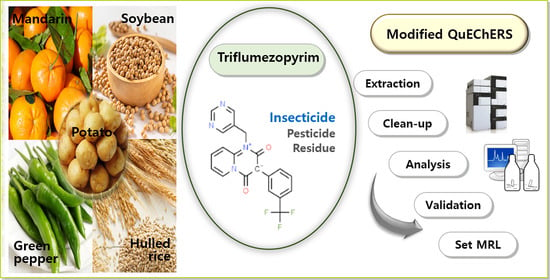
Determination of Residual Triflumezopyrim Insecticide in Agricultural Products through a Modified QuEChERS Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. UPLC-MS/MS Analysis
2.4. Method Validation
3. Results and Discussion
3.1. Optimization of Instrument Conditions and Chromatograms
3.2. Optimization of Extraction and Clean-Up
3.3. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holyoke, C.W., Jr.; Zhang, W.; Pahutski, T.F., Jr.; Lahm, G.P.; Tong, M.H.; Cordova, D.; Schroeder, M.E.; Benner, E.A.; Rauh, J.J.; Dietrich, R.F.; et al. Triflumezopyrim: Discovery and Optimization of a Mesoionic Insecticide for Rice. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; pp. 366–378. [Google Scholar]
- Zhang, W. Mesoionic Pyrido[1,2-a]pyrimidinone Insecticides: From Discovery to Triflumezopyrim and Dicloromezotiaz. Acc. Chem. Res. 2017, 50, 2381–2388. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, F.; Li, J.; Tao, Q.; Gao, J.; Lu, Y.-Y.; Wang, L. Effects of maximum residue limit of triflumezopyrim exposure on fitness of the red imported fire ant Solenopsis invicta. PeerJ 2019, 7, e8241. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Schroeder, M.E.; Holyoke, C.W., Jr.; Zhang, W.; Pahutski, T.F.; Leighty, R.M.; Vincent, D.R.; Hamm, J.C. Mode of action of triflumezopyrim: A novel mesoionic insecticide which inhibits the nicotinic acetylcholine receptor. Insect Biochem. Mol. Biol. 2016, 74, 32–41. [Google Scholar] [CrossRef]
- Holyoke, C.W., Jr.; Cordova, D.; Zhang, W.; Barry, J.D.; Leighty, R.M.; Dietrich, R.F.; Rauh, J.J.; Pahutski, T.F., Jr.; Lahm, G.P.; Tong, M.H.; et al. Mesoionic insecticides: A novel class of insecticides that modulate nicotinic acetylcholine receptors. Pest Manag. Sci. 2017, 73, 796–806. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Jiang, H.; Liu, C.; Lu, W.; Dai, W.; Xu, J.; Liu, F. Selective toxicity of the mesoionic insecticide, triflumezopyrim, to rice planthoppers and beneficial arthropods. Ecotoxicology 2018, 27, 411–419. [Google Scholar] [CrossRef]
- Risk Assessment Report Triflumezopyrim (Pesticide); Pesticides FS/663/2017; Food Safety Commission of Japan (FSCJ): Tokyo, Japan, 2017.
- Pesticide residues in food 2017, Report 2017. In Proceedings of the Joint FAO/WHO Meeting on Pesticide Residues, Geneva, Switzerland, 12–21 September 2017.
- Codex Allimentarius Commission. Pesticide Database-Maximum Residue Limits. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticide-detail/en/?p_id=303 (accessed on 10 August 2021).
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and determination of pesticides in fruit and vegetables. Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Chapter 15—Challenges of Biopesticides Under the European Regulation (EC) No. 1107/2009: An Overview of New Trends in Residue Analysis. Stud. Nat. Prod. Chem. 2014, 43, 437–482. [Google Scholar]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803–812. [Google Scholar] [CrossRef]
- Steinborn, A.; Alder, L.; Spitzke, M.; Dörk, D.; Anastassiades, M. Development of a QuEChERS-Based Method for the Simultaneous Determination of Acidic Pesticides, Their Esters, and Conjugates Following Alkaline Hydrolysis. J. Agric. Food Chem. 2017, 65, 1296–1305. [Google Scholar] [CrossRef] [Green Version]
- Musarurwa, H.; Chimuka, L.; Pakade, V.E.; Tavengwa, N.T. Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food Compos. Anal. 2019, 84, 103314. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Computational-Based Study of QuEChERS Extraction of Cyclohexanedione Herbicide Residues in Soil by Chemometric Modeling. Molecules 2018, 23, 2009. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, G.; Kemmerich, M.; Ribeiro, L.C.; Adaime, M.B.; Zanella, R.; Prestes, O.D. An effective method for pesticide residues determination in tobacco by GC-MS/MS and UHPLC-MS/MS employing acetonitrile extraction with low-temperature precipitation and d-SPE clean-up. Talanta 2016, 161, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Chen, X.; Xu, Z.; Liu, L.; Shen, D.; Dong, S.; Zhang, Q. Uptake and Translocation of Triflumezopyrim in Rice Plants. J. Agric. Food Chem. 2020, 68, 7086–7092. [Google Scholar] [CrossRef]
- Ortega, N.; Romero, M.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.; Motilva, M. Comparative study of UPLC–MS/MS and HPLC–MS/MS to determine procyanidins and alkaloids in cocoa samples. J. Food Compos. Anal. 2010, 23, 298–305. [Google Scholar] [CrossRef]
- Junza, A.; Amatya, R.; Barrón, D.; Barbosa, J. Comparative study of the LC-MS/MS and UPLC-MS/MS for the multi-residue analysis of quinolones, penicillins and cephalosporins in cow milk, and validation according to the regulation 2002/657/EC. J. Chromatogr. B 2011, 879, 2601–2610. [Google Scholar] [CrossRef] [Green Version]
- Codex Alimentarius Commission (CAC). Guidelines on Good Laboratory Practice in Pesticide Residue Analysis, CAC/GL 40-1993; Rev.1-2003; CAC: Rome, Italy.
- SANTE/12682/2019—Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 30 August 2021).
- Lehotay, S.J. QuEChERS sample preparation approach for mass spectrometric analysis of pesticide residues in foods. Methods Mol. Biol. 2011, 747, 65–91. [Google Scholar]
- Pizzutti, I.R.; de Kok, A.; Hiemstra, M.; Wickert, C.; Prestes, O.D. Method validation and comparison of acetonitrile and acetone extraction for the analysis of 169 pesticides in soya grain by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 4539–4552. [Google Scholar] [CrossRef]
- Lee, S.J.; McClements, D.J. Fabrication of protein-stabilized nanoemulsions using a combined homogenization and amphiphilic solvent dissolution/evaporation approach. Food Hydrocoll. 2010, 24, 560–569. [Google Scholar] [CrossRef]
- Diez, C.; Traag, W.; Zommer, P.; Marinero, P.; Atienza, J. Comparison of an acetonitrile extraction/partitioning and “dispersive solid-phase extraction” method with classical multi-residue methods for the extraction of herbicide residues in barley samples. J. Chromatogr. A 2006, 1131, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Romero-González, R. Detection of Residual Pesticides in Foods. Foods 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Liu, P.; Jia, Q.; Han, H.; Jiang, C.; Qiu, J. Determination of Three Typical Metabolites of Pyrethroid Pesticides in Tea Using a Modified QuEChERS Sample Preparation by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Foods 2021, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, L.C.; Caldas, S.S.; Prestes, O.D.; Primel, E.G.; Zanella, R. Evaluation of alternative sorbents for dispersive solid-phase extraction clean-up in the QuEChERS method for the determination of pesticide residues in rice by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1945–1954. [Google Scholar] [CrossRef]
- Song, N.E.; Seo, D.-H.; Choi, J.Y.; Yoo, M.; Koo, M.; Nam, T.G. Dispersive Solid–Liquid Extraction Coupled with LC-MS/MS for the Determination of Sulfonylurea Herbicides in Strawberries. Foods 2019, 8, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, V.C.; Luts, W.; Delerue-Matos, C.; Domingues, V.F. Improved QuEChERS for Analysis of Polybrominated Diphenyl Ethers and Novel Brominated Flame Retardants in Capsicum Cultivars Using Gas Chromatography. J. Agric. Food Chem. 2020, 68, 3260–3266. [Google Scholar] [CrossRef]
- Kim, L.; Lee, D.; Cho, H.K.; Choi, S.D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Checchini, L.; Carlo, R.M.D.; Orlandini, S.; Rivoira, L.; Bubba, M.D. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, Y.; Zheng, J.; Zhang, Y.; Yang, L.; Liao, C.; Su, L.; Zhou, Y.; Gong, D.; Chen, L.; et al. The application of the QuEChERS methodology in the determination of antibiotics in food: A review. Trends Anal. Chem. 2019, 118, 517–537. [Google Scholar] [CrossRef]
- Meng, Z.; Li, Q.; Cong, J.; Huang, Y.; Wang, D.; Pan, C.; Fan, S.; Zhang, Y. Rapid Screening of 350 Pesticide Residues in Vegetable and Fruit Juices by Multi-Plug Filtration Cleanup Method Combined with Gas Chromatography-Electrostatic Field Orbitrap High Resolution Mass Spectrometry. Foods 2021, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Kittlaus, S.; Schimanke, J.; Kempe, G.; Speer, K. Assessment of sample cleanup and matrix effects in the pesticide residue analysis of foods using postcolumn infusion in liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 8399–8410. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruit and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Guidelines on Performance Criteria for Methods of Analysis for the Determination of Pesticide Residues in Food and Feed. Available online: http://www.fao.org/fao-who-codexalimentarius/thematic-areas/pesticides/en/ (accessed on 30 August 2021).




| Molecular Weight | Exact Mass | Ionization Mode ESI | Retention Time (min) | Precursor Ion (m/z) | Product Ions (m/z) | CE (1) (eV) |
|---|---|---|---|---|---|---|
| 398.3 | 398.09 | (+) | 5.3 | 399.10 | 278.2 (2) | 28 |
| 121.25 | 30 | |||||
| 305.9 | 26 |
| Matrix | Linear Equation | R2 | Linear Range (mg/kg) | LOD (mg/kg) | LOQ (mg/kg) | Matrix Effect (%) |
|---|---|---|---|---|---|---|
| Solvent | y = 7,722,939x − 14,567 | 0.9996 | 0.003–0.30 | 0.003 | 0.01 | - |
| Mandarin | y = 7,296,289x − 19,541 | 0.9998 | −5.5 | |||
| Potato | y = 7,027,745x − 11,321 | 0.9992 | −9.0 | |||
| Green pepper | y = 7,191,487x − 18,409 | 0.9998 | −6.9 | |||
| Hulled rice | y = 6,759,340x − 10,407 | 0.9997 | −12.5 | |||
| Soybean | y = 7,350,651x − 21,557 | 0.9998 | −4.8 |
| Matrix | Spiked Level (mg/kg) | Recovery (%) ± RSD (%) | Average Recovery (%) ± RSD (%) | |
|---|---|---|---|---|
| Intra-Day | Inter-Day | |||
| Mandarin | 0.01 | 102.1 ± 5.4 | 101.1 ± 5.2 | 101.4 ± 5.4 |
| 0.1 | 96.2 ± 1.5 | 98.3 ± 4.4 | 97.8 ± 4.0 | |
| 0.5 | 97.1 ± 0.9 | 98.4 ± 3.8 | 98.1 ± 3.4 | |
| Potato | 0.01 | 103.7 ± 1.0 | 104.5 ± 1.8 | 104.3 ± 1.7 |
| 0.1 | 84.5 ± 5.1 | 98.1 ± 9.0 | 94.7 ± 9.8 | |
| 0.5 | 86.6 ± 2.9 | 90.7 ± 9.3 | 89.7 ± 8.5 | |
| Green pepper | 0.01 | 99.5 ± 1.5 | 99.8 ± 4.5 | 99.7 ± 4.0 |
| 0.1 | 94.7 ± 0.9 | 98.4 ± 3.0 | 97.4 ± 3.2 | |
| 0.5 | 85.0 ± 1.4 | 95.2 ± 8.3 | 92.6 ± 8.8 | |
| Hulled rice | 0.01 | 97.4 ± 2.6 | 100.6 ± 5.1 | 99.8 ± 4.9 |
| 0.1 | 103.7 ± 3.2 | 98.6 ± 3.8 | 99.9 ± 4.3 | |
| 0.5 | 99.5 ± 1.2 | 101.4 ± 2.8 | 101.0 ± 2.6 | |
| Soybean | 0.01 | 103.1 ± 3.9 | 100.4 ± 6.3 | 101.1 ± 6.0 |
| 0.1 | 90.8 ± 1.1 | 97.5 ± 6.6 | 95.8 ± 6.6 | |
| 0.5 | 92.1 ± 1.5 | 91.6 ± 1.6 | 91.7 ± 1.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-M.; Lee, H.-S.; Park, J.-S.; Lee, S.-J.; Shin, H.-S.; Chung, Y.-M.; Choi, H.-N.; Jung, Y.-H.; Oh, J.-H.; Yun, S.-S. Determination of Residual Triflumezopyrim Insecticide in Agricultural Products through a Modified QuEChERS Method. Foods 2021, 10, 2090. https://doi.org/10.3390/foods10092090
Cho S-M, Lee H-S, Park J-S, Lee S-J, Shin H-S, Chung Y-M, Choi H-N, Jung Y-H, Oh J-H, Yun S-S. Determination of Residual Triflumezopyrim Insecticide in Agricultural Products through a Modified QuEChERS Method. Foods. 2021; 10(9):2090. https://doi.org/10.3390/foods10092090
Chicago/Turabian StyleCho, Sung-Min, Han-Sol Lee, Ji-Su Park, Su-Jung Lee, Hye-Sun Shin, Yun-Mi Chung, Ha-Na Choi, Yong-Hyun Jung, Jae-Ho Oh, and Sang-Soon Yun. 2021. "Determination of Residual Triflumezopyrim Insecticide in Agricultural Products through a Modified QuEChERS Method" Foods 10, no. 9: 2090. https://doi.org/10.3390/foods10092090
APA StyleCho, S.-M., Lee, H.-S., Park, J.-S., Lee, S.-J., Shin, H.-S., Chung, Y.-M., Choi, H.-N., Jung, Y.-H., Oh, J.-H., & Yun, S.-S. (2021). Determination of Residual Triflumezopyrim Insecticide in Agricultural Products through a Modified QuEChERS Method. Foods, 10(9), 2090. https://doi.org/10.3390/foods10092090