A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
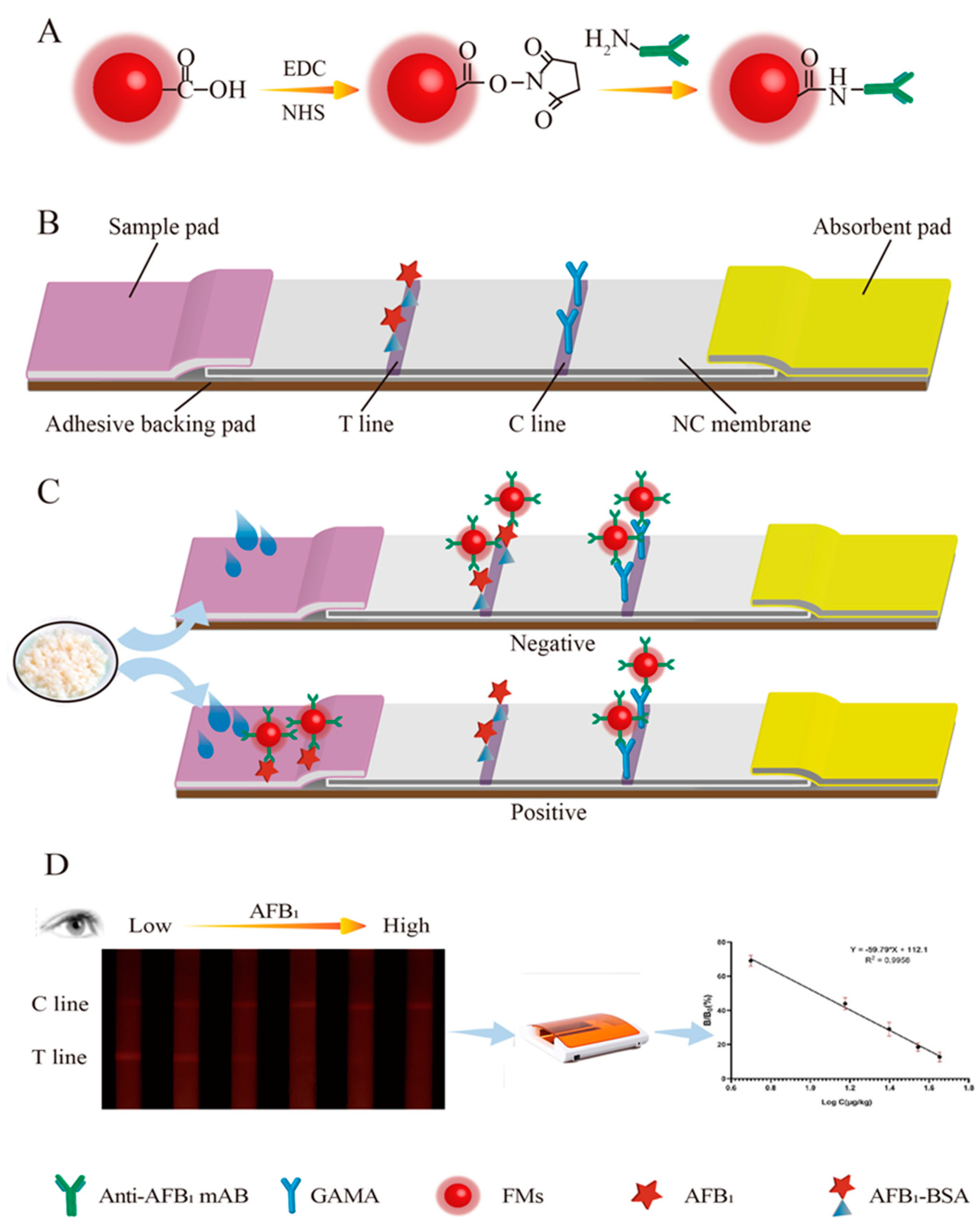
2.2. Preparation of FM Immunoprobe
2.3. Fabrication of the Immunochromatographic Strip
2.4. Sample Pretreatments
2.5. Immunochromatographic Assay Procedure
2.6. Method Evaluation
2.6.1. Sensitivity
2.6.2. Specificity
2.6.3. Accuracy and Precision
2.6.4. Confirmatory Test
3. Results and Discussion
3.1. Optimization of the FM Immunoprobe
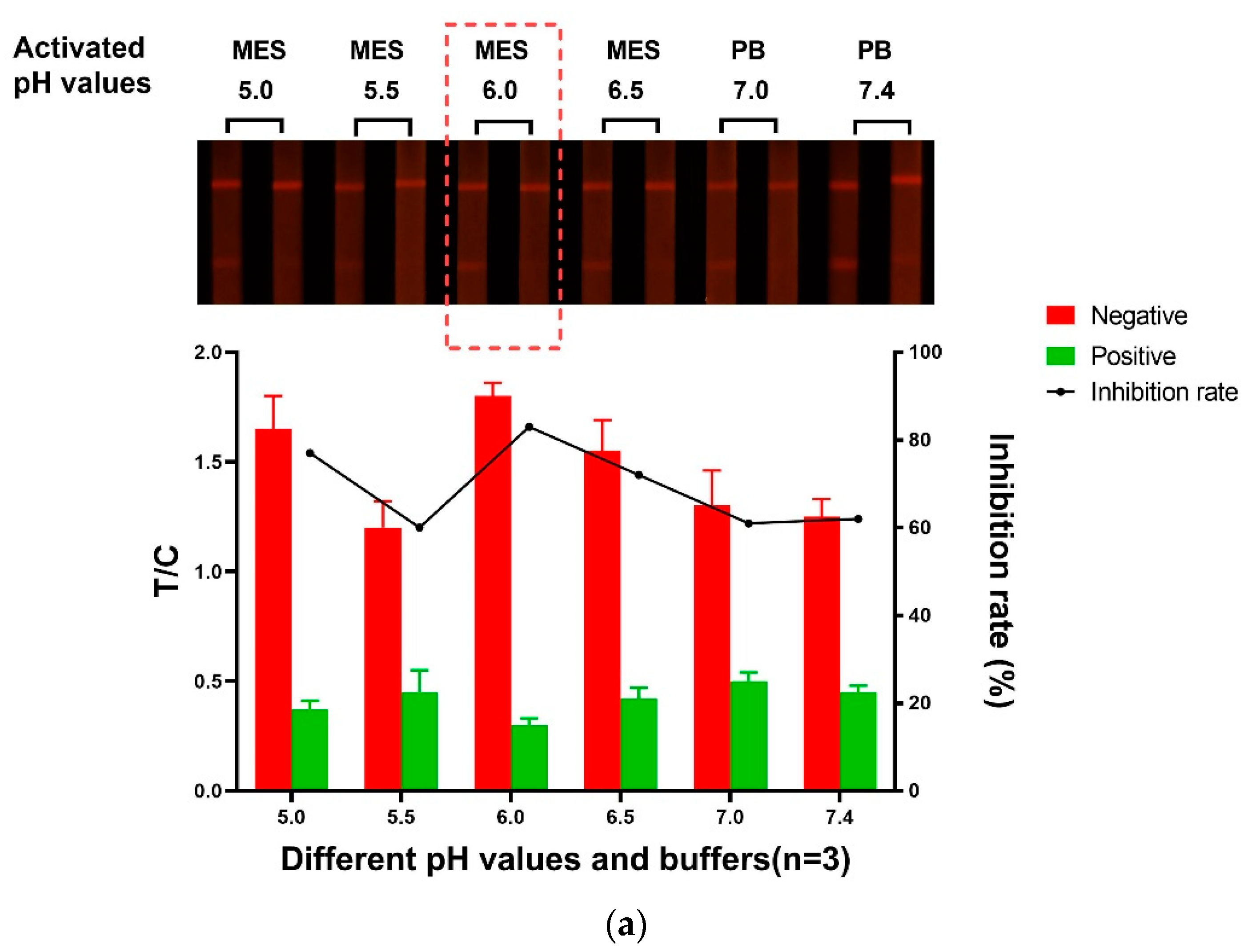
3.1.1. The Activated pH Value of FM
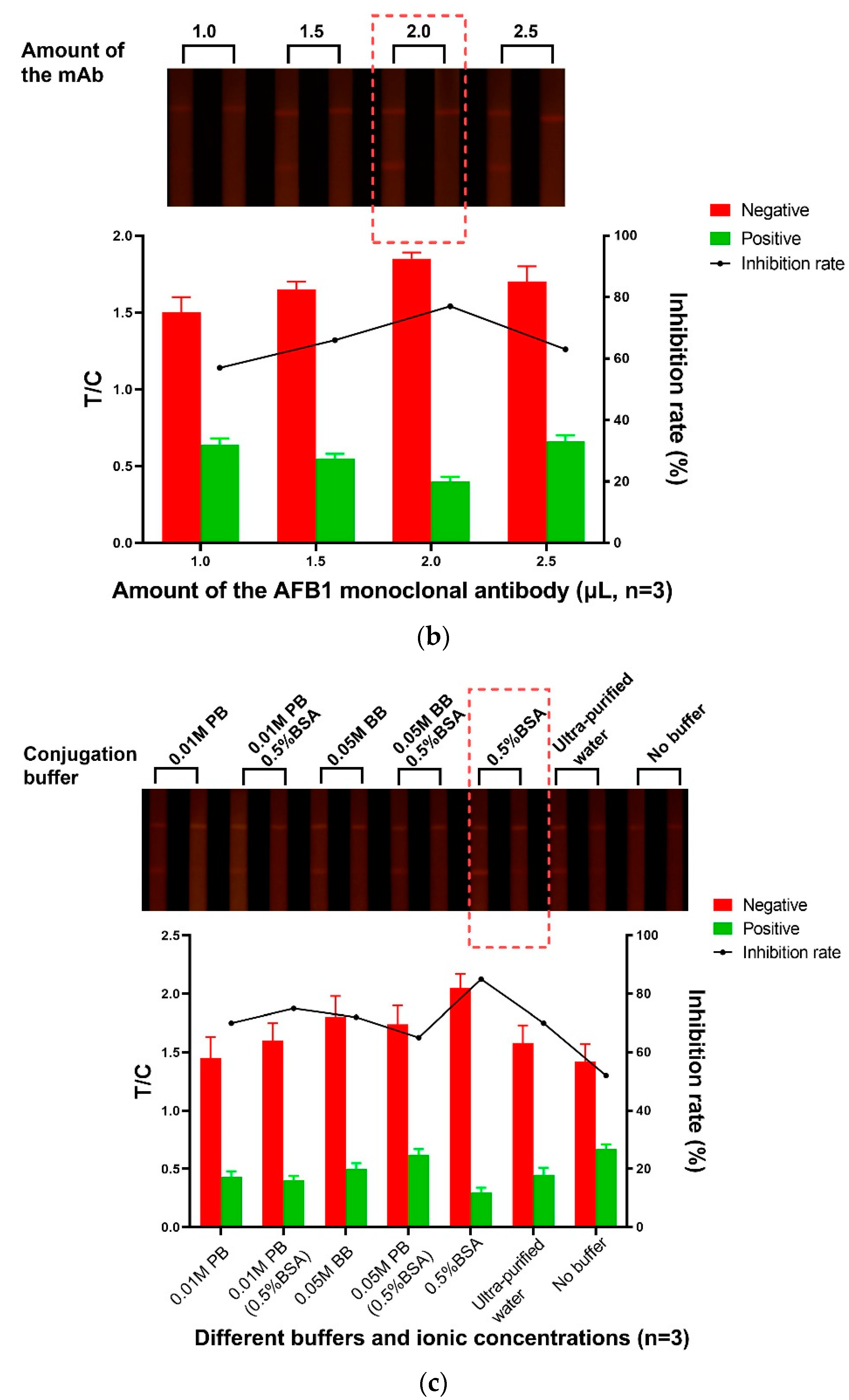
3.1.2. The Amount of Abs
3.1.3. Selection of the Coupling Buffer
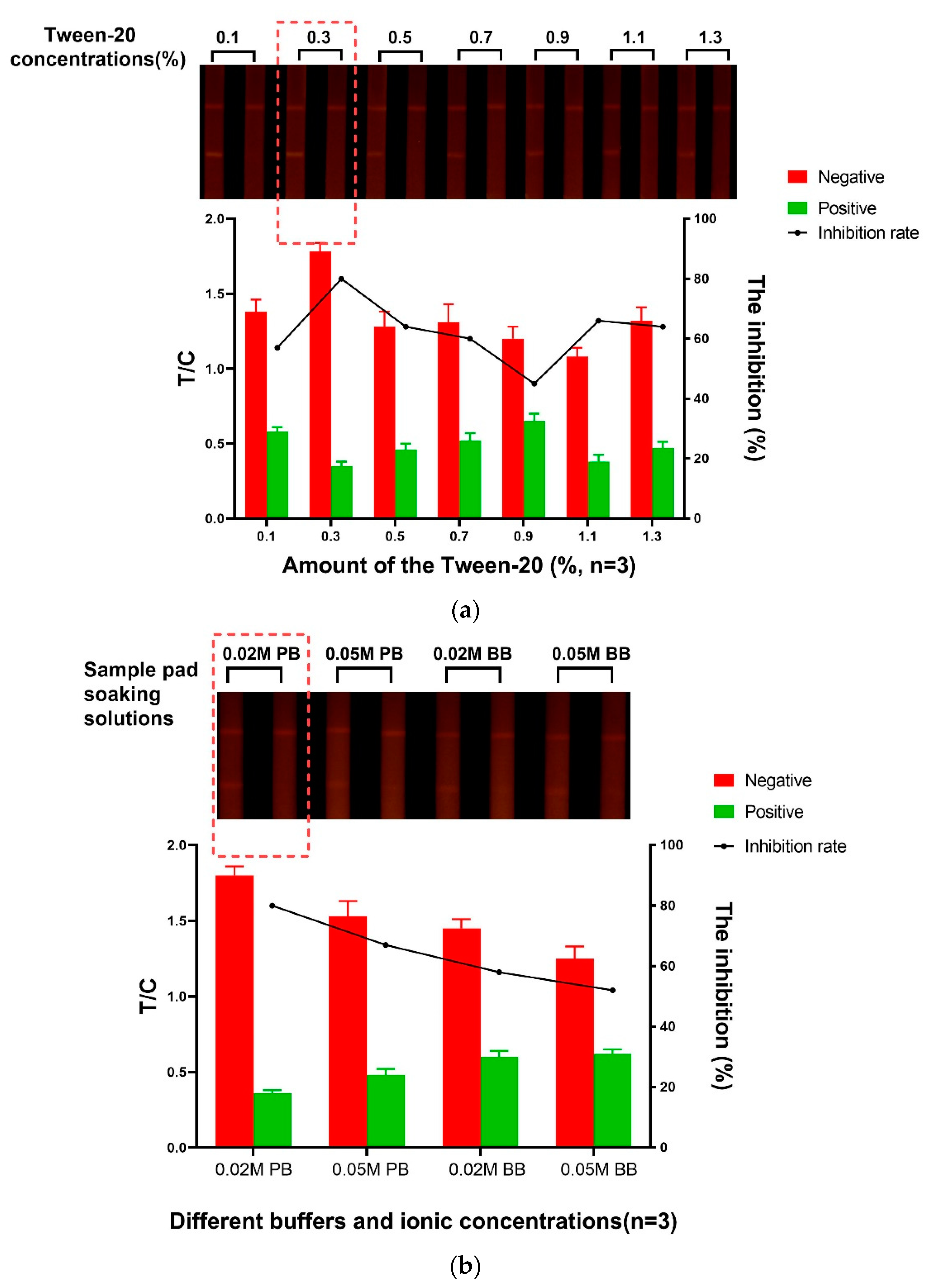
3.2. Optimization of the Sample Pad Treatment Solution
3.3. Evaluation of the Test Strip
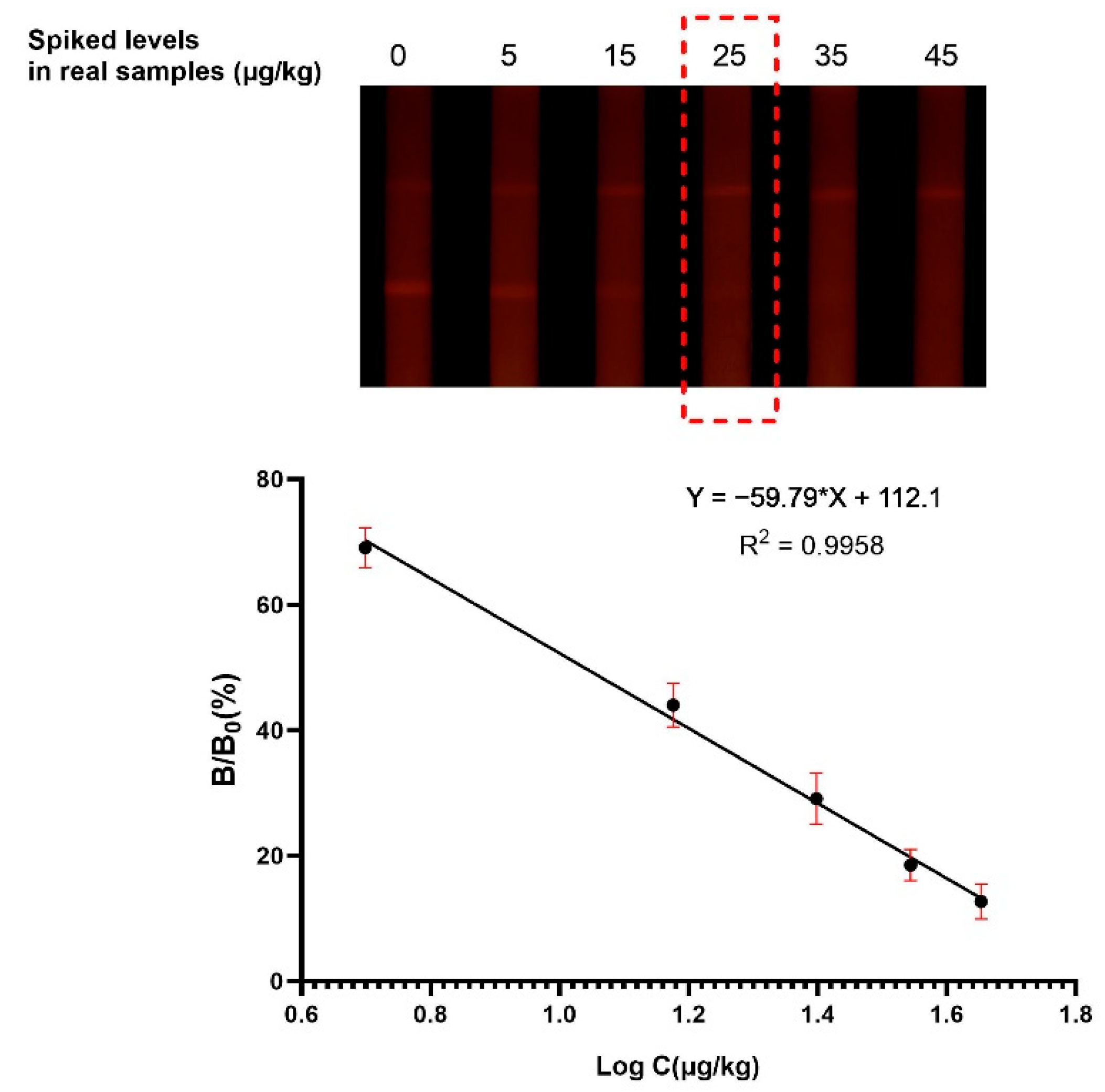
3.3.1. Sensitivity
3.3.2. Selectivity
3.3.3. Accuracy and Precision
3.4. A Comparison between the Test Strip and HPLC
3.5. Comparison of Different Immunoassays for AFB1 Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Al-Jaal, B.A.; Jaganjac, M.; Barcaru, A.; Horvatovich, P.; Latiff, A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: A systematic literature review, 2001-2018. Food Chem. Toxicol. 2019, 129, 211–228. [Google Scholar] [CrossRef]
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. Nutr. 2013, 53, 887–901. [Google Scholar] [CrossRef]
- Darwish, W.S.; Ikenaka, Y.; Nakayama, S.M.; Ishizuka, M. An overview on mycotoxin contamination of foods in Africa. J. Vet. Med. Sci. 2014, 76, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Al-Atiyat, R.M.; Khan, R.U. A survey of mycotoxin contamination and chemical composition of distiller’s dried grains with solubles (DDGS) imported from the USA into Saudi Arabia. Environ. Sci. Pollut. Res. Int. 2017, 24, 15401–15405. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Zhong, X.Z.; Wang, T.T.; Sun, Z.Y.; Tang, Y.Q.; Kida, K. Aerobic composting of distilled grain waste eluted from a Chinese spirit-making process: The effects of initial pH adjustment. Bioresour. Technol. 2017, 245, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Tansakul, N.; Jala, P.; Laopiem, S.; Tangmunkhong, P.; Limsuwan, S. Co-occurrence of five Fusarium toxins in corn-Dried Distiller’s Grains with Solubles in Thailand and comparison of ELISA and LC-MS/MS for fumonisin analysis. Mycotoxin Res. 2013, 29, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, G.H.; Han, G.P.; Kil, D.Y. Effect of feeding corn distillers dried grains with solubles naturally contaminated with deoxynivalenol on growth performance, meat quality, intestinal permeability, and utilization of energy and nutrients in broiler chickens. Poult. Sci. 2021, 100, 101215. [Google Scholar] [CrossRef]
- Anfossi, L.; Baggiani, C.; Giovannoli, C.; D’Arco, G.; Giraudi, G. Lateral-flow immunoassays for mycotoxins and phycotoxins: A review. Anal. Bioanal. Chem. 2013, 405, 467–480. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]
- Miklos, G.; Angeli, C.; Ambrus, A.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jozwiak, A.; Bartok, T. Detection of Aflatoxins in Different Matrices and Food-Chain Positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmed, A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Walker, G. Incidence of aflatoxins contamination in dry fruits and edible nuts collected from Pakistan. Food Control 2017, 78, 169–175. [Google Scholar] [CrossRef]
- Andrade, G.C.R.M.; Pimpinato, R.F.; Francisco, J.G.; Monteiro, S.H.; Calori-Domingues, M.A.; Tornisielo, V.L. Evaluation of mycotoxins and their estimated daily intake in popcorn and cornflakes using LC-MS techniques. LWT 2018, 95, 240–246. [Google Scholar] [CrossRef]
- Weaver, A.C.; Adams, N.; Yiannikouris, A. Invited Review: Use of technology to assess and monitor multimycotoxin and emerging mycotoxin challenges in feedstuffs. Appl. Anim. Sci. 2020, 36, 19–25. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Edupuganti, S.R.; Edupuganti, O.P.; Hearty, S.; O’Kennedy, R. A highly stable, sensitive, regenerable and rapid immunoassay for detecting aflatoxin B1 in corn incorporating covalent AFB1 immobilization and a recombinant Fab antibody. Talanta 2013, 115, 329–335. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Liu, Z.; Wang, J.; Hua, Q.; Liang, J.; Shen, X.; Xu, Z.; Lei, H.; Sun, Y. Latex microsphere immunochromatography for quantitative detection of dexamethasone in milk and pork. Food Chem. 2021, 345, 128607. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Wu, J.; Liu, Z.; Wang, J.; Song, C.; Zhao, S.; Lei, H.; Sun, Y. Portable, Rapid, and Sensitive Time-Resolved Fluorescence Immunochromatography for On-Site Detection of Dexamethasone in Milk and Pork. Foods 2021, 10, 1339. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; Rusanova, T.Y.; Burmistrova, N.A.; De Saeger, S. Immunochemical methods for the determination of mycotoxins. J. Anal. Chem. 2009, 64, 768–785. [Google Scholar] [CrossRef]
- Kavanagh, O.; Elliott, C.T.; Campbell, K. Progress in the development of immunoanalytical methods incorporating recombinant antibodies to small molecular weight biotoxins. Anal. Bioanal. Chem. 2015, 407, 2749–2770. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Cunha, S.C.; Fernandes, J.O. Validation of an Enzyme-Linked Immunosorbent Assay (ELISA) Test Kit for Determination of Aflatoxin B1 in Corn Feed and Comparison with Liquid-Chromatography Tandem Mass Spectrometry (LC-MS/MS) Method. Food Anal. Methods 2020, 13, 1806–1816. [Google Scholar] [CrossRef]
- Xing, K.-Y.; Shan, S.; Liu, D.-F.; Lai, W.-H. Recent advances of lateral flow immunoassay for mycotoxins detection. TrAC Trends Anal. Chem. 2020, 133, 116087. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal. Chim. Acta 2019, 1069, 1–27. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, B.; Zhang, Y.; Wang, J.; Lu, Y.; Deng, J.; Wang, S. Application of CdTe/CdS/ZnS quantum dot in immunoassay for aflatoxin B1 and molecular modeling of antibody recognition. Anal. Chim. Acta 2019, 1047, 139–149. [Google Scholar] [CrossRef]
- Shu, Q.; Wu, Y.; Wang, L.; Fu, Z. A label-free immunoassay protocol for aflatoxin B1 based on UV-induced fluorescence enhancement. Talanta 2019, 204, 261–265. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Wang, J.; Hua, Q.; Wu, J.; Shen, X.; Sun, Y.; Lei, H. Three lateral flow immunochromatographic assays based on different nanoparticle probes for on-site detection of tylosin and tilmicosin in milk and pork. Sens. Actuators B Chem. 2019, 301, 127059. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef]
- Kong, D.; Liu, L.; Song, S.; Suryoprabowo, S.; Li, A.; Kuang, H.; Wang, L.; Xu, C. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 2016, 8, 5245–5253. [Google Scholar] [CrossRef]
- Chen, X.; Miao, X.; Ma, T.; Leng, Y.; Hao, L.; Duan, H.; Yuan, J.; Li, Y.; Huang, X.; Xiong, Y. Gold Nanobeads with Enhanced Absorbance for Improved Sensitivity in Competitive Lateral Flow Immunoassays. Foods 2021, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, Y.-K.; Chen, M.; Wu, D.-Z.; Cai, S.-X.; Liu, M.-M.; He, W.-H.; Chen, J.-H. A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with Zn(II)-ion signal-enhancement for simultaneous detection of OTA and AFB1. Sens. Actuators B Chem. 2016, 235, 79–85. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Li, P.; Zhang, Q.; Zhang, W. Time-Resolved Fluorescent Immunochromatography of Aflatoxin B1 in Soybean Sauce: A Rapid and Sensitive Quantitative Analysis. Sensors 2016, 16, 1094. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.M.; Andersen, T.L.; Lindhardt, A.T.; de Almeida, M.V.; Skrydstrup, T. General method for the preparation of active esters by palladium-catalyzed alkoxycarbonylation of aryl bromides. J. Org. Chem 2015, 80, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, A.; Gee, S.J.; Hammock, B.D. An immunoassay for the detection of triclosan-O-glucuronide, a primary human urinary metabolite of triclosan. Anal. Bioanal. Chem. 2015, 407, 7263–7273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majdinasab, M.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Li, P.; Zhang, Q.; Li, X.; Tang, X.; Li, J. A reliable and sensitive time-resolved fluorescent immunochromatographic assay (TRFICA) for ochratoxin A in agro-products. Food Control 2015, 47, 126–134. [Google Scholar] [CrossRef]
- Ibrahim, M.I.M. Immunochromatographic techniques for mycotoxin analysis. In Nanomycotoxicology; Academic Press: London, UK, 2020; pp. 71–86. [Google Scholar]
- Xie, F.; Lai, W.; Saini, J.; Shan, S.; Cui, X.; Liu, D. Rapid pretreatment and detection of trace aflatoxin B1 in traditional soybean sauce. Food Chem. 2014, 150, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Chin, L. A comprehensive survey on the occurrence of mycotoxins in maize dried distillers’ grain and solubles sourced worldwide. World Mycotoxin J. 2012, 5, 83–88. [Google Scholar] [CrossRef]
- Lee, S.; Kim, G.; Moon, J. Performance improvement of the one-dot lateral flow immunoassay for aflatoxin B1 by using a smartphone-based reading system. Sensors 2013, 13, 5109–5116. [Google Scholar] [CrossRef]
- Yu, S.; He, L.; Yu, F.; Liu, L.; Qu, C.; Qu, L.; Liu, J.; Wu, Y.; Wu, Y. A lateral flow assay for simultaneous detection of Deoxynivalenol, Fumonisin B1 and Aflatoxin B1. Toxicon 2018, 156, 23–27. [Google Scholar] [CrossRef]
| Mycotoxins | Structure | CR (%) |
|---|---|---|
| AFB1 | 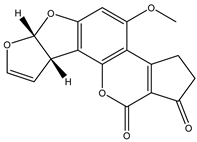 | 100 |
| AFB2 |  | 5.2 |
| AFG1 | 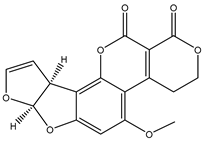 | 0.02 |
| AFG2 |  | 0.01 |
| AFM1 | 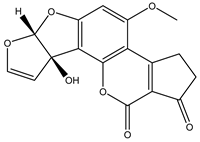 | 0.04 |
| Spiked Levels (μg/kg) | Mean ± SD (μg/kg) | Recovery (%) | CV (%) |
|---|---|---|---|
| 5 | 5.3 ± 0.3 | 95.2–113.0 | 6.1 |
| 15 | 14.8 ± 0.5 | 96.1–104.3 | 3.1 |
| 30 | 31.3 ± 0.7 | 102.0–108.5 | 2.1 |
| Spiked Levels (μg/kg) | Intra-Day Precision, n = 6 | Inter-Day Precision, n = 6 | ||
|---|---|---|---|---|
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | |
| 5 | 89.0–106.5 | 6.5 | 93.0–105.2 | 1.3 |
| 15 | 90.5–103.5 | 5.1 | 96.2–103.1 | 1.4 |
| 30 | 96.7–106.7 | 3.8 | 96.6–106.8 | 4.4 |
| Samples | AFB1 Concentration (μg/kg) | |
|---|---|---|
| LFIA | HPLC | |
| Sample 1 | N.D. 1 | N.D. |
| Sample 2 | 6.14 | 5.95 |
| Sample 3 | N.D. | N.D. |
| Sample 4 | 7.45 | 7.81 |
| Sample 5 | N.D. | N.D. |
| Sample 6 | N.D. | N.D. |
| Sample 7 | 8.21 | 8.13 |
| Sample 8 | N.D. | N.D. |
| Sample 9 | N.D. | N.D. |
| Sample 10 | N.D. | N.D. |
| Sample 11 | 5.82 | 5.69 |
| Sample 12 | N.D. | N.D. |
| Sample 13 | N.D. | N.D. |
| Sample 14 | N.D. | N.D. |
| Sample 15 | N.D. | N.D. |
| Sample 16 | N.D. | N.D. |
| Sample 17 | 7.21 | 7.38 |
| Sample 18 | N.D. | N.D. |
| Sample 19 | 4.95 | 5.12 |
| Sample 20 | N.D. | N.D. |
| Method | Sample | Sensitivity | Reference |
|---|---|---|---|
| Smartphone-Based LFIA | Maize | The detection limit for AFB1 was 5 μg/kg | [39] |
| ELISA | Corn | The limit of detection (LOD) for AFB1 was 12 ± 2.3 ng/mL | [17] |
| Au-particles-based LFIA | Corn | The visual LOD for AFB1 was 10 ng/mL | [40] |
| Quantum-dot based fluorescence immunoassay | Cereal | The LOD for AFB1 was 0.01 ng/mL | [25] |
| ELISA test kit | Corn feed | The LOD for AFB1 was 1.1 μg/kg and limit of quantification was 2.5 μg/kg | [22] |
| FM-based LFIA | Distillers’ grain | The cut-off value for AFB1 was 25.0 μg/kg, and the qLOD was 3.4 μg/kg | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Luo, P.; Zheng, B. A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains. Foods 2021, 10, 2109. https://doi.org/10.3390/foods10092109
Wang Z, Luo P, Zheng B. A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains. Foods. 2021; 10(9):2109. https://doi.org/10.3390/foods10092109
Chicago/Turabian StyleWang, Zifei, Pengjie Luo, and Baodong Zheng. 2021. "A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains" Foods 10, no. 9: 2109. https://doi.org/10.3390/foods10092109
APA StyleWang, Z., Luo, P., & Zheng, B. (2021). A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains. Foods, 10(9), 2109. https://doi.org/10.3390/foods10092109






