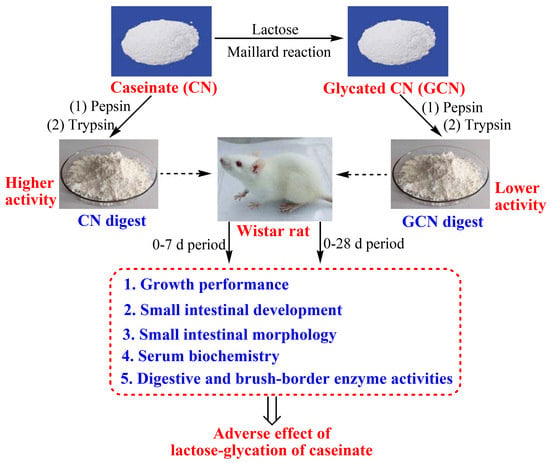
Lactose Glycation of the Maillard-Type Impairs the Benefits of Caseinate Digest to the Weaned Rats for Intestinal Morphology and Serum Biochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation and Chemical Analyses of the Digests
2.3. Animals, Diets, and Housing
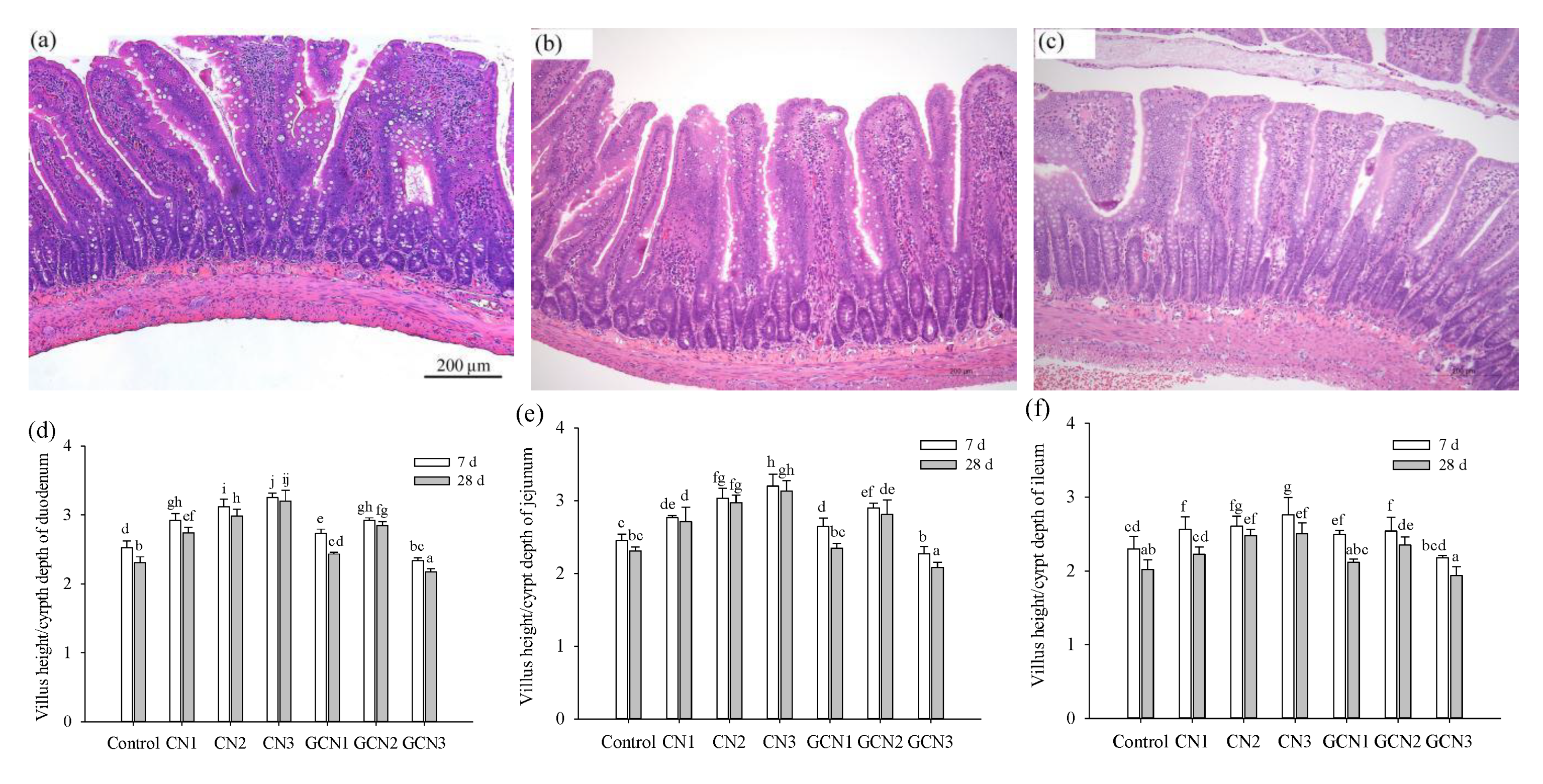
2.4. Assay of Intestinal Morphology
2.5. Assays of Digestive and Brush-Border Enzyme Activities
2.6. Assays of Serum Chemical Indices
2.7. Statistical Analysis
3. Results
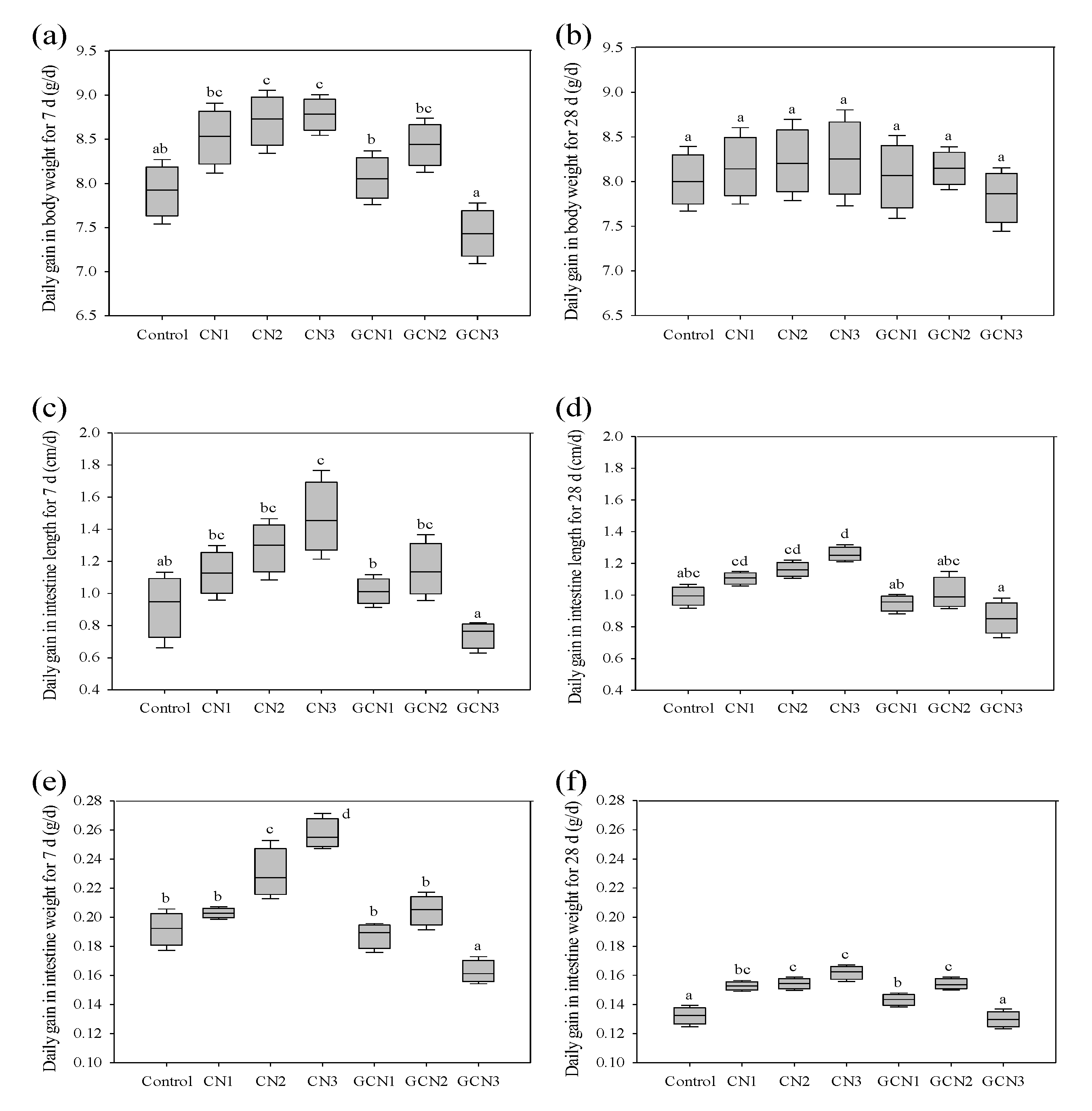
3.1. The Body Weight Gain and Intestinal Indices of the Rats in Response to the Two Digests
3.2. The Morphology Changes of Intestinal Tissues in Response to the Two Digests
3.3. The Changes of Digestive and Brush-Border Enzyme Activities in Response to the Two Digests
3.4. Serum Biochemical Indices of the Rats in Response to the Two Digests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, B.M.; Deeth, H.C. Blocked Lysine in dairy products: Formation, occurrence, analysis, and nutritional implications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 206–218. [Google Scholar] [CrossRef]
- Gasparini, A.; Buhler, S.; Faccini, A.; Sforza, S.; Tedeschi, T. Thermally-induced lactosylation of whey proteins: Identification and synthesis of lactosylated β-lactoglobulin epitope. Molecules 2020, 25, 1294. [Google Scholar] [CrossRef] [Green Version]
- Turner, L.G.; Swaisgood, H.E.; Hansen, A.P. Interaction of lactose and proteins of skim milk during ultra-high-temperature processing. J. Dairy Sci. 1978, 61, 384–392. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, H.G.; Li, P.; Li, M.; Yao, Q.Q.; Li, M.; Zhang, Y.D.; Wang, J.Q.; Zheng, N. Maillard reaction products with furan ring, like furosine, cause kidney injury through triggering ferroptosis pathway. Food Chem. 2020, 319, e126368. [Google Scholar] [CrossRef]
- Bhatt, H.; Cucheval, A.; Coker, C.; Patel, H.; Carr, A.; Bennett, R. Effect of lactosylation on plasmin-induced hydrolysis of β-casein. Int. Dairy J. 2014, 38, 213–218. [Google Scholar] [CrossRef]
- Aktağ, I.G.; Hamzalıoğlu, A.; Gökmen, V. Lactose hydrolysis and protein fortification pose an increased risk for the formation of Maillard reaction products in UHT treated milk products. J. Food Compos. Anal. 2019, 84, e103308. [Google Scholar] [CrossRef]
- Yang, W.; Tu, Z.; Li, Q.; Kaltashov, I.A.; McClements, D.J. Utilization of sonication-glycation to improve the functional properties of ovalbumin: A high-resolution mass spectrometry study. Food Hydrocoll. 2021, 119, e106822. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Zhou, F.; Guo, J.; Tang, J.; Han, Y.; Li, Z.; Fu, C. Preparation, bioactivities and applications in food industry of chitosan-based Maillard products: A review. Molecules 2020, 26, 166. [Google Scholar] [CrossRef] [PubMed]
- Ottum, M.S.; Mistry, A.M. Advanced glycation end-products: Modifiable environmental factors profoundly mediate insulin resistance. J. Clin. Biochem. Nutr. 2015, 57, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.M.; Ulaszewska, M.M.; Gobba, C.D.; Rinnan, Å.; Poulsen, M.W.; Chen, J.; Mattivi, F.; Hedegaard, R.V.; Skibsted, L.H.; Dragsted, L.O. New advanced glycation end products observed in rat urine by untargeted metabolomics after feeding with heat-treated skimmed milk powder. Mol. Nutr. Food Res. 2021, 65, e2001049. [Google Scholar] [CrossRef]
- Arena, S.; Salzano, A.M.; Renzone, G.; D’Ambrosio, C.; Scaloni, A. Non-enzymatic glycation and glycoxidation protein products in foods and diseases: An interconnected, complex scenario fully open to innovative proteomic studies. Mass Spectrom. Rev. 2014, 33, 49–77. [Google Scholar] [CrossRef]
- Henle, T.; Miyata, T. Advanced glycation end products in uremia. Adv. Ren. Replace Ther. 2003, 10, 321–331. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sun, W.; Sell, D.R.; Fan, X.; Nemet, I.; Genuth, S. Glucosepane: A poorly understood advanced glycation end product of growing importance for diabetes and its complications. Clin. Chem. Lab. Med. 2014, 52, 21–32. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.F.; Kondrashina, A.; Greco, I.; Gamon, L.F.; Lund, M.N.; Giblin, L.; Davies, M.J. Effects of protein-derived amino acid modification products present in infant formula on metabolic function, oxidative stress, and intestinal permeability in cell models. J. Agric. Food Chem. 2019, 67, 5634–5646. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Shimizu, M. Interaction between food substances and the intestinal epithelium. Biosci. Biotechnol. Biochem. 2010, 74, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug. Anal. 2017, 25, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.P.; Zhao, X.H. Using an enzymatic galactose assay to detect lactose glycation extents of caseinate and soybean protein isolate via the Maillard reaction. J. Sci. Food Agric. 2017, 97, 2617–2622. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, X.H. Influence of the Maillard-type caseinate glycation with lactose on the intestinal barrier activity of the caseinate digest in IEC-6 cells. Food Funct. 2019, 10, 2010–2021. [Google Scholar] [CrossRef]
- Wang, X.P.; Zhao, X.H. Prior lactose glycation of caseinate via the Maillard reaction affects in vitro activities of the pepsin-trypsin digest toward intestinal epithelial cells. J. Dairy Sci. 2017, 100, 5125–5138. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, DC, USA, 2005. [Google Scholar]
- Hong, C.O.; Rhee, C.H.; Pyo, M.C.; Lee, K.W. Anti-inflammatory effect of glucose-lysine Maillard reaction products on intestinal inflammation model in vivo. Int. Immunopharmacol. 2017, 52, 324–332. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; He, S.D.; Cao, X.D.; Ye, Y.K.; Yang, L.; Wang, J.H.; Liu, H.Y.; Sun, H.J. Potential prebiotic activities of soybean peptides Maillard reaction products on modulating gut microbiota to alleviate aging-related disorders in D-galactose-induced ICR mice. J. Funct. Foods 2020, 65, e103729. [Google Scholar] [CrossRef]
- Lainé, J.; Beattie, M.; Lebel, D. Simultaneous kinetic determinations of lipase, chymotrypsin, trypsin, elastase, and amylase on the same microtiter plate. Pancreas 1993, 8, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Buccigrossi, V.; de Marco, G.; Bruzzese, E.; Ombrato, L.; Bracale, I.; Polito, G.; Guarino, A. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr. Res. 2007, 61, 410–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, R.; Klein, T.; Freier, S.; Menczel, J. Alkaline phosphatase and disaccharidase activities in the rat intestine from birth to weaning. I. Effect of diet on enzyme development. Am. J. Clin. Nutr. 1971, 24, 1224–1231. [Google Scholar] [CrossRef]
- Zou, L.J.; Xiong, X.; Liu, H.N.; Zhou, J.; Liu, Y.H.; Yin, Y.L. Effects of dietary lysozyme levels on growth performance, intestinal morphology, immunity response and microbiota community of growing pigs. J. Sci. Food Agric. 2019, 99, 1643–1650. [Google Scholar] [CrossRef]
- Zhong, G.; Shao, D.; Wang, Q.; Tong, H.B.; Shi, S.R. Effects of dietary supplemented of γ-amino butyric acid on growth performance, blood biochemical indices and intestinal morphology of yellow-feathered broilers exposed to a high temperature environment. Ital. J. Anim. Sci. 2020, 19, 431–438. [Google Scholar] [CrossRef]
- Shi, J.; Fu, Y.; Zhao, X.H.; Lametsch, R. Glycation sites and bioactivity of the lactose-glycated caseinate hydrolysate towards the lipopolysaccharide-injured rat intestinal epithelial cells. J. Dairy Sci. 2021, 104, 1351–1363. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.Á.; Delgado-Andrade, C. Effects of long-term consumption of standard diets including glucose-lysine model glycated compounds on the antioxidant status of adult rats. Food Chem. 2015, 183, 283–290. [Google Scholar] [CrossRef]
- Hillman, M.; Weström, B.; Aalaei, K.; Erlanson-Albertsson, C.; Wolinski, J.; Lozinska, L.; Sjöholm, I.; Rayner, M.; Landin-Olsson, M. Skim milk powder with high content of Maillard reaction products affect weight gain, organ development and intestinal inflammation in early life in rats. Food Chem. Toxicol. 2019, 125, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Yang, H.S.; Wang, X.C.; Hu, Q.; Liu, C.X.; Wu, X.; Deng, D.; Hou, Y.Q.; Nyachoti, C.M.; Xiao, D.F. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015, 93, 1089–1097. [Google Scholar] [CrossRef]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and fecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef]
- Kamboh, A.A. Flavonoids: Health promoting phytochemicals for animal production-a review. J. Anim. Health Prod. 2015, 3, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Caspary, W.F. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 1992, 55, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.K.; Croom, J.; Christensen, V.L.; Black, B.L.; Bird, A.R.; Daniel, L.R.; Mcbride, B.W.; Eisen, E.J. Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poul. Sci. 1997, 76, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Zhao, J.S.; Qu, W.T.; Zhang, Y.X.; Jia, B.P.; Fan, Z.Y.; He, Q.H.; Li, J.X. Accumulation and effects of dietary advanced glycation end products on the gastrointestinal tract in rats. Int. J. Food Sci. Technol. 2018, 53, 2273–2281. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Roncero-Ramos, I.; Alonso-Olalla, R.; Seiquer, I.; Navarro, M.P. Maillard product consumption and nitrogen digestibility in young and adult rats. Czech J. Food Sci. 2014, 32, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Shang, Q.H.; Ma, X.K.; Li, M.; Zhang, L.H.; Hu, J.X.; Piao, X.S. Effects of α-galactosidase supplementation on nutrient digestibility, growth performance, intestinal morphology and digestive enzyme activities in weaned piglets. Anim. Feed Sci. Technol. 2018, 236, 48–56. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.S.; Fang, S.L.; Yang, X.J.; Feng, J. Betaine improves intestinal functions by enhancing digestive enzymes, ameliorating intestinal morphology, and enriching intestinal microbiota in high-salt stressed rats. Nutrients 2018, 10, 907. [Google Scholar] [CrossRef] [Green Version]
- Oste, R.E.; Miller, R.; Sjostrom, H.; Noren, O. Effect of Maillard reaction products on protein digestion. Studies on pure compounds. J. Agric. Food Chem. 1987, 35, 938–942. [Google Scholar] [CrossRef]
- Chung, S.Y.; Han, S.H.; Lee, S.W.; Rhee, C. Effect of Maillard reaction products prepared from glucose-glycine model systems on starch digestibility. Starch 2012, 64, 657–664. [Google Scholar] [CrossRef]
- Serrano, X.; Morales, G.A.; Hernández, A.J.; Díaz, M.; Moyano, F.J.; Márquez, L. Effects of glucose-glycine melanoidins on the digestive trypsin-like activity of the rainbow trout oncorhynchus mykiss. Aquaculture 2019, 516, e734513. [Google Scholar] [CrossRef]
- Scheijen, J.L.J.M.; Hanssen, N.M.J.; Van Greevenbroek, M.M.; Van der Kallen, C.J.; Feskens, E.J.M.; Stehouwer, C.D.A.; Schalkwijk, C.G. Dietary intake of advanced glycation end products is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef]
- Angoorani, P.; Ejtahed, H.S.; Mirmiran, P.; Mirzaei, S.; Azizi, F. Dietary consumption of advanced glycation end products and risk of metabolic syndrome. Int. J. Food Sci. Nutr. 2016, 67, 170–176. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D.P.; Tian, X.; Zhang, Z.M.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Abey, N.O. Cannabis sativa (Marijuana) alters blood chemistry and the cytoarchitecture of some organs in Sprague Dawley rat models. Food Chem. Toxicol. 2019, 16, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, M.; Sarbakhsh, P.; Mobasseri, M.; Noshad, H.; Esfandiari, A.; Khodadadi, B.; Gargari, B.P. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. 2019, 13, 542–547. [Google Scholar] [CrossRef]
- Harsij, M.; Kanani, H.G.; Adineh, H. Effects of antioxidant supplementation (nano-selenium, vitamin C and E) on growth performance, blood biochemistry, immune status and body composition of rainbow trout (Oncorhynchus mykiss) under sub-lethal ammonia exposure. Aquaculture 2020, 521, e734942. [Google Scholar] [CrossRef]
- Kamely, M.; Torshizi, M.A.K.; Rahimi, S. Blood biochemistry, thyroid hormones, and performance in broilers with ascites caused by caffeine. Poult. Sci. 2016, 95, 2673–2678. [Google Scholar] [CrossRef]
| Index | Time (d) | Control | CN Digest (mg/kg BW/d) | GCN Digest (mg/kg BW/d) | ||||
|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 400 | 100 | 200 | 400 | |||
| Duodenum | ||||||||
| Lipase | 7 | 126.90 ± 5.46 b | 135.04 ± 6.52 bc | 156.39 ± 6.69 d | 204.35 ± 11.17 e | 127.83 ± 6.24 b | 136.99 ± 5.34 c | 115.30 ± 6.53 a |
| 28 | 165.56 ± 5.86 b,* | 196.48 ± 9.19 c,* | 209.89 ± 10.72 d,* | 231.48 ± 10.15 e,* | 167.46 ± 4.13 b,* | 198.85 ± 8.97 c,* | 143.91 ± 8.57 a,* | |
| α-Amylase | 7 | 4.67 ± 0.16 b | 5.56 ± 0.12 e | 5.88 ± 0.15 f | 7.21 ± 0.20 g | 4.89 ± 0.16 c | 5.29 ± 0.12 d | 4.13 ± 0.09 a |
| 28 | 5.76 ± 0.15 a,* | 7.16 ± 0.20 c,* | 8.54 ± 0.27 d,* | 8.74 ± 0.13 d,* | 6.27 ± 0.12 b,* | 7.04 ± 0.24 c,* | 5.57 ± 0.14 a,* | |
| Trypsin | 7 | 2.82 ± 0.03 b | 3.64 ± 0.09 c | 4.24 ± 0.10 e | 4.99 ± 0.06 f | 2.89 ± 0.04 b | 3.74 ± 0.03 d | 2.71 ± 0.08 a |
| 28 | 2.94 ± 0.10 a,* | 4.29 ± 0.18 c,* | 4.76 ± 0.18 d,* | 5.32 ± 0.09 e,* | 3.23 ± 0.06 b,* | 4.34 ± 0.07 c,* | 2.81 ± 0.10 a,* | |
| Jejunum | ||||||||
| Lipase | 7 | 104.28 ± 5.43 a | 109.89 ± 6.01 a b | 136.45 ± 6.12 c | 187.53 ± 6.00 d | 107.35 ± 5.68 a | 122.82 ± 9.71 b | 101.16 ± 6.54 a |
| 28 | 148.65 ± 6.47 b,* | 161.49 ± 6.01 c,* | 177.40 ± 8.09 d,* | 202.05 ± 11.56 e,* | 154.04 ± 6.02 b c,* | 176.87 ± 7.37 d,* | 123.20 ± 4.04 a,* | |
| α-Amylase | 7 | 4.81 ± 0.14 b | 6.52 ± 0.13 d | 6.67 ± 0.10 d | 7.77 ± 0.24 e | 5.58 ± 0.07 c | 6.65 ± 0.11 d | 4.55 ± 0.17 a |
| 28 | 6.25 ± 0.13 b,* | 8.38 ± 0.09 d,* | 9.23 ± 0.09 e,* | 10.14 ± 0.13 f,* | 7.66 ± 0.22 c,* | 9.11 ± 0.17 e,* | 5.96 ± 0.10 a,* | |
| Trypsin | 7 | 2.23 ± 0.06 b | 3.17 ± 0.12 d | 3.74 ± 0.10 f | 4.39 ± 0.07 g | 2.36 ± 0.11 c | 3.29 ± 0.08 e | 2.06 ± 0.04 a |
| 28 | 2.47 ± 0.12 b,* | 3.78 ± 0.14 d,* | 4.28 ± 0.06 e,* | 4.72 ± 0.16 f,* | 2.84 ± 0.09 c,* | 3.72 ± 0.08 d,* | 2.00 ± 0.11 a,* | |
| Ileum | ||||||||
| Lipase | 7 | 78.92 ± 4.13 a | 95.39 ± 4.53 b | 122.70 ± 9.13 d | 164.17 ± 9.57 e | 85.20 ± 5.86 a | 113.49 ± 8.13 c | 86.56 ± 4.29 a,b |
| 28 | 114.66 ± 10.45 b,* | 126.25 ± 10.88 c,* | 146.10 ± 8.09 d,* | 184.82 ± 7.86 e,* | 121.71 ± 5.71 b,c,* | 144.29 ± 9.51 d,* | 95.31 ± 5.14 a,* | |
| α-Amylase | 7 | 4.34 ± 0.09 b | 5.14 ± 0.14 d | 5.58 ± 0.18 e | 7.12 ± 0.21 f | 4.74 ± 0.09 c | 5.25 ± 0.08 d | 4.07 ± 0.15 a |
| 28 | 5.55 ± 0.23 b,* | 6.02 ± 0.17 c,* | 7.94 ± 0.09 d,* | 8.47 ± 0.30 e,* | 5.86 ± 0.09 b,c,* | 6.07 ± 0.13 c,* | 5.26 ± 0.12 a,* | |
| Trypsin | 7 | 1.82 ± 0.05 a b | 2.48 ± 0.08 c | 3.43 ± 0.05 e | 3.76 ± 0.05 f | 1.89 ± 0.02 b | 2.84 ± 0.05 d | 1.77 ± 0.04 a |
| 28 | 2.25 ± 0.14 b,* | 3.21 ± 0.08 d,* | 3.70 ± 0.10 e,* | 3.63 ± 0.06 e,* | 2.26 ± 0.15 b,* | 3.03 ± 0.26 c | 1.81 ± 0.06 a,* | |
| Index | Time (d) | Control | CN Digest (mg/kg BW/d) | GCN (mg/kg BW/d) | ||||
|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 400 | 100 | 200 | 400 | |||
| Duodenum | ||||||||
| Lactase | 7 | 9.81 ± 0.13 a,b | 10.44 ± 0.34 b,c | 12.16 ± 0.62 d | 12.46 ± 0.77 d | 10.30 ± 0.57 a,b,c | 10.90 ± 0.53 c | 9.65 ± 0.30 a |
| 28 | 8.50 ± 0.39 b,* | 9.40 ± 0.48 c,* | 10.11 ± 0.22 d,* | 11.90 ± 0.29 e | 8.64 ± 0.29 b,* | 9.60 ± 0.33 c,* | 8.05 ± 0.20 a,* | |
| Sucrase | 7 | 23.01 ± 1.36 b | 27.61 ± 1.37 c | 32.20 ± 2.38 d | 42.62 ± 1.48 e | 24.10 ± 1.62 b | 28.62 ± 1.86 c | 20.23 ± 1.52 a |
| 28 | 30.20 ± 1.32 a,* | 37.26 ± 1.61 c,* | 46.47 ± 2.35 d,* | 52.60 ± 1.55 e,* | 33.42 ± 1.63 b,* | 39.31 ± 2.11 c,* | 28.22 ± 1.16 a,* | |
| Alkaline phosphatase | 7 | 141.93 ± 10.06 a b | 173.31 ± 9.58 c | 223.54 ± 10.77 d | 232.87 ± 8.43 d | 151.06 ± 7.51 b | 173.54 ± 13.70 c | 135.44 ± 7.00 a |
| 28 | 198.35 ± 11.89 b,* | 224.68 ± 15.16 c,* | 242.57 ± 17.74 d | 264.94 ± 11.74 e,* | 204.08 ± 11.41 b,* | 227.34 ± 6.55 c d,* | 176.80 ± 5.60 a,* | |
| Jejunum | ||||||||
| Lactase | 7 | 8.75 ± 0.16 a | 9.76 ± 0.11 b | 11.14 ± 0.24 c | 11.43 ± 0.32 c | 9.55 ± 0.43 b | 9.98 ± 0.79 b | 8.27 ± 0.46 a |
| 28 | 7.55 ± 0.23 a,* | 8.97 ± 0.25 b,* | 9.13 ± 0.39 b,* | 10.70 ± 0.22 c,* | 7.82 ± 0.37 a,* | 9.16 ± 0.32 b,* | 7.78 ± 0.22 a,* | |
| Sucrase | 7 | 29.71 ± 1.25 a | 35.58 ± 1.28 b | 44.07 ± 2.09 c | 50.30 ± 2.43 d | 30.43 ± 1.39 a | 37.9 ± 2.42 b | 27.97 ± 1.57 a |
| 28 | 38.45 ± 1.38 b,* | 54.45 ± 2.36 d,* | 60.46 ± 3.13 e,* | 62.35 ± 2.31 e,* | 41.25 ± 1.74 c,* | 54.88 ± 1.77 d,* | 33.27 ± 1.65 a,* | |
| Alkaline phosphatase | 7 | 113.37 ± 8.72 a,b | 133.86 ± 6.88 c | 173.89 ± 6.61 e | 198.70 ± 10.85 f | 121.25 ± 7.49 b | 147.22 ± 8.04 d | 107.90 ± 6.91 a |
| 28 | 161.49 ± 6.70 a,* | 172.37 ± 6.16 b,* | 226.82 ± 4.87 d,* | 232.30 ± 8.26 d,* | 171.04 ± 7.18 b,* | 188.85 ± 7.76 b,c,* | 158.67 ± 6.21 a,* | |
| Ileum | ||||||||
| Lactase | 7 | 7.84 ± 0.04 a | 8.80 ± 0.12 b | 9.05 ± 0.51 b c | 9.51 ± 0.54 c | 8.57 ± 0.42 b | 8.84 ± 0.58 b | 7.64 ± 0.40 a |
| 28 | 6.93 ± 0.15 b,* | 8.03 ± 0.24 c,* | 8.30 ± 0.32 c,* | 9.01 ± 0.34 d,* | 7.00 ± 0.45 b,* | 8.05 ± 0.46 c,* | 6.34 ± 0.15 a,* | |
| Sucrase | 7 | 16.50 ± 0.65 a | 22.41 ± 1.03 c | 26.66 ± 1.74 d | 37.27 ± 0.72 e | 18.66 ± 1.62 b | 23.62 ± 1.14 c | 16.50 ± 0.47 a |
| 28 | 23.65 ± 1.31 a,* | 33.41 ± 1.70 c,* | 38.14 ± 0.83 d,e,* | 41.21 ± 2.05 e,* | 29.81 ± 1.72 b,* | 36.39 ± 2.03 d,* | 21.60 ± 1.40 a,* | |
| Alkaline phosphatase | 7 | 99.95 ± 5.47 a,b | 120.5 ± 7.13 c | 147.63 ± 5.30 e | 156.78 ± 11.72 e | 108.19 ± 7.81 b | 131.06 ± 7.59 d | 94.38 ± 3.15 a |
| 28 | 145.16 ± 9.92 a,b,* | 152.85 ± 5.77 b,* | 176.78 ± 5.46 c,* | 201.60 ± 6.87 d,* | 151.29 ± 5.35 b,* | 152.18 ± 5.90 b,* | 139.56 ± 7.58 a,* | |
| Index | Time (d) | Control | CN Digest (mg/kg BW/d) | GCN Digest (mg/kg BW/d) | ||||
|---|---|---|---|---|---|---|---|---|
| 100 | 200 | 400 | 100 | 200 | 400 | |||
| Low-density lipoprotein | 7 | 0.68 ± 0.04 a | 0.70 ± 0.04 a,b | 0.72 ± 0.02 a,b | 0.72 ± 0.03 a,b | 0.73 ± 0.02 b | 0.79 ± 0.02 c | 0.82 ± 0.04 c |
| 28 | 0.71 ± 0.03 a | 0.73 ± 0.05 a,b | 0.74 ± 0.03 a,b,* | 0.75 ± 0.06 a,b | 0.74 ± 0.04 a,b | 0.80 ± 0.05 b,c | 0.85 ± 0.07 c | |
| Glucose | 7 | 3.98 ± 0.11 a | 4.05 ± 0.13 a,b | 4.17 ± 0.07 b,c | 4.23 ± 0.13 c | 4.54 ± 0.11 d | 5.04 ± 0.15 e | 5.43 ± 0.14 f |
| 28 | 4.06 ± 0.09 a,* | 4.17 ± 0.12 a,b,* | 4.33 ± 0.14 b,c | 4.41 ± 0.11 c,* | 4.56 ± 0.12 c | 5.21 ± 0.17 d,* | 5.51 ± 0.15 e | |
| Triglyceride | 7 | 1.18 ± 0.05 a | 1.20 ± 0.06 a | 1.27 ± 0.05 a,b | 1.32 ± 0.05 b | 1.46 ± 0.09 c | 1.53 ± 0.08 c | 1.82 ± 0.06 d |
| 28 | 1.23 ± 0.04 a,* | 1.24 ± 0.04 a,* | 1.28 ± 0.04 a,b | 1.35 ± 0.05 b,* | 1.51 ± 0.05 c,* | 1.56 ± 0.10 c | 1.88 ± 0.06 d | |
| Cholesterol | 7 | 1.72 ± 0.04 a | 1.77 ± 0.04 a | 1.89 ± 0.08 b | 2.02 ± 0.13 c | 2.14 ± 0.07 d | 2.38 ± 0.08 e | 2.58 ± 0.05 f |
| 28 | 1.88 ± 0.05 a,* | 1.91 ± 0.06 a,* | 2.02 ± 0.06 b,* | 2.13 ± 0.08 c,* | 2.08 ± 0.08 b,c,* | 2.40 ± 0.09 d | 2.65 ± 0.11 e | |
| Blood urea nitrogen | 7 | 5.54 ± 0.08 a | 5.66 ± 0.19 a | 5.76 ± 0.19 a,b | 5.95 ± 0.15 b | 6.28 ± 0.24 c | 6.62 ± 0.23 d | 6.84 ± 0.16 d |
| 28 | 6.13 ± 0.33 a,* | 6.36 ± 0.17 a,b,* | 6.56 ± 0.16 b,c,* | 6.76 ± 0.17 c,* | 6.65 ± 0.18 b,c,* | 7.49 ± 0.24 d,* | 7.83 ± 0.21 e,* | |
| Alanine transaminase | 7 | 26.86 ± 1.29 a | 27.56 ± 1.11 a | 28.71 ± 1.72 a,b | 29.60 ± 1.53 b | 30.47 ± 1.27 b,c | 31.97 ± 1.45 c | 34.51 ± 1.84 d |
| 28 | 28.34 ± 2.15 a | 29.48 ± 1.92 a,b | 30.53 ± 1.53 a,b,* | 31.67 ± 1.89 b,c,* | 31.33 ± 2.78 a,b | 34.55 ± 2.95 c,d,* | 37.36 ± 2.43 d,* | |
| Aspartate amino transferase | 7 | 172.28 ± 4.29 a | 175.61 ± 6.67 a | 179.37 ± 5.58 a | 187.57 ± 8.84 b | 192.27 ± 6.47 b | 209.74 ± 5.47 c | 221.34 ± 3.24 d |
| 28 | 180.95 ± 5.28 a,* | 183.77 ± 5.22 a,b,* | 185.31 ± 4.69 a,b,* | 189.22 ± 5.57 b | 196.48 ± 2.84 b | 209.66 ± 3.36 c | 225.29 ± 6.76 d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-P.; Zhao, X.-H. Lactose Glycation of the Maillard-Type Impairs the Benefits of Caseinate Digest to the Weaned Rats for Intestinal Morphology and Serum Biochemistry. Foods 2021, 10, 2104. https://doi.org/10.3390/foods10092104
Wang X-P, Zhao X-H. Lactose Glycation of the Maillard-Type Impairs the Benefits of Caseinate Digest to the Weaned Rats for Intestinal Morphology and Serum Biochemistry. Foods. 2021; 10(9):2104. https://doi.org/10.3390/foods10092104
Chicago/Turabian StyleWang, Xiao-Peng, and Xin-Huai Zhao. 2021. "Lactose Glycation of the Maillard-Type Impairs the Benefits of Caseinate Digest to the Weaned Rats for Intestinal Morphology and Serum Biochemistry" Foods 10, no. 9: 2104. https://doi.org/10.3390/foods10092104
APA StyleWang, X.-P., & Zhao, X.-H. (2021). Lactose Glycation of the Maillard-Type Impairs the Benefits of Caseinate Digest to the Weaned Rats for Intestinal Morphology and Serum Biochemistry. Foods, 10(9), 2104. https://doi.org/10.3390/foods10092104