Migration of Various Nanoparticles into Food Samples: A Review
Abstract
1. Introduction
2. General Rules for Using Nano-Materials in the Food Packaging
3. Silver Nanoparticles
4. Clay Nanoparticles
5. Copper (Cu) Nanoparticles
6. Detection and Evaluation Methods of Nano-Materials
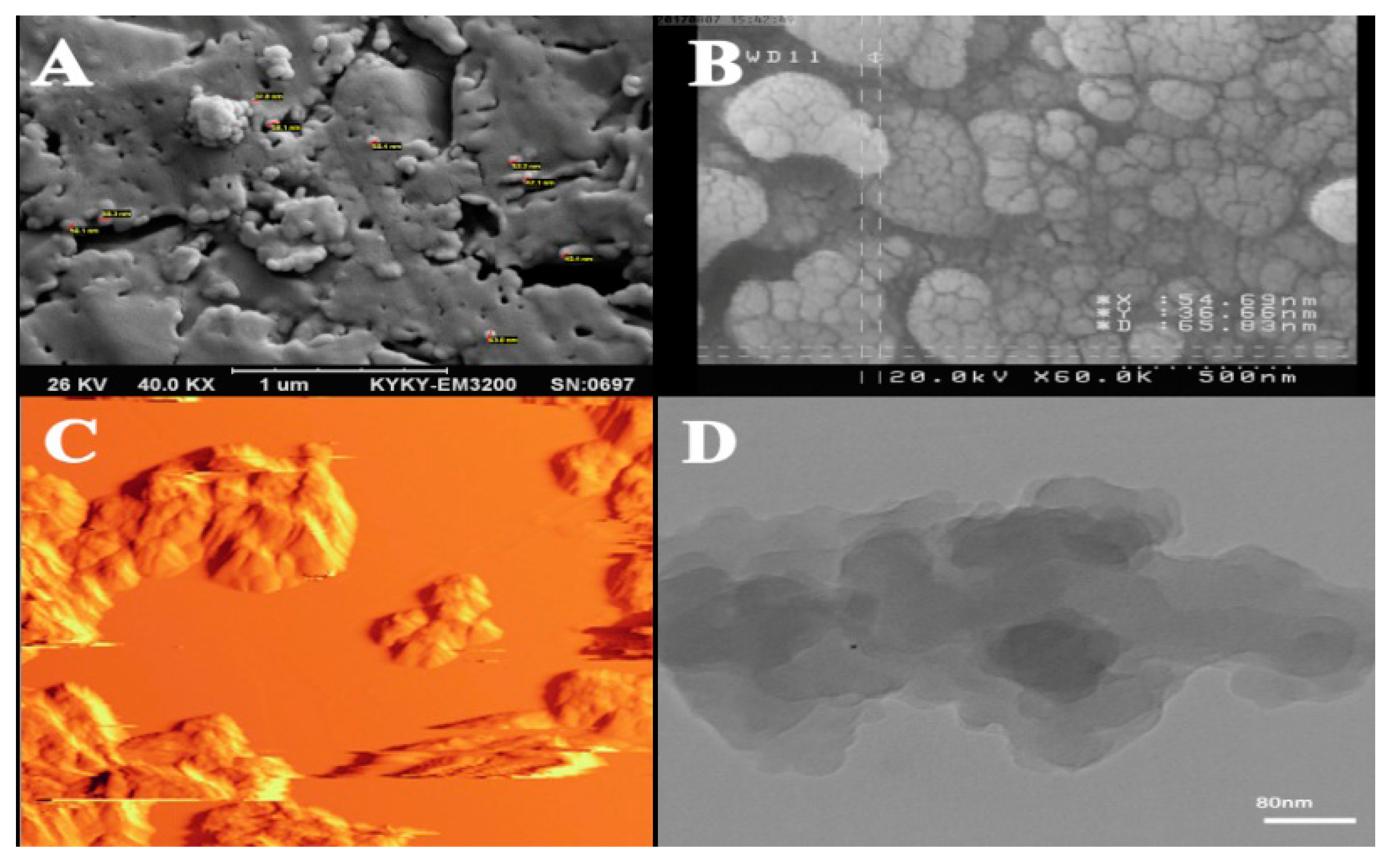
6.1. Microscopic Methods
6.2. Quantitative Analysis Methods
6.3. Spectroscopy Methods
D-Titration and Migration
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tewari, G.; Juneja, V. Advances in Thermal and Non-Thermal Food Preservation; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Han, J.; Wang, Y. Proteomics: Present and future in food science and technology. Trends Food Sci. Technol. 2008, 19, 26–30. [Google Scholar] [CrossRef]
- Kazemi, M.M.; Hashemi-Moghaddam, H.; Mohammadi Nafchi, A.; Ajodnifar, H. Application of modified packaging and nano ZnO for extending the shelf life of fresh pistachio. J. Food Process. Eng. 2020, 43, e13548. [Google Scholar] [CrossRef]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, antioxidant and antibacterial activities of chitosan nanoparticles loaded with nettle essential oil. J. Food Meas. Charact. 2020, 15, 1395–1402. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Rizzello, C. Design of a “Clean-Label” Gluten-Free Bread to Meet Consumers Demand. Foods 2021, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Akelah, A. Functionalized Polymeric Materials in Agriculture and the Food Industry; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ahari, H.; Karim, G.; Anvar, S.A.; Paidari, S.; Mostaghim, S.A.; Mazinani, A.S. Method for Producing Antimicrobial Nanofilms Packaging Cover Based on Titanium Nano-Dioxide Through Extrusion for Extension of Food Shelf-Life. U.S. Patent 16/726,718, 2 July 2020. [Google Scholar]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Kumar, U.S.U.; Khalil, H.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Arora, A.; Padua, G. Nanocomposites in food packaging. J. Food Sci. 2010, 75, R43–R49. [Google Scholar] [CrossRef] [PubMed]
- Mahalik, N.P.; Nambiar, A.N. Trends in food packaging and manufacturing systems and technology. Trends Food Sci. Technol. 2010, 21, 117–128. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Nafchi, A.M. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Paidari, S.; Ibrahim, S.A. Potential application of gold nanoparticles in food packaging: A mini review. Gold Bull. 2021, 54, 31–36. [Google Scholar] [CrossRef]
- Jokar, M.; Löschner, K.; Nafchi, A.M. Modeling of Silver Migration from Polyethylene Nanocomposite Packaging into a Food Model System Using Response Surface Methodology. ETP Int. J. Food Eng. 2016, 2, 13. [Google Scholar] [CrossRef][Green Version]
- Hyeon, D.J.; Kim, T.H.; Park, L.S. Effect of nano-silica filler on the uniform packaging of white light emitting diodes. J. Nanosci. Nanotechnol. 2013, 13, 5976–5981. [Google Scholar] [CrossRef]
- Marcel, N.R.; Patrick, Y.; Seraphine, E.; Robert, N. Utilization of overripe banana/plantain-maize composite flours for making doughnuts: Physicochemical, functional, rheological and sensory characterization. J. Food Meas. Charact. 2021, 15, 59–70. [Google Scholar] [CrossRef]
- Yagoubi, A.S.; Shahidi, F.; Mohebbi, M.; Varidi, M.; Golmohammadzadeh, S. Preparation, characterization and evaluation of physicochemical properties of phycocyanin-loaded solid lipid nanoparticles and nanostructured lipid carriers. J. Food Meas. Charact. 2018, 12, 378–385. [Google Scholar] [CrossRef]
- Sadeghi, K.; Shahedi, M. Physical, mechanical, and antimicrobial properties of ethylene vinyl alcohol copolymer/chitosan/nano-ZnO (ECNZn) nanocomposite films incorporating glycerol plasticizer. J. Food Meas. Charact. 2016, 10, 137–147. [Google Scholar] [CrossRef]
- Pilevar, Z.; Bahrami, A.; Beikzadeh, S.; Hosseini, H.; Jafari, S.M. Migration of styrene monomer from polystyrene packaging materials into foods: Characterization and safety evaluation. Trends Food Sci. Technol. 2019, 91, 248–261. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Emam-Djomeh, Z. Development and characterization of pH-sensitive and antioxidant edible films based on mung bean protein enriched with Echium amoenum anthocyanins. J. Food Meas. Charact. 2021, 15, 2984–2994. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, Y.S. Effect of storage conditions on the absorption kinetics of non-metallic oxygen scavenger suitable for moist food packaging. J. Food Meas. Charact. 2017, 11, 965–971. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Martin, O.; Montalvo, G. Recent advances in polymer-metallic composites for food packaging applications. Trends Food Sci. Technol. 2021, 109, 230–244. [Google Scholar] [CrossRef]
- Haghighatpanah, N.; Mirzaee, H.; Khodaiyan, F.; Kennedy, J.F.; Aghakhani, A.; Hosseini, S.S.; Jahanbin, K. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020, 152, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Rückerl, R.; Schneider, A.; Breitner, S.; Cyrys, J.; Peters, A. Health effects of particulate air pollution: A review of epidemiological evidence. Inhal. Toxicol. 2011, 23, 555–592. [Google Scholar] [CrossRef] [PubMed]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Drobne, D. Nanotoxicology for safe and sustainable nanotechnology. Arch. Ind. Hyg. Toxicol. 2007, 58, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Paidari, S.; Goli, M.; Anari, E.; Haghdoust, N. Evaluation the effects of nanosilver composites synthesized using sol-gel method on inoculated Vibrio parahaemolyticus to pink shrimp. Acta Sci. Nutr. Health 2019, 3, 47–51. [Google Scholar]
- Ahari, H.; Fahimi, B.; Sheikhi, N.; Anvar, A.; Paidari, S. Use of Real-Time PCR and High-Resolution Melting Analysis for Detection and Discrimination of Salmonella typhimurium and Salmonella enteritidis in Contaminated Raw-Egg Samples. J. Food Biosci. Technol. 2021, 11, 59–68. [Google Scholar]
- Kalaee, M.; Karami, M. Review of degradation kinetics of epoxy nanocomposites in the presence of clay nanoparticles. Basparesh 2021. [Google Scholar] [CrossRef]
- Asadi, G.; Mousavi, S. Application of Nanotechnology in Food Packaging. In 13th World Congress of Food Science & Technology; EDP Sciences: Les Ulis, France, 2006; p. 739. [Google Scholar]
- Zamindar, N.; Anari, E.S.; Bathaei, S.S.; Shirani, N.; Tabatabaei, L.; Mahdavi-Asl, N.; Khalili, A.; Paidari, S. Application of Copper Nano Particles in Antimicrobial Packaging: A Mini Review. Acta Sci. Nutr. Health 2020, 4, 14–18. [Google Scholar] [CrossRef]
- Efatian, H.; Ahari, H.; Shahbazzadeh, D.; Nowruzi, B.; Yousefi, S. Fabrication and characterization of LDPE/silver-copper/titanium dioxide nanocomposite films for application in Nile Tilapia (Oreochromis niloticus) packaging. J. Food Meas. Charact. 2021, 15, 2430–2439. [Google Scholar] [CrossRef]
- Ahari, H.; Amanolah Nejad, Z.; Magharehei, M.; Paidari, S. Incresing Shelf Life of Penaeus semisulcatus in NanoSilver Coatings Based on Titanium Dioxide. J. Food Technol. Nutr. 2020, 17, 91–98. [Google Scholar]
- Foghara, S.K.; Jafarian, S.; Zomorodi, S.; Asl, A.K.; Nasiraei, L.R. Fabrication and characterization of an active bionanocomposite film based on basil seed mucilage and ZnO nanoparticles. J. Food Meas. Charact. 2020, 14, 3542–3550. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Rodríguez, S.; Nerín, C. Nanoclay migration from food packaging materials. Food Addit. Contam. Part A 2016, 33, 530–539. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: Human and animal health. EFSA J. 2021, 19, e06768. [Google Scholar] [PubMed]
- Magri, A.; Petriccione, M.; Gutiérrez, T.J. Metal-organic frameworks for food applications: A review. Food Chem. 2021, 354, 129533. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, M.; Verma, N. Electrochemical preparation of Fe3O4/MWCNT-polyaniline nanocomposite film for development of urea biosensor and its application in milk sample. J. Food Meas. Charact. 2019, 14, 163–175. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Assessing the Effects of Significant Manufacturing Process Changes, Including Emerging Technologies, on the Safety and Regulatory Status of FOOD ingredients and Food Contact Substances, Including Food Ingredients that Are Color Additives; Food and Drug Administration: Washington, DC, USA, 2014.
- Adrah, K.; Ananey-Obiri, D.; Tahergorabi, R. Physicochemical Changes of Deep-Fat-Fried Chicken Drumsticks Treated with Quercetin-in-Edible Coating during Storage Time. Foods 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Warheit, D.B.; Borm, P.J.A.; Hennes, C.; Lademann, J. Testing strategies to establish the safety of nanomaterials: Conclusions of an ECETOC workshop. Inhal. Toxicol. 2007, 19, 631–643. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Salehabadi, A.; Nafchi, A.M.; Oladzadabbasabadi, N.; Jafari, S.M. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends in Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Razavi, R.; Rasouli, Y.; Ghorbani, M.; Divsalar, E.; Tajik, H.; Guimarães, J.T.; Ibrahim, S.A. Review of microbiological methods for testing protein and carbohydrate-based antimicrobial food packaging. Trends Food Sci. Technol. 2021, 111, 595–609. [Google Scholar] [CrossRef]
- Gong, J.; Tong, F.; Wang, B.; Ma, D.; Zhang, C.; Jiang, J.; Zhang, L. Zeolite A enhanced chitosan films with high water absorption ability and antimicrobial activity. Chin. J. Chem. Eng. 2021, 33, 337–343. [Google Scholar] [CrossRef]
- Boschetto, D.L.; Lerin, L.; Cansian, R.; Pergher, S.B.C.; Di Luccio, M. Preparation and antimicrobial activity of polyethylene composite films with silver exchanged zeolite-Y. Chem. Eng. J. 2012, 204, 210–216. [Google Scholar] [CrossRef]
- Fankhauser-Noti, A.; Grob, K. Migration of plasticizers from PVC gaskets of lids for glass jars into oily foods: Amount of gasket material in food contact, proportion of plasticizer migrating into food and compliance testing by simulation. Trends Food Sci. Technol. 2006, 17, 105–112. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar]
- Singh, J.; Dhaliwal, A.S. Novel Green Synthesis and Characterization of the Antioxidant Activity of Silver Nanoparticles Prepared from Nepeta leucophylla Root Extract. Anal. Lett. 2018, 52, 213–230. [Google Scholar] [CrossRef]
- Zapata, P.; Tamayo, L.; Páez, M.; Cerda, E.; Azocar, M.; Rabagliati, F.M. Nanocomposites based on polyethylene and nanosilver particles produced by metallocenic “in situ” polymerization: Synthesis, characterization, and antimicrobial behavior. Eur. Polym. J. 2011, 47, 1541–1549. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Zaghloul, S.; Hashem, M.; El-Shafei, A. A green approach to improve the antibacterial properties of cellulose based fabrics using Moringa oleifera extract in presence of silver nanoparticles. Cellulose 2021, 28, 549–564. [Google Scholar] [CrossRef]
- Manikandan, D.B.; Sridhar, A.; Sekar, R.K.; Perumalsamy, B.; Veeran, S.; Arumugam, M.; Karuppaiah, P.; Ramasamy, T. Green fabrication, characterization of silver nanoparticles using aqueous leaf extract of Ocimum americanum (Hoary Basil) and investigation of its in vitro antibacterial, antioxidant, anticancer and photocatalytic reduction. J. Environ. Chem. Eng. 2021, 9, 104845. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; McClements, D. Functional Materials in Food Nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S. Review of Mechanical Properties, Migration, and Potential Applications in Active Food Packaging Systems Containing Nanoclays and Nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Nafiseh, Z.; Samira, N.; Saeed, P.; Mohammad, G.; Hajar, A. Evaluationhe shelf life of minimally processed lettuce packed in modified atmosphere packaging treated with calcium lactate and heat shock, cysteine and ascorbic acid and sodium hypochlorite. J. Food Meas. Charact. 2021. [Google Scholar] [CrossRef]
- Martinez-Abad, A.; Lagarón, J.M.; Ocio, M.J. Characterization of transparent silver loaded poly(l-lactide) films produced by melt-compounding for the sustained release of antimicrobial silver ions in food applications. Food Control 2014, 43, 238–244. [Google Scholar] [CrossRef]
- Paidari, S.; Zamindar, N.; Tahergorabi, R.; Kargar, M.; Ezzati, S.; Shirani, N.; Musavi, S.H. Edible coating and films as promising packaging: A mini review. J. Food Meas. Charact. 2021, 1–10. [Google Scholar] [CrossRef]
- Asl, N.M.; Ahari, H.; Moghanjoghi, A.A.M.; Paidari, S. Assessment of nanochitosan packaging containing silver NPs on improving the shelf life of caviar (Acipenser persicus) and evaluation of nanoparticles migration. J. Food Meas. Charact. 2021, 1–9. [Google Scholar] [CrossRef]
- Farhoodi, M.; Mousavi, S.M.; Sotudeh-Gharebagh, R.; Emam-Djomeh, Z.; Oromiehie, A. Migration of aluminum and silicon from PET/clay nanocomposite bottles into acidic food simulant. Packag. Technol. Sci. 2014, 27, 161–168. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; Nedaeinia, R.; Goli, M.; Hosseini Teshnizi, S.; Sarkarizi, H.; Sarkarizi, K.; Nedaeinia, M.; Faraji, H. Novel application of Nanotechnology in drug and Gene delivery: Emphasis on Liposomes. Int. J. Pharm. Phytopharm. Res. 2018, 8, 81–91. [Google Scholar]
- Ahari, H.; Lahijani, L. Migration of Silver and Copper Nanoparticles from Food Coating. Coatings 2021, 11, 380. [Google Scholar] [CrossRef]
- Jeziórska, R.; Zielecka, M.; Gutarowska, B.; Żakowska, Z. High-Density Polyethylene Composites Filled with Nanosilica Containing Immobilized Nanosilver or Nanocopper: Thermal, Mechanical, and Bactericidal Properties and Morphology and Interphase Characterization. Int. J. Polym. Sci. 2014, 2014, 183724. [Google Scholar] [CrossRef]
- Palza, H.; Delgado, K.; Moraga, N.; Wang Molina, S.H. Polypropylene in the melt state as a medium for in situ synthesis of copper nanoparticles. AIChE J. 2014, 60, 3406–3411. [Google Scholar] [CrossRef]
- Hannon, J.C.; Kerry, J.P.; Cruz-Romero, M.; Azlin-Hasim, S.; Morris, M.; Cummins, E. Human exposure assessment of silver and copper migrating from an antimicrobial nanocoated packaging material into an acidic food simulant. Food Chem. Toxicol. 2016, 95, 128–136. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Evaluation and Simulation of Silver and Copper Nanoparticle Migration from Polyethylene Nanocomposites to Food and an Associated Exposure Assessment. J. Agric. Food Chem. 2014, 62, 1403–1411. [Google Scholar] [CrossRef]
- Hosseini, R.; Ahari, H.; Mahasti, P.; Paidari, S. Measuring the migration of silver from silver nanocomposite polyethylene packaging based on (TiO2) into Penaeus semisulcatus using titration comparison with migration methods. Fish. Sci. 2017, 83, 649–659. [Google Scholar] [CrossRef]
- Anvar, A.; Haghighat Kajavi, S.; Ahari, H.; Sharifan, A.; Motallebi, A.; Kakoolaki, S.; Paidari, S. Evaluation of the antibacterial effects of Ag-Tio2 nanoparticles and optimization of its migration to sturgeon caviar (Beluga). Iran. J. Fish. Sci. 2019, 18, 954–967. [Google Scholar]
- Panea, B.; Ripoll, G.; González, J.; Fernández-Cuello, Á.; Albertí, P. Effect of nanocomposite packaging containing different proportions of ZnO and Ag on chicken breast meat quality. J. Food Eng. 2014, 123, 104–112. [Google Scholar] [CrossRef]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.A.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Von Goetz, N.; Fabricius, L.; Glaus, R.; Weitbrecht, V.; Günther, D.; Hungerbühler, K. Migration of silver from commercial plastic food containers and implications for consumer exposure assessment. Food Addit. Contam. Part A 2013, 30, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Mackevica, A.; Olsson, M.E.; Hansen, S.F. Quantitative characterization of TiO2 nanoparticle release from textiles by conventional and single particle ICP-MS. J. Nanopart. Res. 2017, 20, 6. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerin, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, M.-J.; Ahmadi, H.; Lesankhosh, R.; Khalaj, G. Study of physical and mechanical properties of polypropylene nanocomposites for food packaging application: Nano-clay modified with iron nanoparticles. Trends Food Sci. Technol. 2016, 51, 41–48. [Google Scholar] [CrossRef]
- Schmidt, B.; Petersen, J.H.; Koch, C.B.; Plackett, D.; Johansen, N.; Katiyar, V.; Larsen, E. Combining asymmetrical flow field-flow fractionation with light-scattering and inductively coupled plasma mass spectrometric detection for characterization of nanoclay used in biopolymer nanocomposites. Food Addit. Contam. Part A 2009, 26, 1619–1627. [Google Scholar] [CrossRef]
- Golja, V.; Dražić, G.; Lorenzetti, M.; Vidmar, J.; Ščančar, J.; Zalaznik, M.; Kalin, M.; Novak, S. Characterisation of food contact non-stick coatings containing TiO2 nanoparticles and study of their possible release into food. Food Addit. Contam. Part A 2017, 34, 421–433. [Google Scholar] [CrossRef]
- Bott, J.; Störmer, A.; Franz, R. A model study into the migration potential of nanoparticles from plastics nanocomposites for food contact. Food Packag. Shelf Life 2014, 2, 73–80. [Google Scholar] [CrossRef]
- Liu, F.; Huai-Ning, Z.; Zhao, Q.; Shi, Y.-J.; Zhong, H.-N. Migration of copper from nanocopper/LDPE composite films. Food Addit. Contam. Part A 2016, 33, 1741–1749. [Google Scholar] [CrossRef]
- Herting, G.; Wallinder, I.O.; Leygraf, C. Corrosion-induced release of chromium and iron from ferritic stainless steel grade AISI 430 in simulated food contact. J. Food Eng. 2008, 87, 291–300. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Li, X.; Zhou, W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Al-Ali, R.M.; Al-Hilifi, S.A.; Rashed, M.M. Fabrication, characterization, and anti-free radical performance of edible packaging-chitosan film synthesized from shrimp shell incorporated with ginger essential oil. J. Food Meas. Charact. 2021, 15, 2951–2962. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Yu, S.-J.; Yin, Y.-G.; Chao, J.-B. Methods for separation, identification, characterization and quantification of silver nanoparticles. TrAC Trends Anal. Chem. 2012, 33, 95–106. [Google Scholar] [CrossRef]
- Song, H.; Li, B.; Lin, Q.-B.; Wu, H.-J.; Chen, Y. Migration of silver from nanosilver–polyethylene composite packaging into food simulants. Food Addit. Contam. Part A 2011, 28, 1–5. [Google Scholar] [CrossRef]
- Shamhari, N.M.; Wee, B.S.; Chin, S.F.; Kok, K.Y. Synthesis and Characterization of Zinc Oxide Nanoparticles with Small Particle Size Distribution. Acta Chim. Slov. 2018, 65, 578–585. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.; Saha, R.; Liu, J.; Chugh, V.; Wang, J.-P. Magnetic Particle Spectroscopy: A Short Review of Applications Using Magnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 4972–4989. [Google Scholar] [CrossRef]
| NP | Matrix | Sample or Simulant | Detection Method | References |
|---|---|---|---|---|
| Silver | PP | Ethanol 10% & Acetic Acid (AA) 3% | Titration | [67] |
| Silver | LDPE | Sturgeon (Bulga) | Titration | [68] |
| Silver | LDPE-PT | Milk | ICP-AES | [65] |
| Silver | LDPE | Chicken Breast | ICP-Ms | [69] |
| Silver | LDPE | Ethanol 10%, AA 3%, Olive Oil | ICP-Ms | [70] |
| Silver | LDPE-PT | Ethanol 10%, AA 3% | ICP-Ms | [71] |
| Silver | LDPE | Ethanol 10%, AA 3% | ICP-Ms | [72] |
| Silver | LDPE-PP | Ethanol 10%, AA 3% | ICP-Ms | [73] |
| ZnO | LDPE | Chicken Breast | ICP-Ms | [69] |
| Clay | PP | Cheese water | ICP-Ms | [74] |
| Clay | LDPE | Ethanol 10%, AA 3% | ICP-Ms | [73] |
| Clay | PLA | AA 3% | ICP-OEs | [60] |
| Clay | PLA | Ethanol 95% | ICP-Ms & AF4 | [75] |
| Ti | LDPE | Deionized water, Ethanol 10%, AA 3% | ICP-MS | [76] |
| Ti | LDPE | 95% Ethanol | ICP-MS | [77] |
| Cu | LDPE | Ethanol 10%, AA 3% | ICP-MS | [78] |
| Cr | Stainless Steel | AA 3% | ICP-Ms | [79] |
| Fe | Stainless Steel | AA 3% | ICP-Ms | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paidari, S.; Tahergorabi, R.; Anari, E.S.; Nafchi, A.M.; Zamindar, N.; Goli, M. Migration of Various Nanoparticles into Food Samples: A Review. Foods 2021, 10, 2114. https://doi.org/10.3390/foods10092114
Paidari S, Tahergorabi R, Anari ES, Nafchi AM, Zamindar N, Goli M. Migration of Various Nanoparticles into Food Samples: A Review. Foods. 2021; 10(9):2114. https://doi.org/10.3390/foods10092114
Chicago/Turabian StylePaidari, Saeed, Reza Tahergorabi, Ensieh Sadat Anari, Abdorezza Moahammdi Nafchi, Nafiseh Zamindar, and Mohammad Goli. 2021. "Migration of Various Nanoparticles into Food Samples: A Review" Foods 10, no. 9: 2114. https://doi.org/10.3390/foods10092114
APA StylePaidari, S., Tahergorabi, R., Anari, E. S., Nafchi, A. M., Zamindar, N., & Goli, M. (2021). Migration of Various Nanoparticles into Food Samples: A Review. Foods, 10(9), 2114. https://doi.org/10.3390/foods10092114








