s) |
|---|---|---|---|---|
| Identification/Quantification | ||||
| Plasma and urine polyphenol metabolites after raspberry drink consumption by human subjects | UPLC | Q-TOF-ESI-MS | Column: Zorbax Eclipse Plus RRHD (50 × 2.1 mm2, 1.8 μm) Guard column: Eclipse Plus (5 × 2.1 mm2, 1.8 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 1% B (0 min); 10% B (5 min); 25% B (8 min); 99 % B (9.1 min); 99% B (10 min); equilibration with 1% B (12 min) Run (t): 10 min Flow rate: 0.4 mL/min | [11] |
| Identification/Quantification | ||||
| Urolithins in plasma after raspberry drink consumption by human subjects | UPLC | Q-TOF-MS | Column: Poroshell 120 EC-C18 (100 × 3.0 mm2, 2.7 μm) Eluents: (A) 0.1% HCOOH in H2O; (B) 0.1% HCOOH in ACN Gradient: 5% B (0 min); 18% B (7 min); 28% B (17 min); 50% B (22 min); 90% B (27–28 min); re-equilibration (29 min); isocratic conditions up to 33 min Run (t): 33 min T: 25 °C Flow rate: 0.5 mL/min | |
| Identification/Quantification | ||||
| Urolithins in urine after raspberry drink consumption by human subjects | UPLC | DAD-Q-MS | Same Conditions with Q-TOF | |
| Targeted analysis | ||||
| Detection of potential ellagic acid derived metabolites | ||||
| Quantification of urolithins Available authentic standards | ||||
| Identification/Quantification | ||||
| Urolithins in extracts of mouse plasma, liver, prostate, colon tissue, and luminal content after consumption of black raspberries | UPLC | TQD-ESI+-MS/MS | Column: BEH C18 RP (2.1 × 50 mm2, 1.7 μm) Eluents: (A) 1.0% HCOOH in H2O; (B) 1.0% HCOOH in AC Gradient: 10% B (0.5 min); 55.5% B (5 min); linear increase to 95% B (6 min); 10% B (9 min) Run (t): 9 min Flow rate: 0.3 mL/min T: 40 °C | [79] |
| Tentative Identification Methyl urolithin C and dimethyl ellagic acid were (Accurate mass and fragmentation) | ||||
| UV absorption of urolithins was also consistent with the MS/MS analysis | ||||
| Identification/Quantification | ||||
| Ellagitannins from blackberry at simulated GI and colonic levels | HPLC | UV-PDA | Column: Phenomenex Gemini (4.6 × 250 mm2, 5.0 μm) Eluents: (A) 2.0% acetic acid in H2O; (B) 0.5% acetic acid in 50% aqueous ACN Gradient: 10% B (0 min); 15% B (13 min); 25% B (20 min); 55% B (50 min); 100% B (54 min); 10% B (60 min) Run (t): 60 min Flow rate: 1.0 mL/min Detection Wavelength: 254 and 280 nm T: 30 °C Quantification of ellagitannins: ellagic acid | [81] |
| Identification/Characterization | ||||
| Urolithins and ellagic acid metabolites produced in different animals after consumption of ellagitannins (blackberries)/Biological samples (feces, urine, plasma, urine, ruminal fluid, and feces) | HPLC | DAD | Column: LiChroCART RP C18 (4.0× 250 mm2, 5 μm) Guard Column: Same packing. Eluents: (A) 5.0% HCOOH in H2O; (B) ACN Gradient: 1–25% A (0–20 min); 25–55% A (20–30 min); 90% (30–35 min); 90–1% (35–45 min) Run (t): 45 min Flow rate: 1.0 mL/min Detection wavelength: 280, 305, 360 nm | [119] |
| Identification/Characterization | ||||
| HPLC | DAD-MS/MS | Conditions similar to HPLC-DAD | ||
| Identification/Characterization | ||||
| HPLC | ESI-Q-TOF-MS/MS | Gradient, Column, Run (t): Same as for HPLC-DAD/HPLC-DAD-MS/MS Eluents: (A) H2O: HCOOH in H2O (99.9: 0.1 (v/v)); (B) ACN Flow rate: 0.7 mL/min | ||
| Identification/Characterization | ||||
| Urine and plasma samples from men consumed blackberry rich products (confection or nectar) | UPLC | TQD-ESI+-MS/MS | Column: BEH C18 RP (2.1 × 50 mm2, 1.7 μm) Eluents: (A) 1.0% HCOOH in H2O; (B) 1.0% HCOOH in ACN Gradient: 10% B (0.5 min); linear increase to 55.5% B (4 min); 88.9% B (5 min); re-equilibration (6.5 min) Run (t): 9 minFlow rate: 0.75 mL/min T: 40 °C | [129] |
| Tentative Identification Q-TOF for methyl urolithin C and DMEA (Accurate mass and fragmentation, UV spectra) Targeted compounds Urolithin A, B, C, D, hydroxyl urolithin C, dimethyl ellagic acid, methyl urolithin A, dimethyl urolithin A | ||||
| Targeted compounds Urolithin A, B, C, D, hydroxyl urolithin C, dimethyl ellagic acid, methyl urolithin A, dimethyl urolithin A |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dall’Asta, M.; Calani, L.; Tedeschi, M.; Jechiu, L.; Brighenti, F.; Del Rio, D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition 2012, 28, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavera, S.; Texier, O.; Gil-Izquierdo, A.; Lamaison, J.L.; Remesy, C. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans. J. Agric. Food Chem. 2005, 53, 7721–7727. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Rugină, D.; Stanilă, A.; Dulf, F.; Bunea, A.; Socaci, S.A.; Socaciu, C.; Pintea, A. Phytochemical characterization of commercial processed blueberry, blackberry, blackcurrant, cranberry, and raspberry and their antioxidant activity. Antioxidants 2019, 8, 540. [Google Scholar] [CrossRef]
- Wang, L.-S.; Hecht, S.; Carmella, S.; Seguin, C.; Rocha, C.; Yu, N.; Stoner, K.; Chiu, S.; Stoner, G. Berry ellagitannins may not be sufficient for prevention of tumors in the rodent esophagus. J. Agric. Food Chem. 2010, 58, 3992–3995. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Mendoza, S.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A.; Villegas-Ochoa, M.A.; Quintero-Vargas, J.T.J.; Wall-Medrano, A. First-pass metabolism of polyphenols from selected berries: A high-throughput bioanalytical approach. Antioxidants 2020, 9, 311. [Google Scholar] [CrossRef]
- Yang, J.; Chiang, Y.-C.; Hsu, T.-W.; Kim, S.-H.; Pak, J.-H.; Kim, S.-C. Characterization and comparative analysis among plastome sequences of eight endemic Rubus (Rosaceae) species in Taiwan. Sci. Rep. 2021, 11, 1152. [Google Scholar] [CrossRef]
- Dai, J.; Gupte, A.; Gates, L.; Mumper, R.J. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: Extraction methods, stability, anticancer properties and mechanisms. Food Chem. Toxicol. 2009, 47, 837–847. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular antioxidant activity of common fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef]
- Istas, G.; Feliciano, R.P.; Weber, T.; Garcia-Villalba, R.; Tomas-Barberan, F.; Heiss, C.; Rodriguez-Mateos, A. Plasma urolithin metabolites correlate with improvements in endothelial function after red raspberry consumption: A double-blind randomized controlled trial. Arch. Biochem. Biophys. 2018, 651, 43–51. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Chaves, V.C.; Soares, M.S.P.; Spohr, L.; Teixeira, F.; Vieira, A.; Constantino, L.S.; Pizzol, F.D.; Lencina, C.L.; Spanevello, R.M.; Freitas, M.P.; et al. Blackberry extract improves behavioral and neurochemical dysfunctions in a ketamine-induced rat model of mania. Neurosci. Lett. 2020, 714, 134566. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Du, J.; Li, M.-L.; Li, C.-M. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT 2020, 128, 109448. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Zhang, L.; Li, Y.; Zheng, X. Wild raspberry subjected to simulated gastrointestinal digestion improves the protective capacity against ethyl carbamate-induced oxidative damage in caco-2 cells. Oxid. Med. Cell Longev. 2016, 2016, 3297363. [Google Scholar] [CrossRef] [PubMed]
- Gruner, L.A.; Kornilov, B.B. Priority trends and prospects of blackberry breeding in conditions of Central Russia. Vavilovskii Zhurnal Genet. Sel. 2020, 24, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Bojkovska, K.; Joshevska, F.; Tosheva, E.; Momirceski, J. Global raspberries market trends and their impact on the Macedonian raspberries market. Int. J. Res. Rev. 2021, 8, 362–369. [Google Scholar] [CrossRef]
- Goodman, C.; Lyon, K.N.; Scotto, A.; Smith, C.; Sebrell, T.A.; Gentry, A.B.; Bala, G.; Stoner, G.D.; Bimczok, D. A high-throughput metabolic microarray assay reveals antibacterial effects of black and red raspberries and blackberries against helicobacter pylori infection. Antibiotics 2021, 10, 845. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical composition, antioxidant and antimicrobial activity of raspberry, blackberry and raspberry-blackberry hybrid leaf buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef]
- Farias-Cervantes, V.S.; Salinas-Moreno, M.Y.; Chávez-Rodríguez, A.; Luna-Solano, G.; Medrano-Roldan, H.; Andrade-González, I. Stickiness and agglomeration of blackberry and raspberry spray dried juices using agave fructans and maltodextrin as carrier agents. Czech J. Food Sci. 2020, 38, 229–236. [Google Scholar] [CrossRef]
- Feresin, R.; Najjar, R.; Simecka, C.; Mu, S. Blackberry and raspberry attenuate the increase in blood pressure elicited by angiotensin II in mice (P06-054-19). Curr. Dev. Nutr. 2019, 3, nzz031. [Google Scholar] [CrossRef]
- Cefali, L.C.; Franco, J.G.; Nicolini, G.F.; Ataide, J.A.; Mazzola, P.G. In vitro antioxidant activity and solar protection factor of blackberry and raspberry extracts in topical formulation. J. Cosmet. Dermatol. 2019, 18, 539–544. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Juvonen, R.; Kössö, T.; Truchado, P.; Westerlund-Wikström, B.; Leppänen, T.; Moilanen, E.; Oksman-Caldentey, K.-M. Fermentation and dry fractionation increase bioactivity of cloudberry (Rubus chamaemorus). Food Chem. 2016, 197, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Azofeifa, G.; Quesada, S.; Navarro, L.; Hidalgo, O.; Portet, K.; Pérez, A.M.; Vaillant, F.; Poucheret, P.; Michel, A. Hypoglycaemic, hypolipidaemic and antioxidant effects of blackberry beverage consumption in streptozotocin-induced diabetic rats. J. Funct. Foods 2016, 26, 330–337. [Google Scholar] [CrossRef]
- Tavares, L.; Figueira, I.; McDougall, G.J.; Vieira, H.L.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective effects of digested polyphenols from wild blackberry species. Eur. J. Nutr. 2013, 52, 225–236. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Denardin, C.C.; Hirsch, G.E.; da Rocha, R.F.; Vizzotto, M.; Henriques, A.T.; Moreira, J.C.F.; Guma, F.T.C.R.; Emanuelli, T. Antioxidant capacity and bioactive compounds of four Brazilian native fruits. J. Food Drug Anal. 2015, 23, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Apel, M.; Raseira, M.C.B.; Zuanazzi, J.N.S.; Henriques, A.T. Polyphneol content and evaluation of antichemotactic, antiedeatogenic, and antioxidant activities of Rubus sp. cultivars. J. Food Biochem. 2011, 35, 1389–1397. [Google Scholar] [CrossRef]
- Ma, H.; Johnson, S.L.; Liu, W.; DaSilva, N.A.; Meschwitz, S.; Dain, J.A.; Seeram, N.P. Evaluation of polyphenol anthocyanin-enriched extracts of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry for free radical scavenging, reactive carbonyl species trapping, anti-glycation, anti-β-amyloid aggregation, and microglial neuroprotective effects. Int. J. Mol. Sci. 2018, 19, 461. [Google Scholar] [CrossRef]
- Rambaran, T.F.; Nembhard, N.; Bowen-Forbes, C.S.; Alexander-Lindo, R.L. Hypoglycemic effect of the fruit extracts of two varieties of Rubus rosifolius. J. Food Biochem. 2020, 44, e13365. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from Polana raspberry (Rubus idaeus L.) fruit and juice—In vitro study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Li, G.; Wei, J.; Chen, H.; Zhang, C.; Zhao, J.; Wang, Y.; Dang, S.; Li, X.; et al. Fresh red raspberry phytochemicals suppress the growth of hepatocellular carcinoma cells by PTEN/AKT pathway. Int. J. Biochem. Cell Biol. 2018, 104, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.M.; Kondoleon, N.; Gutierrez, E.; Wolforth, J.; Bolling, S. The intake of red raspberry fruit is inversely related to cardiac risk factors associated with metabolic syndrome. J. Funct. Foods 2018, 41, 83–89. [Google Scholar] [CrossRef]
- Gowd, V.; Bao, T.; Wang, L.; Huang, Y.; Chen, S.; Zheng, X.; Cui, S.; Chen, W. Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chem. 2018, 269, 618–627. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Zafrilla, P.; Ferreres, F.; Tomás-Barberán, F.A. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of red raspberry (Rubus idaeus) jams. J. Agric. Food Chem. 2001, 49, 3651–3655. [Google Scholar] [CrossRef]
- García-Muñoz, C.; Hernández, L.; Pérez, A.; Vaillant, F. Diversity of urinary excretion patterns of main ellagitannins’ colonic metabolites after ingestion of tropical highland blackberry (Rubus adenotrichus) juice. Food Res. Int. 2014, 55, 161–169. [Google Scholar] [CrossRef]
- González-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In vivo and in vitro studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Scalbert, A.; Brennan, L.; Fiehn, O.; Hankemeier, T.; Kristal, B.S.; van Ommen, B.; Pujos-Guillot, E.; Verheij, E.; Wishart, D.; Wopereis, S. Mass-spectrometry-based metabolomics: Limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics 2009, 5, 435. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography-tandem mass spectrometry technique (LC-MS/MS): A review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Martínez-Huélamo, M.; Vallverdú-Queralt, A. Microbial phenolic metabolites: Which molecules actually have an effect on human health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef] [PubMed]
- Koistinen, V.M.; Bento da Silva, A.; Abrankó, L.; Low, D.; Garcia Villalba, R.; Tomás Barberán, F.; Landberg, R.; Savolainen, O.; Alvarez-Acero, I.; De Pascual-Teresa, S.; et al. Interlaboratory coverage test on plant food bioactive compounds and their metabolites by mass spectrometry-based untargeted metabolomics. Metabolites 2018, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, R.C.; Dew, T.; Figueira, M.E.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C.N.; Williamson, G. Urinary metabolite profiling identifies novel colonic metabolites and conjugates of phenolics in healthy volunteers. Mol. Nutr. Food Res. 2014, 58, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.-M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Barron, D.; Smarrito-Menozzi, C.; Viton, F. (Bio)chemical labelling tools for studying absorption and metabolism of dietary phenols—An overview. Curr. Org. Chem. 2012, 16, 663–690. [Google Scholar] [CrossRef][Green Version]
- Ottaviani, J.I.; Fong, R.Y.; Borges, G.; Schroeter, H.; Crozier, A. Use of LC-MS for the quantitative analysis of (poly)phenol metabolites does not necessarily yield accurate results: Implications for assessing existing data and conducting future research. Free Radic. Biol. Med. 2018, 124, 97–103. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicka, E.; Sójka, M. Structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Carregosa, D.; Carecho, R.; Figueira, I.; Santos, C. Low-molecular weight metabolites from polyphenols as effectors for attenuating neuroinflammation. J. Agric. Food Chem. 2020, 68, 1790–1807. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Nusrat, A. Regulation of Paracellular Transport across Tight Junctions by the Actin Cytoskeleton Tight Junctions; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Pimpão, R.C.; Ventura, M.R.; Ferreira, R.B.; Williamson, G.; Santos, C.N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 2015, 113, 454–463. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Kosińska, A.; Andlauer, W. Modulation of tight junction integrity by food components. Food Res. Int. 2013, 54, 951–960. [Google Scholar] [CrossRef]
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of polyphenols and flavonoids in the era of precision medicine. Mol. Pharm. 2017, 14, 2861–2863. [Google Scholar] [CrossRef]
- Chen, G. Chapter 3—Xenobiotic metabolism and disposition. In An Introduction to Interdisciplinary Toxicology; Pope, C.N., Liu, J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 31–42. [Google Scholar] [CrossRef]
- Gómez-Juaristi, M.; Sarria, B.; Goya, L.; Bravo-Clemente, L.; Mateos, R. Experimental confounding factors affecting stability, transport and metabolism of flavanols and hydroxycinnamic acids in Caco-2 cells. Food Res. Int. 2020, 129, 108797. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Meireles, M.; Fernandes, I.; Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; de Freitas, V.; Mateus, N.; Calhau, C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014, 149, 190–196. [Google Scholar] [CrossRef]
- Pearce, S.C.; Coia, H.G.; Karl, J.P.; Pantoja-Feliciano, I.G.; Zachos, N.C.; Racicot, K. Intestinal in vitro and ex vivo models to study host-microbiome interactions and acute stressors. Front. Physiol. 2018, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Martins, N.; Barros, L. Chapter 1—Phenolic compounds and its bioavailability: In vitro bioactive compounds or health promoters. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 1–44. [Google Scholar]
- Gowd, V.; Bao, T.; Chen, W. Antioxidant potential and phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation. Food Res. Int. 2019, 120, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Dietary anthocyanin-rich plants: Biochemical basis and recent progress in health benefits studies. Mol. Nutr. Food Res. 2012, 56, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W. Anthocyanins and their C(6)-C(3)-C(6) metabolites in humans and animals. Molecules 2019, 24, 4024. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Lucini, L.; Giuberti, G.; Bhumireddy, S.R.; Mandal, R.; Trevisan, M.; Wishart, D.S. Transformation of polyphenols found in pigmented gluten-free flours during in vitro large intestinal fermentation. Food Chem. 2019, 298, 125068. [Google Scholar] [CrossRef]
- Azofeifa, G.; Quesada, S.; Pérez, A.M.; Vaillant, F.; Michel, A. Effect of an in vitro digestion on the antioxidant capacity of a microfiltrated blackberry juice (Rubus adenotrichos). Beverages 2018, 4, 30. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol. Nutr. Food Res. 2019, 63, e1800636. [Google Scholar] [CrossRef]
- Edwards, M.; Czank, C.; Woodward, G.M.; Cassidy, A.; Kay, C.D. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J. Agric. Food Chem. 2015, 63, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Garcia, G.; Pais, T.F.; Pinto, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Santos, C.N. Bioaccessible raspberry extracts enriched in ellagitannins and ellagic acid derivatives have anti-neuroinflammatory properties. Antioxidants 2020, 9, 970. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Garcin, P.; Besson, C.; Lamaison, J.L.; Scalbert, A. Tissue distribution of anthocyanins in rats fed a blackberry anthocyanin-enriched diet. Mol. Nutr. Food Res. 2009, 53, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. An exploratory study of red raspberry (Rubus idaeus L.) (poly)phenols/metabolites in human biological samples. Food Funct. 2018, 9, 806–818. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef]
- Zhang, X.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B.M. Plasma and urinary (poly)phenolic profiles after 4-week red raspberry (Rubus idaeus L.) intake with or without fructo-oligosaccharide supplementation. Molecules 2020, 25, 4777. [Google Scholar] [CrossRef] [PubMed]
- Julie, A.R.; Julianne, E.B.; Phillip, G.; Ronald, B.P. Blackberry polyphenols: Review of composition, quantity, and health impacts from in vitro and in vivo studies. J. Food Bioact. 2020, 9, 9217. [Google Scholar] [CrossRef]
- Tomas, M.; Rocchetti, G.; Ghisoni, S.; Giuberti, G.; Capanoglu, E.; Lucini, L. Effect of different soluble dietary fibres on the phenolic profile of blackberry puree subjected to in vitro gastrointestinal digestion and large intestine fermentation. Food Res. Int. 2020, 130, 108954. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wu, X.; Zhao, T.; Zhao, J.; Li, F.; Zou, Y.; Mao, G.; Yang, L. In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res. Int. 2012, 46, 76–82. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. Part VI—In vitro fermentation models: General introduction. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Agrawal, O.D.; Kulkarni, Y.A. Mini-review of analytical methods used in quantification of ellagic acid. Rev. Anal. Chem. 2020, 39, 31–44. [Google Scholar] [CrossRef]
- Vinaixa, M.; Samino, S.; Saez, I.; Duran, J.; Guinovart, J.J.; Yanes, O. A guideline to univariate statistical analysis for LC/MS-based untargeted metabolomics-derived data. Metabolites 2012, 2, 775–795. [Google Scholar] [CrossRef]
- Figueira, I.; Tavares, L.; Jardim, C.; Costa, I.; Terrasso, A.P.; Almeida, A.F.; Govers, C.; Mes, J.J.; Gardner, R.; Becker, J.D.; et al. Blood-brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: An in vitro study. Eur. J. Nutr. 2019, 58, 113–130. [Google Scholar] [CrossRef]
- Bernas, M.J.; Cardoso, F.L.; Daley, S.K.; Weinand, M.E.; Campos, A.R.; Ferreira, A.J.G.; Hoying, J.B.; Witte, M.H.; Brites, D.; Persidsky, Y.; et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier. Nat. Protoc. 2010, 5, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, F.; Pirovani, M.E.; Drago, S.R. Bioaccessibility analysis of anthocyanins and ellagitannins from blackberry at simulated gastrointestinal and colonic levels. J. Food Compos. Anal. 2018, 72, 22–31. [Google Scholar] [CrossRef]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the antioxidant features of polyphenols by spectroscopic and electrochemical methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Magarelli, G.; Da Silva, J.G.; Sousa Filho, I.A.D.; Lopes, I.S.D.; Souza, J.R.; Hoffmann, L.V.; de Castro, C.S.P. Development and validation of a voltammetric method for determination of total phenolic acids in cotton cultivars. Microchem. J. 2013, 109, 23–28. [Google Scholar] [CrossRef]
- Garcia, G.; Nanni, S.; Figueira, I.; Ivanov, I.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Pinto, P.; Silva, R.F.; Brites, D.; et al. Bioaccessible (poly)phenol metabolites from raspberry protect neural cells from oxidative stress and attenuate microglia activation. Food Chem. 2017, 215, 274–283. [Google Scholar] [CrossRef]
- Gu, L.; House, S.E.; Prior, R.L.; Fang, N.; Ronis, M.J.; Clarkson, T.B.; Wilson, M.E.; Badger, T.M. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J. Nutr. 2006, 136, 1215–1221. [Google Scholar] [CrossRef]
- Blais, E.M.; Rawls, K.D.; Dougherty, B.V.; Li, Z.I.; Kolling, G.L.; Ye, P.; Wallqvist, A.; Papin, J.A. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat. Commun. 2017, 8, 14250. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005, 53, 7029–7034. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, J.L.; Pereira-Caro, G.; Ludwig, I.; Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Crozier, A.; Moreno-Rojas, J.M. A critical evaluation of the use of gas chromatography and high performance liquid chromatography-mass spectrometry techniques for the analysis of microbial metabolites in human urine after consumption of orange juice. J. Chromatogr. A 2018, 1575, 100–112. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Conner, S.; Pereira-Caro, G.; Gonzalez-Barrio, R.; Brown, E.M.; Verrall, S.; Stewart, D.; Moffet, T.; Ibars, M.; Lawther, R.; et al. Tracking (Poly)phenol components from raspberries in ileal fluid. J. Agric. Food Chem. 2014, 62, 7631–7641. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada, F.; Ibáñez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trends Anal. Chem. 2019, 112, 87–101. [Google Scholar] [CrossRef]
- Cambiaghi, A.; Ferrario, M.; Masseroli, M. Analysis of metabolomic data: Tools, current strategies and future challenges for omics data integration. Brief. Bioinform. 2016, 18, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef]
- Sójka, M.; Janowski, M.; Grzelak-Błaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from rubus berries for the control of gastric inflammation: In vitro and in vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef]
- Garcia-Muñoz, C.; Vaillant, F. Metabolic fate of ellagitannins: Implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef]
- Bobowska, A.; Granica, S.; Filipek, A.; Melzig, M.F.; Moeslinger, T.; Zentek, J.; Kruk, A.; Piwowarski, J.P. Comparative studies of urolithins and their phase II metabolites on macrophage and neutrophil functions. Eur. J. Nutr. 2021, 60, 1957–1972. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Stanisławska, I.; Granica, S.; Stefańska, J.; Kiss, A.K. Phase II conjugates of urolithins isolated from human urine and potential role of β-glucuronidases in their disposition. Drug Metab. Dispos. 2017, 45, 657–665. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Tomás-Barberan, F.A.; Espín, J.C.; García-Conesa, M.T. Bioavailability and metabolism of ellagic acid and ellagitannins. In Chemistry and Biology of Ellagitannins; Quideau, S.E., Ed.; World Scientific: London, UK, 2009. [Google Scholar]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Truchado, P.; Ito, H.; Espín, J.C.; Tomás-Barberán, F.A. UV and MS identification of Urolithins and Nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals. J. Agric. Food Chem. 2011, 59, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Beltrán, D.; García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014, 5, 1779–1784. [Google Scholar] [CrossRef]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomás-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, J.; Yan, L.; Yang, L.; Sun, L.; Shi, L.; Ma, C.; Liu, Y. Urolithin C, a gut metabolite of ellagic acid, induces apoptosis in PC12 cells through a mitochondria-mediated pathway. RSC Adv. 2017, 7, 17254–17263. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Bolca, S.; Verstraete, W.; Heyerick, A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 2011, 82, 53–66. [Google Scholar] [CrossRef]
- Selma, M.V.; Beltrán, D.; Luna, M.C.; Romo-Vaquero, M.; García-Villalba, R.; Mira, A.; Espín, J.C.; Tomás-Barberán, F.A. Isolation of human intestinal bacteria capable of producing the bioactive metabolite Isourolithin A from ellagic acid. Front. Microbiol. 2017, 8, 1521. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong interindividual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Grainger, E.M.; Thomas-Ahner, J.M.; Hinton, A.; Gu, J.; Riedl, K.; Vodovotz, Y.; Abaza, R.; Schwartz, S.J.; Clinton, S.K. Dose-dependent increases in ellagitannin metabolites as biomarkers of intake in humans consuming standardized black raspberry food products designed for clinical trials. Mol. Nutr. Food Res. 2020, 64, 1900800. [Google Scholar] [CrossRef]
- Madji, H.B.; Blasco, H.; Nadal-Desbarats, L.; Diémé, B.; Montigny, F.; Andres, C.R.; Emond, P.; Mavel, S. Analytical methodology for metabolomics study of adherent mammalian cells using NMR, GC-MS and LC-HRMS. Anal. Bioanal. Chem. 2015, 407, 8861–8872. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Jones, H.; Thijssen, D.; Cable, N.T.; Atkinson, G. Flow-mediated dilation and cardiovascular event prediction. Hypertension 2011, 57, 363–369. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, D.K.; Tzima, K. A Review on Chromatography–Mass Spectrometry Applications on Anthocyanin and Ellagitannin Metabolites of Blackberries and Raspberries. Foods 2021, 10, 2150. https://doi.org/10.3390/foods10092150
Rai DK, Tzima K. A Review on Chromatography–Mass Spectrometry Applications on Anthocyanin and Ellagitannin Metabolites of Blackberries and Raspberries. Foods. 2021; 10(9):2150. https://doi.org/10.3390/foods10092150
Chicago/Turabian StyleRai, Dilip K., and Katerina Tzima. 2021. "A Review on Chromatography–Mass Spectrometry Applications on Anthocyanin and Ellagitannin Metabolites of Blackberries and Raspberries" Foods 10, no. 9: 2150. https://doi.org/10.3390/foods10092150
APA StyleRai, D. K., & Tzima, K. (2021). A Review on Chromatography–Mass Spectrometry Applications on Anthocyanin and Ellagitannin Metabolites of Blackberries and Raspberries. Foods, 10(9), 2150. https://doi.org/10.3390/foods10092150







