Postharvest Treatments with Sulfur-Containing Food Additives to Control Major Fungal Pathogens of Stone Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Additives
2.2. Fungal Pathogens
2.3. In Vitro Antifungal Activity
2.4. In Vivo Curative Activity
2.4.1. Fruit
2.4.2. Fungal Inoculation
2.4.3. In Vivo Primary Screenings
2.4.4. Dip Treatment Conditions
2.4.5. Disease and Phytotoxicity Assessments
2.5. Statistical Analysis
3. Results
3.1. Inhibition of In Vitro Mycelial Growth
3.2. In Vivo Curative Activity
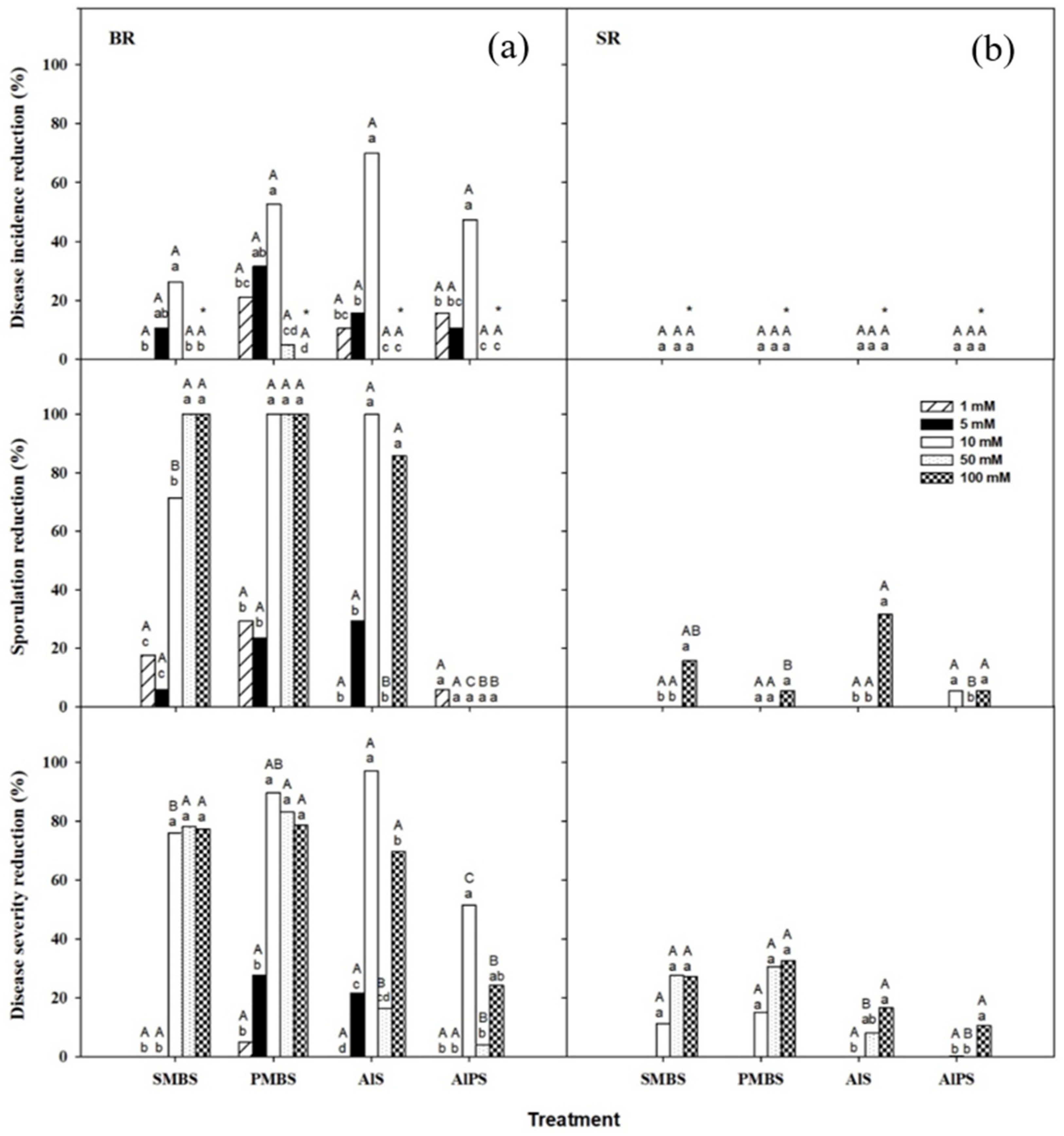
3.2.1. Primary Screenings
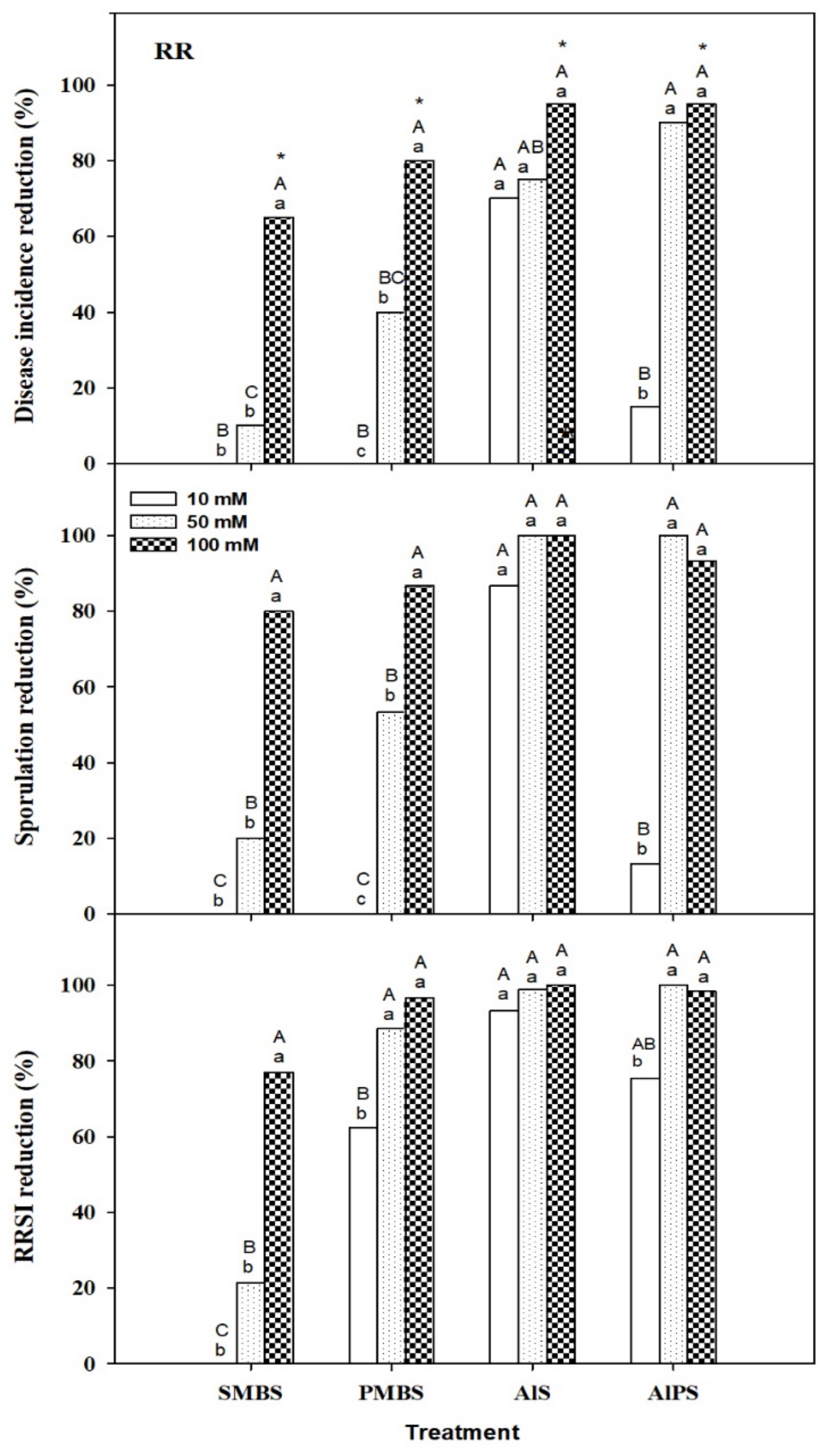
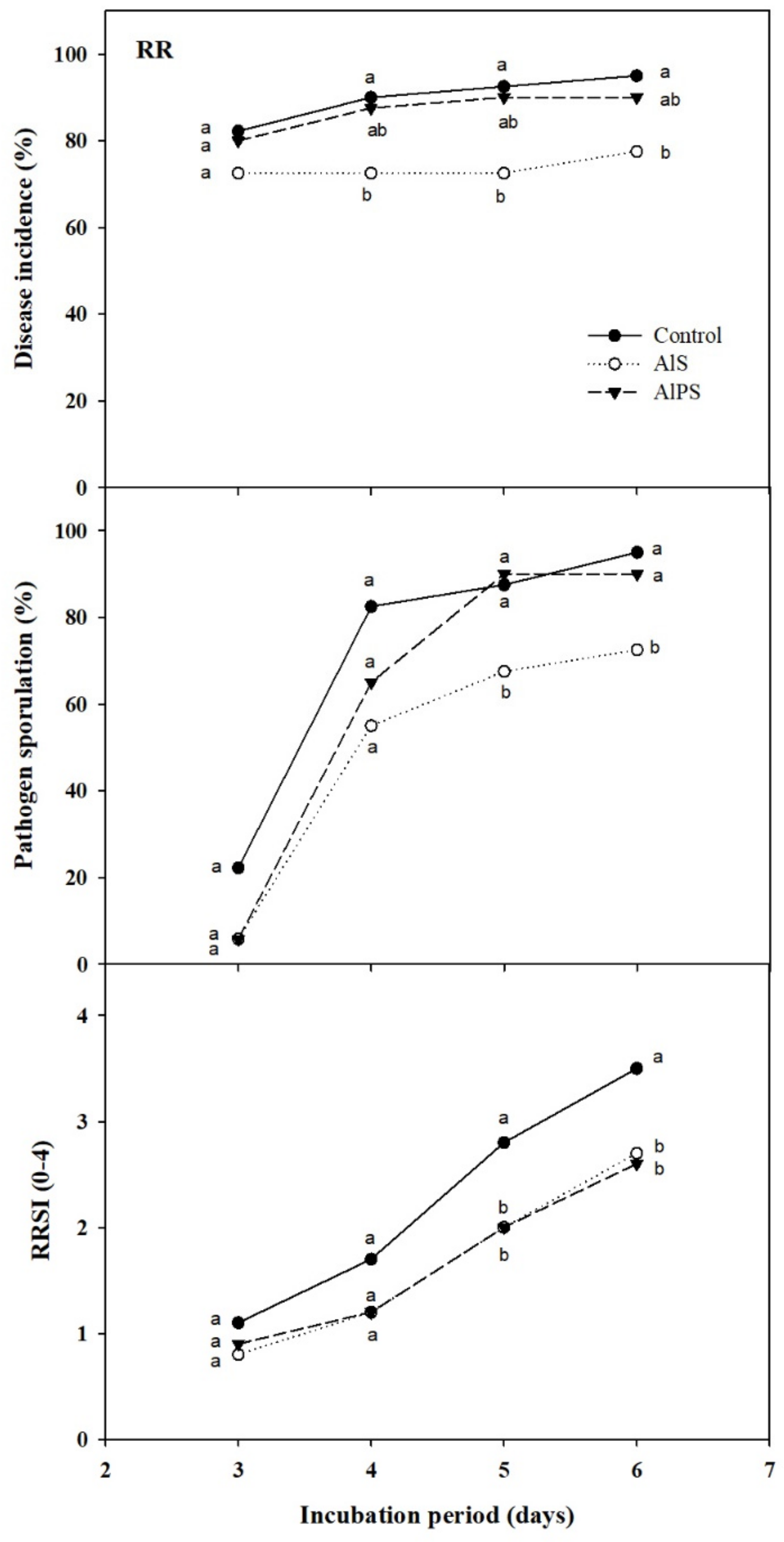
3.2.2. Dip Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United Nations. Statistics. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 5 June 2021).
- Kader, A.; Mitchell, F.G. Maturity and quality. In Peaches, Plums and Nectarines: Growing and Handling for Fresh Market; La Rue, J.H., Johnson, R., Eds.; University of California Department of Agriculture and Natural Resources Publication: Oakland, CA, USA, 1989; pp. 191–196. [Google Scholar]
- Mari, M.; Spadaro, D.; Casals, C.; Collina, M.; De Cal, A.; Usall, J. Stone Fruits. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 111–140. ISBN 9781315209180. [Google Scholar]
- Barkai-Golan, R. Each fruit or vegetable and its characteristic pathogens. In Postharvest Diseases of Fruits and Vegetables; Barkai-Golan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 25–32. ISBN 978-0-444-50584-2. [Google Scholar]
- Eckert, J.W.; Ogawa, J.M. The chemical control of postharvest diseases: Deciduous fruits, berries, vegetables and root/tuber crops. Annu. Rev. Phytopathol. 1988, 26, 433–469. [Google Scholar] [CrossRef]
- Förster, H.; Driever, G.F.; Thompson, D.C.; Adaskaveg, J.E. Postharvest decay management for stone fruit crops in California using the “reduced-risk” fungicides fludioxonil and fenhexamid. Plant Dis. 2007, 91, 209–215. [Google Scholar] [CrossRef][Green Version]
- Avis, T.J. Antifungal compounds that target fungal membranes: Applications in plant disease control. Can. J. Plant Pathol. 2007, 29, 323–329. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef]
- Usall, J.; Casals, C.; Sisquella, M.; Palou, L.; De Cal, A. Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev. 2015, 11, 1–6. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A. Reducing or replacing conventional postharvest fungicides with low toxicity acids and salts. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2020; pp. 595–632. ISBN 9781315209180. [Google Scholar]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Prot. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Guimarães, J.E.R.; de la Fuente, B.; Pérez-Gago, M.B.; Andradas, C.; Carbó, R.; Mattiuz, B.-H.; Palou, L. Antifungal activity of GRAS salts against Lasiodiplodia theobromae in vitro and as ingredients of hydroxypropyl methylcellulose-lipid composite edible coatings to control Diplodia stem-end rot and maintain postharvest quality of citrus fruit. Int. J. Food Microbiol. 2019, 301, 9–18. [Google Scholar] [CrossRef]
- Karaca, H.; Pérez-Gago, M.B.; Taberner, V.; Palou, L. Evaluating food additives as antifungal agents against Monilinia fructicola in vitro and in hydroxypropyl methylcellulose-lipid composite edible coatings for plums. Int. J. Food Microbiol. 2014, 179, 72–79. [Google Scholar] [CrossRef]
- Moscoso-Ramírez, P.A.; Montesinos-Herrero, C.; Palou, L. Characterization of postharvest treatments with sodium methylparaben to control citrus green and blue molds. Postharvest Biol. Technol. 2013, 77, 128–137. [Google Scholar] [CrossRef]
- Soto-Muñoz, L.; Taberner, V.; de la Fuente, B.; Jerbi, N.; Palou, L. Curative activity of postharvest GRAS salt treatments to control citrus sour rot caused by Geotrichum citri-aurantii. Int. J. Food Microbiol. 2020, 335, 108860. [Google Scholar] [CrossRef]
- Di Millo, B.; Martínez-Blay, V.; Pérez-Gago, M.B.; Argente-Sanchis, M.; Grimal, A.; Baraldi, E.; Palou, L. Antifungal hydroxypropyl methylcellulose (HPMC)-lipid composite edible coatings and modified atmosphere packaging (MAP) to reduce postharvest decay and improve storability of ‘Mollar De Elche’ pomegranates. Coatings 2021, 11, 308. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Crisosto, C.H. Evaluation of food additives as alternative or complementary chemicals to conventional fungicides for the control of major postharvest diseases of stone fruit. J. Food Prot. 2009, 72, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Gregori, R.; Borsetti, F.; Neri, F.; Mari, M.; Bertolini, P. Effects of potassium sorbate on postharvest brown rot of stone fruit. J. Food Prot. 2008, 71, 1626–1631. [Google Scholar] [CrossRef]
- Casals, C.; Teixidó, N.; Viñas, I.; Silvera, E.; Lamarca, N.; Usall, J. Combination of hot water, Bacillus subtilis CPA-8 and sodium bicarbonate treatments to control postharvest brown rot on peaches and nectarines. Eur. J. Plant Pathol. 2010, 128, 51–63. [Google Scholar] [CrossRef]
- Feliziani, E.; Santini, M.; Landi, L.; Romanazzi, G. Pre- and postharvest treatment with alternatives to synthetic fungicides to control postharvest decay of sweet cherry. Postharvest Biol. Technol. 2013, 78, 133–138. [Google Scholar] [CrossRef]
- Howland, W.C.; Simon, R.A. Sulfite-treated lettuce challenges in sulfite-sensitive subjects with asthma. J. Allergy Clin. Immunol. 1990, 83, 1079–1082. [Google Scholar] [CrossRef]
- Vena, G.A.; Foti, C.; Angelini, G. Sulfite contact allergy. Contact Dermat. 1994, 31, 172–175. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite. EFSA J. 2016, 14, 4438. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Re-evaluation of aluminium sulphates (E 520–523) and sodium aluminium phosphate (E 541) as food additives. EFSA J. 2018, 16, 5372. [Google Scholar] [CrossRef]
- Chaves Junior, O.J.; Youssef, K.; Koyama, R.; Ahmed, S.; Dominguez, A.R.; Mühlbeier, D.T.; Roberto, S.R. Control of gray mold on clamshell-packaged ‘benitaka’ table grapes using sulphur dioxide pads and perforated liners. Pathogens 2019, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Palou, L.; Serrano, M.; Martínez-Romero, D.; Valero, D. New approaches for postharvest quality retention of table grapes. Fresh Prod. 2010, 4, 103–110. [Google Scholar]
- Cantín, C.M.; Palou, L.; Bremer, V.; Michailides, T.J.; Crisosto, C.H. Evaluation of the use of sulfur dioxide to reduce postharvest losses on dark and green figs. Postharvest Biol. Technol. 2011, 59, 150–158. [Google Scholar] [CrossRef]
- Pols, S.; Williams, E.; Botes, A.; Vries, F. Development of novel postharvest techniques for the preservation of blueberry fruit. Acta Hortic. 2019, 1256, 575–581. [Google Scholar] [CrossRef]
- Hervieux, V.; Yaganza, E.S.; Arul, J.; Tweddell, R.J. Effect of organic and inorganic salts on the development of Helminthosporium solani, the causal agent of potato silver scurf. Plant Dis. 2002, 86, 1014–1018. [Google Scholar] [CrossRef]
- Kolaei, E.A.; Tweddell, R.J.; Avis, T.J. Antifungal activity of sulfur-containing salts against the development of carrot cavity spot and potato dry rot. Postharvest Biol. Technol. 2012, 63, 55–59. [Google Scholar] [CrossRef]
- Kolaei, E.A.; Cenatus, C.; Tweddell, R.J.; Avis, T.J. Antifungal activity of aluminium-containing salts against the development of carrot cavity spot and potato dry rot. Ann. Appl. Biol. 2013, 163, 311–317. [Google Scholar] [CrossRef]
- Martínez-Blay, V.; Taberner, V.; Pérez-Gago, M.B.; Palou, L. Control of major citrus postharvest diseases by sulfur-containing food additives. Int. J. Food Microbiol. 2020, 330, 108713. [Google Scholar] [CrossRef] [PubMed]
- Mecteau, M.R.; Arul, J.; Tweddell, R.J. Effect of different salts on the development of Fusarium solani var. coeruleum, a causal agent of potato dry rot. Phytoprotection 2008, 89, 1–6. [Google Scholar] [CrossRef]
- Mills, A.A.S.; Platt, H.W.; Hurta, R.A.R. Effect of salt compounds on mycelial growth, sporulation and spore germination of various potato pathogens. Postharvest Biol. Technol. 2004, 34, 341–350. [Google Scholar] [CrossRef]
- Mills, A.A.S.; Platt, H.W.; Hurta, R.A.R. Salt compounds as control agents of late blight and pink rot of potatoes in storage. Can. J. Plant Pathol. 2005, 27, 204–209. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M.; El Ghaouth, A.; Wilson, C. Influence of food additives on the control of postharvest rots of apple and peach and efficacy of the yeast-based biocontrol product Aspire. Postharvest Biol. Technol. 2003, 27, 127–135. [Google Scholar] [CrossRef]
- Mari, M.; Gregori, R.; Donati, I. Postharvest control of Monilinia laxa and Rhizopus stolonifer in stone fruit by peracetic acid. Postharvest Biol. Technol. 2004, 33, 319–325. [Google Scholar] [CrossRef]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Aoumar, A.A.B. In vitro and in vivo antifungal activities of organic and inorganic salts against citrus sour rot agent Geotrichum candidum. Plant Pathol. J. 2011, 10, 138–145. [Google Scholar] [CrossRef]
- Prusky, D.; Yakoby, N. Pathogenic fungi: Leading or led by ambient pH? Mol. Plant Pathol. 2003, 4, 509–516. [Google Scholar] [CrossRef]
- Prusky, D.; Lichter, A. Mechanisms modulating fungal attack in post-harvest pathogen interactions and their control. Eur. J. Plant Pathol. 2008, 121, 281–289. [Google Scholar] [CrossRef]
- Zhang, Z.; Dvir, O.; Pesis, E.; Pick, U.; Lichter, A. Weak organic acids and inhibitors of pH homeostasis suppress growth of Penicillium infesting litchi fruits. J. Phytopathol. 2005, 153, 667–673. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship between host acidification and virulence of Penicillium spp. on apple and citrus fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Divol, B.; Du Toit, M.; Duckitt, E. Surviving in the presence of sulphur dioxide: Strategies developed by wine yeasts. Appl. Microbiol. Biotechnol. 2012, 95, 601–613. [Google Scholar] [CrossRef]
- Davidson, P.M.; Junja, V.K.; Branen, A.L. Antimicrobial agents. In Food Additives; Branen, A.L., Davidson, P.M., Salminen, S., Thorngate, J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 563–620. ISBN 0824793439. [Google Scholar]
- Avis, T.J.; Michaud, M.; Tweddell, R.J. Role of lipid composition and lipid peroxidation in the sensitivity of fungal plant pathogens to aluminum chloride and sodium metabisulfite. Appl. Environ. Microbiol. 2007, 73, 2820–2824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Avis, T.J.; Rioux, D.; Simard, M.; Michaud, M.; Tweddell, R.J. Ultrastructural alterations in Fusarium sambucinum and Heterobasidion annosum treated with aluminum chloride and sodium metabisulfite. Phytopathology 2009, 99, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Smilanick, J.L.; Hartsell, P.I.; Henson, D.; Fouse, D.C.; Assemi, M.; Harris, C. Inhibitory activity of sulfur dioxide on the germination of spores of Botrytis cinerea. Phytopathology 1990, 80, 217–220. [Google Scholar] [CrossRef]
- Mecteau, M.R.; Arul, J.; Tweddell, R.J. Effect of organic and inorganic salts on the growth and development of Fusarium sambucinum, a causal agent of potato dry rot. Mycol. Res. 2002, 106, 688–696. [Google Scholar] [CrossRef]
- Jeandet, P.; Adrian, M.; Breuil, A.C.; Sbaghi, M.; Debord, S.; Bessis, R.; Weston, L.A.; Harmon, R. Chemical induction of phytoalexin synthesis in gravepines: Application to the control of grey mould in the vineyard. Acta Hortic. 2000, 528, 591–596. [Google Scholar] [CrossRef]
- US FDA, United States Food and Drug Administration. Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 15 April 2021).
- Gad, S.E. Generally Recognized as Safe (GRAS). In Encyclopedia of Toxicology; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 417–420. ISBN 9780123694003. [Google Scholar]
- Smilanick, J.L.; Margosan, D.A.; Henson, D.J. Evaluation of heated solutions of sulfur dioxide, ethanol, and hydrogen peroxide to control postharvest green mold of lemons. Plant Dis. 1995, 79, 742–747. [Google Scholar] [CrossRef]

| Food Additive b | Concentration (mM) | Inhibition of Mycelial Growth a (%) | ||
|---|---|---|---|---|
| Monilinia fructicola c | Geotrichum candidum c | Rhizopus stolonifer c | ||
| SMBS | 10 | 100 a | 100 a | 100 a |
| 20 | 100 a | 100 a | 100 a | |
| 30 | 100 a | 100 a | 100 a | |
| 50 | 100 a | 100 a | 100 a | |
| 100 | 100 a | 100 a | 100 a | |
| PMBS | 10 | 100 a | 100 a | 100 a |
| 20 | 100 a | 100 a | 100 a | |
| 30 | 100 a | 100 a | 100 a | |
| 50 | 100 a | 100 a | 100 a | |
| 100 | 100 a | 100 a | 100 a | |
| AlS | 10 | 100 a | 100 a | 78.95 d |
| 20 | 100 a | 100 a | 100 a | |
| 30 | 100 a | 100 a | 100 a | |
| 50 | 100 a | 100 a | 100 a | |
| 100 | 100 a | 100 a | 100 a | |
| AlPS | 10 | 80.47 c | 65.47 b | 82.56 c |
| 20 | 86.86 b | 100 a | 90.47 b | |
| 30 | 100 a | 100 a | 100 a | |
| 50 | 100 a | 100 a | 100 a | |
| 100 | 100 a | 100 a | 100 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Blay, V.; Taberner, V.; Pérez-Gago, M.B.; Palou, L. Postharvest Treatments with Sulfur-Containing Food Additives to Control Major Fungal Pathogens of Stone Fruits. Foods 2021, 10, 2115. https://doi.org/10.3390/foods10092115
Martínez-Blay V, Taberner V, Pérez-Gago MB, Palou L. Postharvest Treatments with Sulfur-Containing Food Additives to Control Major Fungal Pathogens of Stone Fruits. Foods. 2021; 10(9):2115. https://doi.org/10.3390/foods10092115
Chicago/Turabian StyleMartínez-Blay, Victoria, Verònica Taberner, María B. Pérez-Gago, and Lluís Palou. 2021. "Postharvest Treatments with Sulfur-Containing Food Additives to Control Major Fungal Pathogens of Stone Fruits" Foods 10, no. 9: 2115. https://doi.org/10.3390/foods10092115
APA StyleMartínez-Blay, V., Taberner, V., Pérez-Gago, M. B., & Palou, L. (2021). Postharvest Treatments with Sulfur-Containing Food Additives to Control Major Fungal Pathogens of Stone Fruits. Foods, 10(9), 2115. https://doi.org/10.3390/foods10092115








