Abstract
This study evaluated the effect of dietary fibre obtained from pomegranate, tomato, grape and broccoli by-products on the gastrointestinal transit survival, growth, and metabolism of six probiotic strains. The results showed that the studied by-products contained variable amounts of polysaccharides that affected the six probiotic microorganisms in different ways. In addition, the protective effect of the fibre obtained on the probiotic strains was more effective in the case of the fibre obtained from tomato peel. In terms of growth, grape stems showed the best results, favouring the growth of lactic acid bacteria. Finally, all fibres were able to increase the content of short-chain fatty acids in the in vitro test, but broccoli stems and pomegranate peel stimulated higher production of short-chain fatty acids. The results of this study demonstrate that plant by-product fibres can improve survival, growth, and metabolism in terms of the fatty acid profiles of probiotic strains, highlighting the desirability of harnessing these by-product fibres to develop new high-value-added ingredients as probiotic carriers.
1. Introduction
Plant-based food production generates large amounts of food waste during processing and storage, which are referred to as by-products that could be reutilized to avoid environmental risks and economic losses [1]. These residues comprise vegetable waste and by-products such as skins, stems, and seeds. Vegetable by-products are rich sources of dietary fibre (DF) and an important source of soluble polysaccharides. Polysaccharides from agro-food industry residues constitute one of the most important renewable resources. The wide variety in their chemical composition and structure, as well as their biodegradability and safety, make them suitable for applications in many fields such as food, pharmaceuticals, cosmetics, tissue engineering, biofuels, and others [2,3]. These are, therefore, bioactive compounds that are gaining increasing interest in their use as food additives or supplements, focusing efforts on improving their biological activity by new extraction methodologies [4].
Polysaccharides from agricultural residues are part of plant cell walls, which are highly diverse in their structure and composition [5]. In general, cell walls are constituted by high-molecular-weight polysaccharides, which are mainly lignin, cellulose, hemicelluloses, pectins, and other non-starch polysaccharides such as inulin and oligosaccharides [6]. Cell-wall-forming polysaccharides are classified into insoluble and soluble polysaccharides based on their solubility in water. Insoluble polysaccharides are lignin, cellulose, hemicelluloses, and pectins (insoluble); the soluble polysaccharide group consists of other pectins, and hemicelluloses [7].
Pectins are mostly considered as soluble fibre and are a polygalacturonic acid-rich part of the plant cell wall that can be composed of up to 17 different monosaccharides [8]. They are also the most structurally complex natural plant polysaccharides [9], and numerous health benefits are attributed to them [10]. They can be obtained from many vegetable sources, primarily citrus peel and apple pomace, but are also present in tomato peel [11,12], pomegranate peel [13], grape skin [14], and broccoli stems [15].
One of the most important activities of DF is its prebiotic activity, which can selectively stimulate the activity or growth of probiotic microorganisms in the colon [16]. In this regard, research efforts have been directed towards the inclusion of these prebiotics in daily dietary foods, most notably in dairy products [17], but also in beverages, processed fruits and vegetables, bakery, confectionery, snacks, sweeteners, and baby formula [18]. Due to this growth-stimulating activity of probiotic microorganisms, the addition of prebiotics and probiotics together to foods is more attractive because it promotes the viability and stability of the microorganisms [19]. Probiotics, aided by prebiotics, have a beneficial effect on consumer health by improving microbial balance at the gut level [20]. To provide these benefits, probiotics must remain viable, with a total number of live organisms above 106 cfu/g of food. The survival of these microorganisms during food processing and storage as well as during intestinal transit is clearly compromised on many occasions [21]. Several technologies have been employed to improve the viability of these microorganisms exposed to environmental stress and intestinal transit, such as encapsulation, in which different methodologies have been used depending on the drying temperature: high temperature drying (spray drying and fluid bed drying) and low temperature drying (ultrasonic vacuum spray drying, spray cooling, electrospinning, supercritical technique, freeze drying, extrusion, emulsion, enzymatic gelation, and impact spray technique) [22].
An alternative method is to combine probiotic cells with fibrous materials where probiotic cells can be stuck to or even form biofilms on the surface. This would confer high viability after gastrointestinal digestion [23,24]. Only a few studies are available in the literature on the use of fibre-rich agronomic by-products as protectors of probiotics as well as prebiotics for growth and metabolism stimulation [25].
The aim of this work was to characterize the fibre obtained from tomato, pomegranate, grape, and broccoli by-products and to investigate the effects of this fibre on the intestinal transit survival, growth, and metabolism of six strains with probiotic properties in order to develop new prebiotic or symbiotic food ingredients.
2. Materials and Methods
2.1. Plant Material
In this study, four agro-industrial by-products were used, including pomegranate peel, tomato peel, grape stalk, and broccoli stem. The vegetable by-products used in this work were provided by horticultural plants from the Extremadura region of Spain. All samples were dried in a forced-air oven at 45 °C for 24–48 h, ground, packed in vacuum bags, and stored at room temperature until use.
2.2. Sample Preparation
DF concentrates were prepared using the alcohol-insoluble residue method described by Femenia et al. [26], with modifications. Briefly, three replicates (5 g each) of dry samples were homogenized with 85% (v/v) ethanol. The mixture was boiled on a shaker for 10 min, and then the solid residue was collected using a Büchner funnel with cellulose-free filters (Whatman, 934-AH ™ glass microfiber filters, Sigma Chemical Co., St Louis, MO, USA). This process was repeated twice, the final time with absolute ethanol. Finally, the insoluble solid residue was washed with acetone, and the excess solvent was removed after 24 h at room temperature.
2.3. Determination of Neutral Sugar and Uronic Acid
To determine the content of uronic acids and the profile of neutral sugars, the DF was previously solubilized (autoclave treatment at 121 °C for 16 min followed by an ultrasound treatment for 1 h), and the soluble content of the DF extract was precipitated by adding to the supernatant 3 times its volume of absolute ethanol at 60 °C. Then, it was centrifuged, and the supernatant was removed. The solid residue was dried in an oven at 45 °C. Once dry, the soluble residue of the DF extract was subjected to a hydrolysis process with 12 M sulfuric acid (3 h at room temperature and 100 °C for 1 h). Finally, the monosaccharides and galacturonic acids released were determined by HPLC. HPLC analyses in this study were conducted using a 1260 Infinity II LC Agilent HPLC System (Waters, Milford, MA, USA), which consisted of a separation module, RI detector. The HPLC system was equipped with a Rezex-ROA column (7.8 mm internal diameter × 150 mm; Phenomenex, Torrance, CA, USA). In isocratic mode, the mobile phase was water, at a flow rate of 0.6 mL/min. In elution mode, the sample injection volume was 10 μL, the column temperature was 80 °C and the detector temperature was 40 °C.
2.4. Survival of Probiotics in the Presence of DF Extracts
Co-incubation of probiotics and DF extracts was performed in MRS broth supplemented with 1% DF extracts. The bacterial strains used were Lactobacillus casei (HL 245, HL 233) (Spanish type culture collection), Lactobacillus reuteri (PL503, PL519), Enterococcus faecium (SE 906, SE 920) [27,28]. Then, 10 mg of DF was weighed out and mixed with 900 µL of (de Man, Rogosa and Sharpe) MRS broth, which was sterilised at 121 °C for 15 min, cooled, and inoculated with 100 μL of MRS broth sterile with probiotic inoculum content of 10% (109 cfu/mL) in aseptic conditions. Co-incubation was carried out over 18 h at 37 °C at 180 rpm with orbital shaking to ensure a homogeneous distribution of the DF extract supply. As a blank, probiotics incubated without DF extracts were used.
A standardised in vitro static digestion method was performed for simulated gastrointestinal digestion [29]. Simulated gastric juice was mixed with an equal volume of probiotic suspensions. A 1 M HCl solution was used to adjust the pH of these samples to pH 2 prior to gastric digestion at 37 °C over 2 h. Following gastric digestion, the gastric mixtures were then mixed with an equal volume of simulated intestinal juice and adjusted to pH 7 with a solution of 1 M NaOH prior to intestinal digestion at 37 °C for 6 h. When gastric and intestinal digestion were completed, viable bacteria were counted in MRS medium in the digested samples after 0, 2 and 6 h. The data were expressed in logarithmic reduction of cfu/mL with respect to the initial inoculum (Time 0 h: 8 Log cfu/mL).
2.5. In Vitro Prebiotic Capacity
The prebiotic capacity of the soluble DF extracts was assessed using the method described previously by Ruiz-Moyano et al. [30]. Inoculum preparations consisted of aliquots of stock cultures of the bacteria strains L. casei (HL 245, HL 233) (Spanish type culture collection), L. reuteri (PL 503, PL 519), and E. faecium (SE 906, SE 920) (Ruiz-Moyano et al. [27,28] grown in Man–Rogosa–Sharpe (MRS; Scharlab, Barcelona, Spain) broth over 24 h at 37 °C. The strains were tested for growth in the presence of solubilized DF (autoclave treatment at 121 °C for 16 min followed by an ultrasound treatment for 1 h). Five microlitres of each bacterial suspension strain was inoculated into 200 μL of semi-solid MRS medium which contained 0.125 g/L agar, glucose-free and supplemented with 2 g/L of each sterile-filtered extract as the exclusive carbohydrate source. Semi-solid MRS supplemented with 2 g/L glucose was used as a positive control for growth, and other DF control was made with short-chain fructo-oligosaccharide (FOS), whereas the negative control was a carbohydrate-free semisolid MRS. Turbidity was measured in a fluorimeter (FLUOstar OPTIMA F), growth was carried out for 48 h at a temperature of 37 °C and readings were taken at a wavelength of 570 nm at 1-h intervals. The ability of each strain to grow was evaluated by comparing, in percentage, the growth in each extract with that in the control.
2.6. Determination of Short-Chain Fatty Acids Produced in the Presence of Fibre Extracts
To determine the ability to produce short-chain fatty acids (SCFAs), lactic acid bacteria (LAB) strains that were grown with the improved fibre extracts were selected. They were grown in modified MRS broth at 37 °C. The MRS was prepared as a commercial MRS without glucose and sodium acetate and complemented with 2 g/L of carbohydrate source (improved fibre extracts). Culture supernatants were obtained by centrifugation of the medium at 8000× g for 5 min before filtration through 0.22-µm filters (Thermo Fisher Scientific, Waltham, MA, USA).
To measure the amount of SCFAs, 500 µL of supernatant was mixed with 500 µL of ultrapure water and 100 µL of internal standard (2-ethylbutyric acid). Then 0.5 µL was injected into a gas chromatograph with a split/split-less injector and a flame ionisation detector (Shimadzu 2010 Plus). SCFAs were separated on a DB-FFAP capillary column (30 m × 0.25 mm id; 0.25 µm). The initial oven temperature was maintained at 80 °C for 2 min, and then increased to 200 °C at 20 °C/min and maintained for 12 min. The injector and detector were set at 250 °C. Helium at 1.8 mL/min was the carrier gas. Individual SCFAs were determined by comparing their retention times with those of the reference standard mixtures from Sigma (Sigma Chemical Co., St Louis, MO, USA). SCFA concentrations were determined as the ratio of the peak area of the analyte to the internal standard (2-ethylbutyric acid), according to Brighenti [31].
2.7. Statistical Analysis
Statistical analysis of the data was carried out using SPSS for Windows, version 21.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics of the data were determined, and the differences within and between groups were studied by one-way analysis of variance (ANOVA) and separated by Tukey’s honestly significant difference test (p ≤ 0.05). Principal component analysis (PCA) was performed on the correlation matrix of the variables. We worked with three biological replicates and each of them were analysed in triplicate.
3. Results and Discussion
3.1. Fibre Constituents: Neutral Sugars and Pectins
The results of the DF composition of by-products of tomato and pomegranate peels, and grape and broccoli stems are shown in Table 1.

Table 1.
Neutral sugar and uronic acid profile of dietary fibre from vegetable by-products (mg/g dry weight).
The most abundant components of the DF extracts were uronic acids (which indicate the pectin content), and their values ranged from 581.24 to 934.72 mg of galacturonic acid per gram of fibre extract. Tomato peel exhibited the highest values, 934.72 mg/g, showing significant differences with the rest of the by-product extracts studied. Regarding the neutral sugar profile, the main monosaccharides were glucose and fucose in tomato and pomegranate peel, mannose in the case of grape stems, showing significant differences with the rest of the by-products, and arabinose and xylose in broccoli stems. In general, it was observed that galactose and rhamnose were the minority sugars in all analysed samples.
The results obtained for pectin concentration were higher than those found by other authors. Depending on the extraction method, Sengar et al. [32] obtained between 675.8 and 913.3 g/kg of galacturonic acid in tomato peel. Pomegranate peel was described with a lower amount of pectins, between 377 and 755 mg/g uronic acids [33]. Lower amounts of pectins were also found in grape and broccoli stems, ranging from 31.09 mg/g in grape stems [14] to 159.5 mg/g in broccoli stems [34].
The profile of the majority of neutral sugars was variable depending on the by-product. Glucose levels in tomato and pomegranate skins were significantly higher than those in grape and broccoli stems. This glucose may come from non-pectic polysaccharides or be a remnant of soluble sugars that were not completely removed during the extraction procedure, and other sugars identified in other studies coincide, although in different amounts, with those found in our study [14,32,33,34].
3.2. Survival of Probiotics in the Presence of DF Extracts
Table 2 shows the results obtained from the assay performed to check the ability of the DF extracts to protect the probiotic strains used from gastrointestinal tract transit. The data are expressed as Log cfu/mL reduction with respect to the initial inoculum (Time 0 h: 8 Log cfu/mL).

Table 2.
Tolerance of bacterial strains to low pH, and complete gastrointestinal transit. Data are expressed in Log cfu/mL reduction with respect to the initial inoculum (Time 0 h: 8 Log cfu/mL).
In the case of E. faecium SE 920, it was observed that tolerance to low pH and gastrointestinal transit was superior in the presence of the different DF extracts, and the best behaviour was observed in the presence of tomato peel extract, although it improved in all cases with respect to the control without fibre. In the same way, tomato peel extracts were able to improve survival through the gastrointestinal tract in all strains of L. casei and L. reuteri. In addition, the survival of all strains was improved more or less in the presence of DF, except that E. faecium SE 906 did not tolerate the low pH and gastrointestinal transit in vitro in the absence of extract, in the control or in the presence of the different DF extracts. Tomato DF extract presented the highest pectin concentration, which may be related to its protective activity. Anal and Singh [35] studied several biopolymers generally recognized as safe as encapsulation materials, including gelatine, pectin, and alginate, for their ability to improve the viability and shelf life of probiotic bacteria. Other authors have shown that multi-layered emulsions including pectins extracted from citrus peels can be used to incorporate probiotics into various products and improve their viability during processing, storage, and after ingestion [36]. Blaiotta et al. [37] demonstrated that chestnut fibre was able to greatly improve the tolerance of Lactobacillus to simulated gastric and bile juice.
Soluble DF can create a viscous environment for probiotic bacteria, providing a protective effect and enhancing cell adhesion or biofilm formation in the fibre matrix [38].
3.3. In Vitro Prebiotic Capacity
Table 3 shows the capacity of the six probiotic bacterial strains tested for in vitro growth on four DF extracts. The results showed that the DF extracts from the grape stem sample achieved the highest growth values for all the strains studied, E. faecium SE 920 showing the highest growth (107.29%). In addition, significant differences were found in the growth of the E. faecium SE 906, E. faecium SE 920, L. casei HL 245, and L. casei HL 233 strains in the presence of the grape stem fibre extracts with respect to the rest of the extracts. However, no significant differences were found in the growth of the two L. reuteri strains. On the one hand, pomegranate peel extracts achieved moderate growth of L. casei HL 233 and E. faecium strains (48.35% and 44.49%, respectively). On the other hand, all the strains studied showed a slight growth in the presence of tomato peel and broccoli stem extracts.

Table 3.
Percentage growth of lactic acid bacteria (LAB) strains, with respect to the positive control, on soluble dietary fibre extracts.
Polysaccharides such as pectin are formed by groups of complex structure, which are metabolised more slowly. Therefore, the ability of bacteria to metabolize fibres of this subgroup as an energy source will be influenced by the degree of polymerization, molecular weight, chain size, and the presence of branching in the molecule [39]. The speed and rate of prebiotic DF fermentation by the gut microbiota depends on several factors such as solubility, chain size, porosity, total particle surface area, and the structure and organization of the fibre cell wall. Furthermore, the fermentation outcome of a fibre mixture is not the same as that of each individual DF [40]. In addition, other dietary components such as proteins, lipids, and phenolic compounds can affect the fermentability and prebiotic effect of DF [41].
3.4. Short-Chain Fatty Acids Produced in the Presence of Fibre Extract Production
Table 4 shows the production of short-chain fatty acids of the probiotic bacteria grown in the presence of the different DF extracts studied. No significant differences were found in the production of the different fatty acids at the strain level. However, significant differences were found according to the type of extract used. Acetic acid was the major acid in all cases, and the values ranged from 14.39 to 355.59 mM. The highest production of acetic acid was observed in the presence of broccoli stem extract, which showed significant differences with respect to the other extracts, followed by grape stem and pomegranate peel samples. By contrast, tomato skin showed the lowest value for acetic acid production. Overall, it can be stated that pomegranate skin achieved the highest values for the other minority fatty acids, followed by the broccoli stem and grape stem samples; however, as in the case of acetic acid, the tomato skin DF extracts showed the lowest values. The glucose and FOS controls obtained average values for all the fatty acids studied.

Table 4.
Production of short-chain fatty acids in mM of lactic acid bacteria (LAB) in the presence of different soluble fibre extracts.
Baenas et al. [42] observed that the production of fatty acids in the presence of DF extracted from raspberry was generally increased during fermentation. Similar to the results obtained by us, previous studies revealed that DF extracted from potato residue could promote the production of acetic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid [43].
3.5. Multivariate Analysis of the Parameters Related to DF Extract Studied from Different Subproducts
PCA was carried out for the entire set of DF data to obtain an interpretable overview of the main information. Figure 1 shows the two-way loadings and score plots, where PC2 was plotted against PC1, explaining 90% of the total variance. According to Figure 1A, PC1 best explains the difference between the variables of bacterial survival to gastrointestinal transit and the production of short-chain fatty acids such as propionic acid, while PC2 shows the variability between the neutral sugars present in the soluble fibre extract.
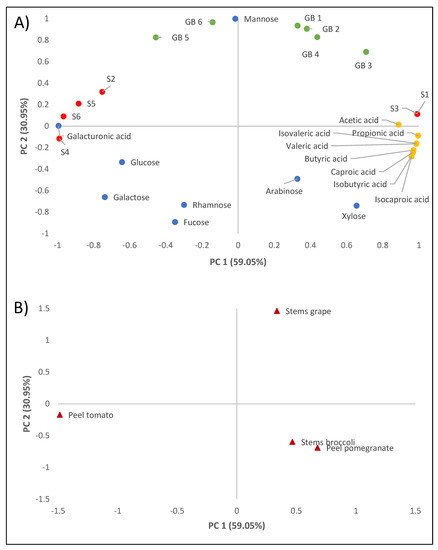
Figure 1.
Principal component analysis of the analytical results of the dietary fibre extracts studied. Loading plot (A). GB1: growth of E. faecium SE 906; GB2: growth of E. faecium SE 920; GB3: growth of L. casei HL 245; GB4: growth of L. casei HL 233; GB5: growth of L. reuteri PL 503; GB6: growth of L. reuteri PL 519; S1: survival in the gastrointestinal tract of E. faecium SE 906; S2: survival in the gastrointestinal tract of E. faecium SE 920; S3: survival in the gastrointestinal tract of L. casei HL 245; S4: survival in the gastrointestinal tract of L. casei HL 233; S5: survival in the gastrointestinal tract of L. reuteri PL 503; S6: survival in the gastrointestinal tract of L. reuteri PL 519. Score plot (B). Grape stem, broccoli stem, pomegranate peel, and tomato peel samples.
With regard to the factors studied, principal component analysis clearly shows how the fibre extracts obtained from the different by-products behave differently with respect to the variables studied. The fibre extracted from grape stems was positively correlated with the percentage of lactic acid bacterial growth with respect to the positive control, while the fibre extracted from broccoli stems and pomegranate peels was correlated with the production of short-chain fatty acids. On the other hand, tolerance to low pH and gastrointestinal transit was superior when tomato peel extract was present.
4. Conclusions
This study presents a characterization of the effect of DF obtained from pomegranate, tomato, grape, and broccoli by-products on the gastrointestinal transit survival, growth, and metabolism of six probiotic strains. These by-products were shown to have high concentrations of polysaccharides that affected the probiotic microorganisms studied in different ways. The protective effect on intestinal transit of the fibre obtained from tomato skin was superior to that of the other extracts of pomegranate, grape, and broccoli. The fibre extracted from grape stems favoured the growth of lactic acid bacteria, and the fibre extracted from broccoli stems and pomegranate peel stimulated the increased production of short-chain fatty acids. The results reveal that these by-products can be considered as high-quality sources of DF for joint applications with probiotic microorganisms by aiding and stimulating their survival, growth, and metabolism, and their use as prebiotics in the food industry would help to develop new high value-added ingredients.
Author Contributions
Conceptualization, R.C., A.M. and M.J.B.; methodology, R.C., S.R.-M. and M.J.B. formal analysis, M.Á.R.; investigation, M.Á.R. and A.V.M.; resources, M.Á.R. and R.C.; data management, R.C. and A.M.; writing—original draft preparation, M.Á.R., R.C. and M.J.B. writing—review and editing, R.C. and M.J.B.; visualization, A.M.; supervision, M.J.B. and M.d.G.C.; funding acquisition, M.d.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
Junta de Extremadura with the projects IB16158, TA18007 and GR18165.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to M. Cabrero and J. Hernández Barreto for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, B.; Dong, F.; Chen, M.; Zhu, J.; Tan, J.; Fu, X.; Wang, Y.; Chen, S. Advances in recycling and utilization of agricultural wastes in China: Based on environmental risk, crucial pathways, influencing factors, policy mechanism. Procedia Environ. Sci. 2016, 31, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Liu, R.H. Microwave-assisted extraction of polysaccharides from Moringa oleifera Lam. leaves: Characterization and hypoglycemic activity. Ind. Crops Prod. 2017, 100, 1–11. [Google Scholar] [CrossRef]
- Santagata, G.; Mallardo, S.; Fasulo, G.; Lavermicocca, P.; Valerio, F.; Di Biase, M.; Di Stasio, M.; Malinconico, M.; Volpe, M.G. Pectin-honey coating as novel dehydrating bioactive agent for cut fruit: Enhancement of the functional properties of coated dried fruits. Food Chem. 2018, 258, 104–110. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Casquete, R.; Martín, A.; Córdoba, M.D.G.; Aranda, E.; Benito, M.J. Strategies to increase the biological and biotechnological value of polysaccharides from agricultural waste for application in healthy nutrition. Int. J. Env. Res. Public Health 2021, 18, 5937. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Nandi, A.K.; Manna, D.K.; Pattanayak, M.; Sen, I.K.; Bhanja, S.K.; Samanta, S.; Panda, B.C.; Paloi, S.; Acharia, K.; et al. Structural characterization and antioxidant activity of a glucan from Meripilus giganteus. Carbohydr. Polym. 2017, 157, 1237–1245. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhang, M.; Zhou, H.L. Microwave-assisted extraction, purification, partial characterization, and bioactivity of polysaccharides from Panax ginseng. Molecules 2019, 24, 1605. [Google Scholar] [CrossRef] [Green Version]
- Flanagan, B.M.; Williams, B.A.; Sonni, F.; Mikkelsen, D.; Gidley, M.J. Fruit and vegetable insoluble dietary fibre in vitro fermentation characteristics depend on cell wall type. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100223. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Bayar, N.; Friji, M.; Kammoun, R. Optimization of enzymatic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal. Food Chem. 2018, 241, 127–134. [Google Scholar] [CrossRef]
- Grassino, A.N.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Halambek, J.; Cindrić, I.; Grassino, A.N. Evaluation of pectin isolated from tomato peel waste as natural tin corrosion inhibitor in sodium chloride/acetic acid solution. Carbohydr. Polym. 2020, 234, 115940. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Makarova, E.N.; Belyy, V.A. Structural studies of biologically active pectin-containing polysaccharides of pomegranate Punica granatum. Int. J. Biol. Macromol. 2019, 122, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.Á.; Casquete, R.; Córdoba, M.D.G.; Ruíz-Moyano, S.; Benito, M.J.; Pérez-Nevado, F.; Martín, A. Chemical composition and functional properties of dietary fibre concentrates from winemaking by-products: Skins, stems and leaves. Foods 2021, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Petkowicz, C.L.; Williams, P.A. Pectins from food waste: Characterization and functional properties of a pectin extracted from broccoli stalk. Food Hydrocoll. 2020, 107, 105930. [Google Scholar] [CrossRef]
- Davis, C.D.; Milner, J.A. Gastrointestinal microflora, food components and colon cancer prevention. J. Nutr. Biochem. 2009, 20, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, M.C.; Carmo, M.R.; Balthazar, C.F.; Guimarães, J.T.; Esmerino, E.A.; Freitas, M.Q.; Silva, M.C.; Pimentel, T.C.; Cruz, A.G. Dairy products with prebiotics: An overview of the health benefits, technological and sensory properties. Int. Dairy J. 2021, 117, 105009. [Google Scholar] [CrossRef]
- Singla, V.; Chakkaravarthi, S. Applications of prebiotics in food industry: A review. Food Sci. Technol. Int. 2017, 23, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci Nutr. 2017, 57, 344–371. [Google Scholar] [CrossRef]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46, S58–S61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkçi, N.; Akdeniz, V.; Akalin, A.S. Survival of probiotics in functional foods during shelf life. In Food Quality and Shelf Life; Academic Press: Cambridge, MA, USA, 2019; pp. 201–233. [Google Scholar] [CrossRef]
- Liu, H.; Cui, S.W.; Chen, M.; Li, Y.; Liang, R.; Xu, F.; Zhong, F. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2863–2878. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Medronho, B.; dos Santos, T.; Nunes-Correia, I.; Granja, P.; Miguel, M.G.; Lindman, B. On the viability, cytotoxicity and stability of probiotic bacteria entrapped in cellulose-based particles. Food Hydrocoll. 2018, 82, 457–465. [Google Scholar] [CrossRef]
- Hu, M.X.; Li, J.N.; Guo, Q.; Zhu, Y.Q.; Niu, H.M. Probiotics biofilm-integrated electrospun nanofiber membranes: A new starter culture for fermented milk production. J. Agr. Food Chem. 2019, 67, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sampers, I.; Raes, K. Dietary fiber concentrates recovered from agro-industrial by-products: Functional properties and application as physical carriers for probiotics. Food Hydrocoll. 2021, 111, 106175. [Google Scholar] [CrossRef]
- Femenia, A.; Garcı́a-Pascual, P.; Simal, S.; Rosselló, C. Effects of heat treatment and dehydration on bioactive polysaccharide acemannanand cell wall polymers from Aloe barbadensis Miller. Carbohydr. Polym. 2003, 51, 397–405. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Casquete, R.; Serradilla, M.J.; de Guía Córdoba, M. Safety and functional aspects of pre-selected lactobacilli for probiotic use in Iberian dry-fermented sausages. Meat Sci. 2009, 83, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Aranda, E.; Casquete, R.; de Guía Cordoba, M. Safety and functional aspects of preselected enterococci for probiotic use in Iberian dry-fermented sausages. J. Food Sci. 2009, 74, M398–M404. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Moyano, S.; dos Santos, M.T.P.G.; Galván, A.I.; Merchán, A.V.; González, E.; de Guía Córdoba, M.; Benito, M.J. Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: Probiotic characteristics and prebiotic metabolism. LWT 2019, 114, 108388. [Google Scholar] [CrossRef]
- Brighenti, F. Simple Method for Quantitative Analysis of Short Chain Fatty Acids in Serum by Gas-Liquid Chrmotography. Plant Polysaccharides in Human Nutrition: Structure, Function, Digestive Fate and Metabolic Affects. 1997, pp. 114–119. Available online: https://ci.nii.ac.jp/naid/10011807480/ (accessed on 23 June 2021).
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef]
- Abid, M.; Renard, C.M.; Watrelot, A.A.; Fendri, I.; Attia, H.; Ayadi, M.A. Yield and composition of pectin extracted from Tunisian pomegranate peel. Int. J. Biol. Macromol. 2016, 93, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydrate Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Zhong, Q. The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Res. Int. 2015, 71, 9–15. [Google Scholar] [CrossRef]
- Blaiotta, G.; La Gatta, B.; Di Capua, M.; Di Luccia, A.; Coppola, R.; Aponte, M. Effect of chestnut extract and chestnut fiber on viability of potential probiotic Lactobacillus strains under gastrointestinal tract conditions. Food Microbiol. 2013, 36, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.H.; Song, A.X.; Yao, Z.P.; Wu, J.Y. Protective effects of natural and partially degraded konjac glucomannan on Bifidobacteria against antibiotic damage. Carbohydr. Polym. 2018, 181, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, B.R.; Tuncil, Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J. Mol. Biol. 2014, 426, 3838–3850. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [Green Version]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Rémésy, C.; Demigné, C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef] [Green Version]
- Baenas, N.; Nuñez-Gómez, V.; Navarro-González, I.; Sánchez-Martínez, L.; García-Alonso, J.; Periago, M.J.; González-Barrio, R. Raspberry dietary fibre: Chemical properties, functional evaluation and prebiotic in vitro effect. LWT 2020, 134, 110140. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, W.; Ma, Z.; Liu, Y.; Mu, J.; Wang, J.; Hui, X. Enzymatic-modified dietary fibre fraction extracted from potato residue regulates the gut microbiotas and production of short-chain fatty acids of C57BL/6 mice. J. Funct. Foods 2021, 84, 104606. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).