Whole or Defatted Sesame Seeds (Sesamum indicum L.)? The Effect of Cold Pressing on Oil and Cake Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Preparation and Analysis
3. Results and Discussion
3.1. Nutritional Value of Seeds and Press Cake
3.2. Vitamin E Profile of Seeds, Cake and Oil
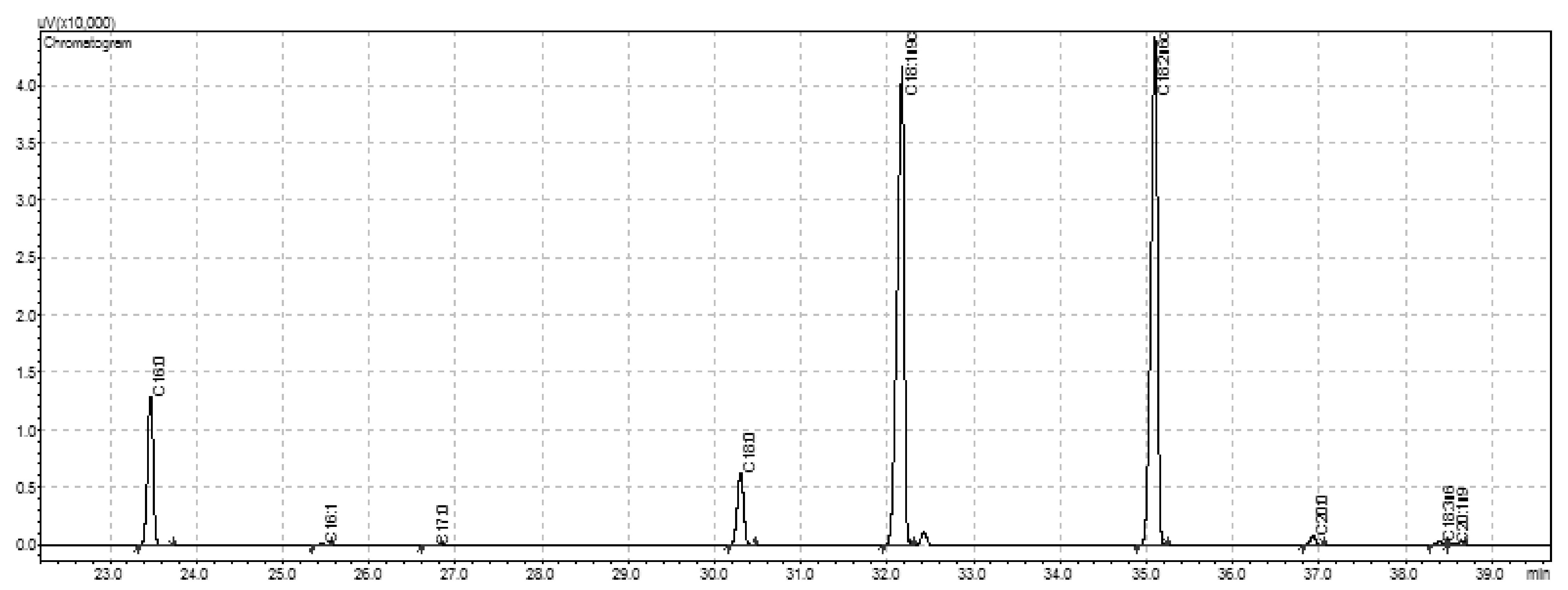
3.3. Fatty Acids Profile of Seeds, Cake and Oil
3.4. Characterization of Cold Pressed Sesame Oil
3.5. Seed’s Label Compliance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kopsahelis, N.; Kachrimanidou, V. Advances in food and byproducts processing towards a sustainable bioeconomy. Foods 2019, 8, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, D.; Machado, T.B.; Oliveira, M.B.P.P. Chia seeds: An ancient grain trending in modern human diets. Food Funct. 2019, 10, 3068–3089. [Google Scholar] [CrossRef]
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Chai, T.-T.; Tan, Y.-N.; Ee, K.-Y.; Xiao, J.; Wong, F.-C. Seeds, fermented foods, and agricultural by-products as sources of plant-derived antibacterial peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, S162–S177. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 23 August 2021).
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of pulsed electric fields and high voltage electrical discharges for oil extraction from sesame seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Fasuan, T.O.; Gbadamosi, S.O.; Omobuwajo, T.O. Characterization of protein isolate from Sesamum indicum seed: In vitro protein digestibility, amino acid profile, and some functional properties. Food Sci. Nutr. 2018, 6, 1715–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capellini, M.C.; Chiavoloni, L.; Giacomini, V.; Rodrigues, C.E.C. Alcoholic extraction of sesame seed cake oil: Influence of the process conditions on the physicochemical characteristics of the oil and defatted meal proteins. J. Food Eng. 2019, 240, 145–152. [Google Scholar] [CrossRef]
- Ji, J.; Liu, Y.; Shi, L.; Wang, N.; Wang, X. Effect of roasting treatment on the chemical composition of sesame oil. LWT Food Sci. Technol. 2019, 101, 191–200. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Bouzoubaa, Z.; Asdadi, A.; El Yadini, A.; Charrouf, Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Sarkis, J.R.; Michel, I.; Tessaro, I.C.; Marczak, L.D.F. Optimization of phenolics extraction from sesame seed cake. Sep. Purif. Technol. 2014, 122, 506–514. [Google Scholar] [CrossRef]
- Shu, Z.; Liu, L.; Geng, P.; Liu, J.; Shen, W.; Tu, M. Sesame cake hydrolysates improved spatial learning and memory of mice. Food Biosci. 2019, 31, 100440. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Tontisirin, K. Chapter 2: Methods of food analysis. In Food Energy: Methods of Analysis and Conversion Factors: Report of a Technical Workshop; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- EC. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, L304, 18–61. [Google Scholar]
- Machado, S.; Costa, A.S.G.; Pimentel, B.F.; Oliveira, M.B.P.P.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Capannesi, C.; Palchetti, I.; Mascini, M.; Parenti, A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000, 71, 553–562. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Alves, R.C.; Costa, A.S.G.; Jerez, M.; Casal, S.; Sineiro, J.; Núñez, M.J.; Oliveira, M.B.P.P. Antiradical activity, phenolics profile, and hydroxymethylfurfural in espresso coffee: Influence of technological factors. J. Agric. Food Chem. 2010, 58, 12221–12229. [Google Scholar] [CrossRef]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, R.; Casal, S.; Oliveira, M.B.P.P. Determination of vitamin E in coffee beans by HPLC using a micro-extraction method. Food Sci. Technol. Int. 2009, 15, 57–63. [Google Scholar] [CrossRef]
- ISO. ISO 12966: Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2—Preparation of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Szydłowska-Czerniak, A.; Karlovits, G.; Hellner, G.; Szłyk, E. Effect of enzymatic and hydrothermal treatments of rapeseeds on quality of the pressed rapeseed oils: Part II—Oil yield and oxidative stability. Process Biochem. 2010, 45, 247–258. [Google Scholar] [CrossRef]
- ISO. NP 937: Edible Fats and Oils—Oils Colour Determination and Their Chromatic Characteristics; International Organization for Standardization: Geneva, Switzerland, 1987. [Google Scholar]
- Malheiro, R.; Oliveira, I.; Vilas-Boas, M.; Falcão, S.; Bento, A.; Pereira, J.A. Effect of microwave heating with different exposure times on physical and chemical parameters of olive oil. Food Chem. Toxicol. 2009, 47, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Kaleem, A.; Aziz, S.; Iqtedar, M.; Abdullah, R.; Aftab, M.; Rashid, F.; Shakoori, F.R.; Naz, S. Investigating changes and effect of peroxide values in cooking oils subject to light and heat. FUUAST J. Biol. 2015, 5, 191–196. [Google Scholar]
- ISO. NP 904: Edible Fats and Oils—Determination of Peroxide Value; International Organization for Standardization: Geneva, Switzerland, 1987. [Google Scholar]
- ISO. ISO 3656: Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- EC. Guidance Document for Competent Authorities for the Control of Compliance with EU Legislation on Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; European Commission, Health and Consumers Directorate-General: Brussels, Belgium, 2012. [Google Scholar]
- Adebisi, A.K.; Stephen, E.C.; Chinedu, I.; Emmanuel, U. Quantification of protein and amino acid composition in some oilseeds. Mol. Biol. 2017, 2, 8–11. [Google Scholar]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Attia, H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007, 103, 641–650. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, M.-H.; Seo, W.D.; Baek, I.-Y.; Kang, J.E.; Lee, J.H. Changes occurring in nutritional components (phytochemicals and free amino acid) of raw and sprouted seeds of white and black sesame (Sesamum indicum L.) and screening of their antioxidant activities. Food Sci. Biotechnol. 2017, 26, 71–78. [Google Scholar] [CrossRef]
- Ong, P.K.; Moreira, A.S.; Daniel-Ribeiro, C.T.; Frangos, J.A.; Carvalho, L.J. Reversal of cerebrovascular constriction in experimental cerebral malaria by L-arginine. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pahlavani, N.; Sadeghi, O.; Ebrahimi, F.; Mazloumi Kiapey, S.S.; Nurany, M. The effects of L-arginine supplementation on athletic performance and risk factors of cardiovascular disease: Review of current evidence. JNKUMS 2019, 11, 8–16. [Google Scholar]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimomura, Y.; Kitaura, Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol. Res. 2018, 133, 215–217. [Google Scholar] [CrossRef]
- Chakraborty, P.; Witt, T.; Harris, D.; Ashton, J.; Stokes, J.R.; Smyth, H.E. Texture and mouthfeel perceptions of a model beverage system containing soluble and insoluble oat bran fibres. Food Res. Int. 2019, 120, 62–72. [Google Scholar] [CrossRef]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New horizons for the study of dietary fiber and health: A review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.M.; Champ, M.M.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.U.; Liu, C.; Sathe, S.K. Functional properties of select seed flours. LWT-Food Sci. Technol. 2015, 60, 325–331. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Smetanska, I.; Ramadan, M.F.; Sarhan, M.A.; Mahmoud, A. Antioxidant potential of sesame (Sesamum indicum) cake extract in stabilization of sunflower and soybean oils. Ind. Crops Prod. 2011, 34, 952–959. [Google Scholar] [CrossRef]
- Sani, I.; Okpalaoka, C.C.; Bello, F.; Warra, A.A.; Abdulhamid, A. Flavonoid content and antioxidant potential of white and brown sesame seed oils. EJBPS 2014, 1, 56–63. [Google Scholar]
- Mohdaly, A.A.A.; Hassanien, M.F.R.; Mahmoud, A.; Sarhan, M.A.; Smetanska, I. Phenolics extracted from potato, sugar beet, and sesame processing by-products. Int. J. Food Prop. 2013, 16, 1148–1168. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.K.; Kumari, R.; Bhat, K.V. Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn. Rev. 2014, 8, 147–155. [Google Scholar] [PubMed] [Green Version]
- Majdalawieh, A.F.; Massri, M.; Nasrallah, G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol. 2017, 815, 512–521. [Google Scholar] [CrossRef]
- Cardoso, C.A.; Oliveira, G.M.M.; Gouveia, L.A.V.; Moreira, A.S.B.; Rosa, G. The effect of dietary intake of sesame (Sesamum indicum L.) derivatives related to the lipid profile and blood pressure: A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 116–125. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [Green Version]
- Pabón-Baquero, L.C.; Otálvaro-Álvarez, Á.M.; Fernández, M.R.R.; Chaparro-González, M.P. Plant extracts as antioxidant additives for food industry. In Antioxidants in Foods and Its Applications; Shalaby, E., Azzam, G.M., Eds.; IntechOpen: London, UK, 2018; pp. 87–116. [Google Scholar]
- Lin, X.; Zhou, L.; Li, T.; Brennan, C.; Fu, X.; Liu, R.H. Phenolic content, antioxidant and antiproliferative activities of six varieties of white sesame seeds (Sesamum indicum L.). RSC Adv. 2017, 7, 5751–5758. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lin, H.; Lin, M.; Lin, P.; Chen, J. Effects of thermal preparation and in vitro digestion on lignan profiles and antioxidant activity in defatted-sesame meal. Food Chem. Toxicol. 2019, 128, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Jannat, B.; Oveisi, M.R.; Sadeghi, N.; Hajimahmoodi, M.; Behzad, M.; Choopankari, E.; Behfar, A.A. Effects of roasting temperature and time on healthy nutraceuticals of antioxidants and total phenolic content in Iranian sesame seeds (Sesamum indicum L.). Iran. J. Environ. Health Sci. Eng. 2010, 7, 97–102. [Google Scholar]
- Munné-Bosch, S. α-Tocopherol: A multifaceted molecule in plants. Vitam. Horm. 2007, 76, 375–392. [Google Scholar] [PubMed]
- Bermúdez, L.; Del Pozo, T.; Lira, B.S.; de Godoy, F.; Boos, I.; Romano, C.; Previtali, V.; Almeida, J.; Bréhélin, C.; Assis, R.; et al. A tomato tocopherol-binding protein sheds light on intracellular α-tocopherol metabolism in plants. Plant Cell Physiol. 2018, 59, 2188–2203. [Google Scholar] [CrossRef] [Green Version]
- Mène-Saffrané, L. Vitamin E Biosynthesis and Its Regulation in Plants. Antioxidants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.R.; O’Brien, N.M. Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods Hum. Nutr. 2007, 62, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Aued-Pimentel, S.; Takemoto, E.; Antoniassi, R.; Badolato, E.S.G. Composition of tocopherols in sesame seed oil: An indicative of adulteration. Grasas Aceites 2006, 57, 205–210. [Google Scholar] [CrossRef]
- Calder, C.C.; Campoy, C.; Eilander, A.; Fleith, M.; Forsyth, S.; Larsson, P.-O.; Schelkle, B.; Lohner, S.; Szommer, A.; de Heijning, B.J.M.; et al. A systematic review of the effects of increasing arachidonic acid intake on PUFA status, metabolism and health-related outcomes in humans. Br. J. Nutr. 2019, 121, 1201–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I.; Badeka, A.V. Prickly Pear Seed Oil by Shelf-Grown Cactus Fruits: Waste or Maste? Processes 2020, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Farhoosh, R.; Tavassoli-Kafrani, M.H.; Sharif, A. Antioxidant activity of sesame, rice bran and bene hull oils and their unsaponifiable matters. Eur. J. Lipid Sci. Technol. 2011, 113, 506–512. [Google Scholar] [CrossRef]
- Ishtiaque, S.; Khan, N.; Siddiqui, M.A.; Siddiqi, R.; Naz, S. Antioxidant potential of the extracts, fractions and oils derived from oilseeds. Antioxidants 2013, 2, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Rangkadilok, N.; Pholphana, N.; Mahidol, C.; Wongyai, W.; Saengsooksree, K.; Nookabkaew, S.; Satayavivad, J. Variation of sesamin, sesamolin and tocopherols in sesame (Sesamum indicum L.) seeds and oil products in Thailand. Food Chem. 2010, 122, 724–730. [Google Scholar] [CrossRef]
- Landete, J.M. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Khalesi, S.; Paukste, E.; Nikbakht, E.; Khosravi-Boroujeni, H. Sesame fractions and lipid profiles: A systematic review and meta-analysis of controlled trials. Br. J. Nutr. 2016, 115, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Vieira, T.M.F.S.; Regitano-D’arce, M.A.B. Stability of oils heated by microwave: UV—Spectrophotometric evaluation. Food Sci. Technol. 1998, 18, 433–437. [Google Scholar] [CrossRef]
- Rabadán, A.; Pardo, J.E.; Gómez, R.; Álvarez-Ortí, M. Influence of temperature in the extraction of nut oils by means of screw pressing. LWT 2018, 93, 354–361. [Google Scholar] [CrossRef]
- Cheung, S.C.M.; Szeto, Y.T.; Benzie, I.F.F. Antioxidant protection of edible oils. Plant Foods Hum. Nutr. 2007, 62, 39–42. [Google Scholar] [CrossRef]
| Parameter | Method of Analysis | Fresh Weight | |
|---|---|---|---|
| Seeds | Cake | ||
| Moisture (%) | Infrared moisture analyser | 2.7 ± 0.1 a | 3.7 ± 0.2 b |
| Ash (%) | Incineration (AOAC 920.153) | 5.1 ± 0.0 a | 7.1 ± 0.0 b |
| Total fat (%) | Soxhlet method (AOAC 991.36) | 53.1 ± 0.0 b | 31.8 ± 0.4 a |
| Fatty acids profile (relative%) | |||
| C16:0 | GC-FID | 10.38 ± 0.20 b | 9.23 ± 0.01 a |
| C16:1 | GC-FID | 0.13 ± 0.00 a | 0.12 ± 0.00 a |
| C17:0 | GC-FID | 0.06 ± 0.01 a | 0.06 ± 0.00 a |
| C18:0 | GC-FID | 5.63 ± 0.07 a | 6.13 ± 0.02 b |
| C18:1n9c | GC-FID | 41.03 ± 0.20 a | 40.92 ± 0.01 a |
| C18:2n6c | GC-FID | 41.67 ± 0.11 a | 42.42 ± 0.02 b |
| C18:3n3 | GC-FID | 0.32 ± 0.01 a | 0.33 ± 0.00 a |
| C20:0 | GC-FID | 0.60 ± 0.02 a | 0.61 ± 0.00 a |
| C20:1n9 | GC-FID | 0.18 ± 0.01 a | 0.17 ± 0.00 a |
| ∑ SFA | GC-FID | 16.67 b | 16.03 a |
| ∑ MUFA | GC-FID | 41.34 a | 41.21 a |
| ∑ PUFA | GC-FID | 41.99 a | 42.76 b |
| 18:2n6/18:3n3 | GC-FID | 130.09 | 127.02 |
| 18:1n9/18:2n6 | GC-FID | 1.02 | 1.04 |
| Total vitamin E (mg/kg) | HPLC-DAD-FLD | 432.03 b | 225.75 a |
| α-Tocopherol (mg/kg) | HPLC-DAD-FLD | 118.43 ± 4.82 b | 57.16 ± 1.36á |
| α-Tocotrienol (mg/kg) | HPLC-DAD-FLD | 23.23 ± 2.29 b | 19.77 ± 1.51 a |
| γ-Tocopherol (mg/kg) | HPLC-DAD-FLD | 290.37 ± 12.81 b | 146.16 ± 6.29 a |
| Protein (%) | Kjeldahl method (AOAC 928.08) × 6.25 [14] | 18.9 ± 0.0 a | 29.6 ± 0.0 b |
| Total amino acids (mg/g) | HPLC-DAD-FLD | 199.44 a | 305.42 b |
| Free amino acids (mg/g) | HPLC-DAD-FLD | 2.02 a | 2.67 b |
| Total Dietary Fibre (%) | Enzymatic-gravimetric (AOAC 985.29) | 19.9 ± 0.0 a | 25.0 ± 0.6 b |
| Insoluble Fibre (%) | Enzymatic-gravimetric (AOAC 991.42) | 18.2 ± 0.0 a | 21.7 ± 0.0 b |
| Soluble Fibre (%) | Calculated by difference [14] | 1.7 ± 0.0 a | 3.3 ± 0.4 b |
| Remaining Carbohydrates (%) | Calculated by difference [14] | 0.3 ± 0.1 a | 2.9 ± 0.3 b |
| Energy value (kJ/100 g) | Calculated [15] | 2452 | 1927 |
| Energy value (kcal/100 g) | Calculated [15] | 595 | 466 |
| Phytochemicals | |||
| TPC (mg GAE/100 g) | Spectrophotometric (765 nm) | 73.6 ± 5.7 a | 153.2 ± 6.1 b |
| TFC (mg ECE/100 g) | Spectrophotometric (510 nm) | 66.7 ± 5.2 a | 74.0 ± 3.8 b |
| Antioxidant activity | |||
| FRAP (mmol FSE/100 g) | Spectrophotometric (595 nm) | 6.8 ± 0.5 a | 8.3 ± 0.3 b |
| DPPH• (mg TE/100 g) | Spectrophotometric (Kinetics reaction, 525 nm) | 86.4 ± 4.4 a | 78.2 ± 12.1 a |
| Total Amino Acids (mg/g of Sample fw) | Free Amino Acids (mg/g of Sample fw) | |||
|---|---|---|---|---|
| Seeds | Cake | Seeds | Cake | |
| Aspartic acid | 16.77 ± 0.68 a | 26.20 ± 0.73 b | 0.25 ± 0.04 A | 0.33 ± 0.04 B |
| Glutamic acid | 39.90 ± 1.51 a | 61.93 ± 1.80 b | 0.33 ± 0.04 A | 0.45 ± 0.04 B |
| Asparagine | ND | ND | 0.46 ± 0.02 A | 0.67 ± 0.03 B |
| Serine | 10.05 ± 0.34 a | 15.20 ± 0.48 b | 0.07 ± 0.00 A | 0.09 ± 0.00 B |
| Glutamine | 1.06 ± 0.02 a | 1.93 ± 0.04 b | 0.03 ± 0.00 A | 0.03 ± 0.00 A |
| * Histidine | 6.93 ± 0.40 a | 10.45 ± 0.12 b | 0.04 ± 0.00 A | 0.06 ± 0.00 B |
| Glycine | 11.21 ± 0.43 a | 17.41 ± 0.53 b | 0.02 ± 0.00 A | 0.03 ± 0.00 B |
| * Threonine | 7.38 ± 0.33 a | 11.31 ± 0.40 b | 0.05 ± 0.00 A | 0.05 ± 0.00 B |
| Arginine | 31.71 ± 1.38 a | 48.31 ± 1.43 b | 0.15 ± 0.00 A | 0.19 ± 0.01 B |
| Alanine | 9.57 ± 0.34 a | 14.60 ± 0.44 b | 0.09 ± 0.01 A | 0.11 ± 0.00 B |
| Tyrosine | 5.98 ± 0.36 a | 9.07 ± 0.21 b | 0.03 ± 0.00 A | 0.05 ± 0.00 B |
| * Valine | 8.40 ± 0.35 a | 13.09 ± 0.33 b | 0.04 ± 0.00 A | 0.06 ± 0.01 B |
| * Methionine | 5.06 ± 0.31 a | 6.97 ± 0.30 b | 0.03 ± 0.00 A | 0.03 ± 0.00 B |
| * Tryptophan | 1.89 ± 0.07 a | 2.19 ± 0.05 b | 0.12 ± 0.00 A | 0.15 ± 0.01 B |
| * Phenylalanine | 9.27 ± 0.36 a | 13.87 ± 0.53 b | 0.14 ± 0.00 A | 0.18 ± 0.02 B |
| * Isoleucine | 7.08 ± 0.29 a | 11.12 ± 0.24 b | 0.03 ± 0.00 A | 0.05 ± 0.00 B |
| * Leucine | 13.63 ± 0.55 a | 20.70 ± 0.57 b | 0.04 ± 0.00 A | 0.05 ± 0.00 B |
| * Lysine | 6.58 ± 0.49 a | 10.32 ± 0.30 b | 0.04 ± 0.00 A | 0.05 ± 0.00 B |
| Hydroxyproline | 0.50 ± 0.03 a | 0.73 ± 0.02 b | 0.01 ± 0.00 A | 0.01 ± 0.00 B |
| Proline | 6.58 ± 0.12 a | 10.04 ± 0.31 b | 0.03 ± 0.00 A | 0.05 ± 0.00 B |
| Total | 199.53 a | 305.45 b | 2.02 A | 2.67 B |
| Parameter | Method of Analysis | Oil |
|---|---|---|
| Fatty acids profile (relative%) | ||
| C16:0 | GC-FID | 9.74 ± 0.06 |
| C16:1 | GC-FID | 0.10 ± 0.03 |
| C18:0 | GC-FID | 5.41 ± 0.09 |
| C18:1n9c | GC-FID | 42.66 ± 0.35 |
| C18:2n6c | GC-FID | 41.25 ± 0.22 |
| C18:3n3 | GC-FID | 0.20 ± 0.03 |
| C20:0 | GC-FID | 0.54 ± 0.04 |
| C20:1n9 | GC-FID | 0.11 ± 0.02 |
| ∑ SFA | GC-FID | 15.68 |
| ∑ MUFA | GC-FID | 42.87 |
| ∑ PUFA | GC-FID | 41.45 |
| 18:2n6/18:3n3 | GC-FID | 203.94 |
| 18:1n9/18:2n6 | GC-FID | 1.03 |
| Total vitamin E (mg/kg) | HPLC-DAD-FLD | 483.95 ± 7.11 |
| α-Tocopherol (mg/kg) | HPLC-DAD-FLD | 13.78 ± 0.85 |
| α-Tocotrienol (mg/kg) | HPLC-DAD-FLD | 14.04 ± 0.29 |
| γ-Tocopherol (mg/kg) | HPLC-DAD-FLD | 456.13 ± 6.05 |
| Phytochemicals | ||
| TPC (mg GAE/100 g) | Spectrophotometric (765 nm) | 13.4 ± 1.3 |
| TFC (mg ECE/100 g) | Spectrophotometric (510 nm) | 20.3 ± 1.7 |
| Antioxidant activity | ||
| FRAP (μmol FSE/100 g) | Spectrophotometric (595 nm) | 215.5 ± 6.7 |
| DPPH• (mg TE/100 g) | Spectrophotometric (Kinetics reaction, 525 nm) | 7.9 ± 0.9 |
| Oxidative stability | ||
| Induction time (h) | Rancimat method | 5.8 ± 0.0 |
| Colour | ||
| Chromatic coordinates (x, y) | Spectrophotometric (445, 495, 560, 595, 625 nm) | (0.3641, 0.3647) |
| Transparency (%) | Spectrophotometric (445, 495, 560, 595, 625 nm) | 73.2 |
| Dominant wavelength (nm) | Spectrophotometric (445, 495, 560, 595, 625 nm) | 579.3 |
| Purity | Spectrophotometric (445, 495, 560, 595, 625 nm) | 27.4 |
| Peroxide value (meqO2/kg) | Titration | 2.6 ± 0.3 |
| K232 nm | Spectrophotometric (232 nm) | 0.03 |
| K270 nm | Spectrophotometric (270 nm) | 0.01 |
| Parameter | Label | Tolerance | Lower Tolerance | Upper Tolerance | Obtained Result | Comparison with the Tolerance Range |
|---|---|---|---|---|---|---|
| Energy value (kJ/100 g) | 2654 | - | - | - | 2518 | - |
| Energy value (kcal/100 g) | 632 | - | - | - | 611 | - |
| Fat (g/100 g) | 55.9 | ±8 g | 47.9 | 63.9 | 54.6 | Within |
| Carbohydrates (g/100 g) | 13.3 | ±20% | 10.6 | 16.0 | 0.3 | Outside |
| Dietary fibre (g/100 g) | 7.6 | ±2 g | 5.6 | 9.6 | 20.4 | Outside |
| Protein (g/100 g) | 17.6 | ±20% | 14.1 | 21.1 | 19.4 | Within |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Costa, A.S.G.; Machado, S.; Alves, R.C.; Pardo, J.E.; Oliveira, M.B.P.P. Whole or Defatted Sesame Seeds (Sesamum indicum L.)? The Effect of Cold Pressing on Oil and Cake Quality. Foods 2021, 10, 2108. https://doi.org/10.3390/foods10092108
Melo D, Álvarez-Ortí M, Nunes MA, Costa ASG, Machado S, Alves RC, Pardo JE, Oliveira MBPP. Whole or Defatted Sesame Seeds (Sesamum indicum L.)? The Effect of Cold Pressing on Oil and Cake Quality. Foods. 2021; 10(9):2108. https://doi.org/10.3390/foods10092108
Chicago/Turabian StyleMelo, Diana, Manuel Álvarez-Ortí, Maria Antónia Nunes, Anabela S. G. Costa, Susana Machado, Rita C. Alves, José E. Pardo, and Maria Beatriz P. P. Oliveira. 2021. "Whole or Defatted Sesame Seeds (Sesamum indicum L.)? The Effect of Cold Pressing on Oil and Cake Quality" Foods 10, no. 9: 2108. https://doi.org/10.3390/foods10092108
APA StyleMelo, D., Álvarez-Ortí, M., Nunes, M. A., Costa, A. S. G., Machado, S., Alves, R. C., Pardo, J. E., & Oliveira, M. B. P. P. (2021). Whole or Defatted Sesame Seeds (Sesamum indicum L.)? The Effect of Cold Pressing on Oil and Cake Quality. Foods, 10(9), 2108. https://doi.org/10.3390/foods10092108











