Nutritional Profile, Antioxidative and Antihyperglycemic Properties of Padina tetrastromatica from Tioman Island, Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection, Identification and Processing of Algal Material
2.2. Molecular Identification of the Sample
2.3. Proximate Analysis
2.4. Fatty Acid Determination
2.5. Amino Acid Analysis
2.6. Mineral and Heavy Metal Analysis
2.7. Nonnutritive Components and Biological Activities
2.7.1. Total Phenolic Content (TPC) and Flavonoid Content (TFC)
2.7.2. Total Antioxidant Activity (TAA)—Phosphomolybdate Assay
2.7.3. Reducing Power Capacity
2.7.4. Hydrogen Peroxide (H2O2) Scavenging Assay
2.8. In Vivo Antidiabetic Study
2.8.1. Experimental Animals
2.8.2. Biochemical and Histological Studies
2.8.3. Oral Glucose Tolerance Test (OGTT)
2.8.4. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Protein and Amino Acid Composition
3.3. Carbohydrate and Dietary Fibre
3.4. Total Lipid and Fatty acids (FA) Profile
3.5. Mineral Profile
3.6. Nonnutritive Components and Biological Activities
3.7. In Vivo Antidiabetic Study
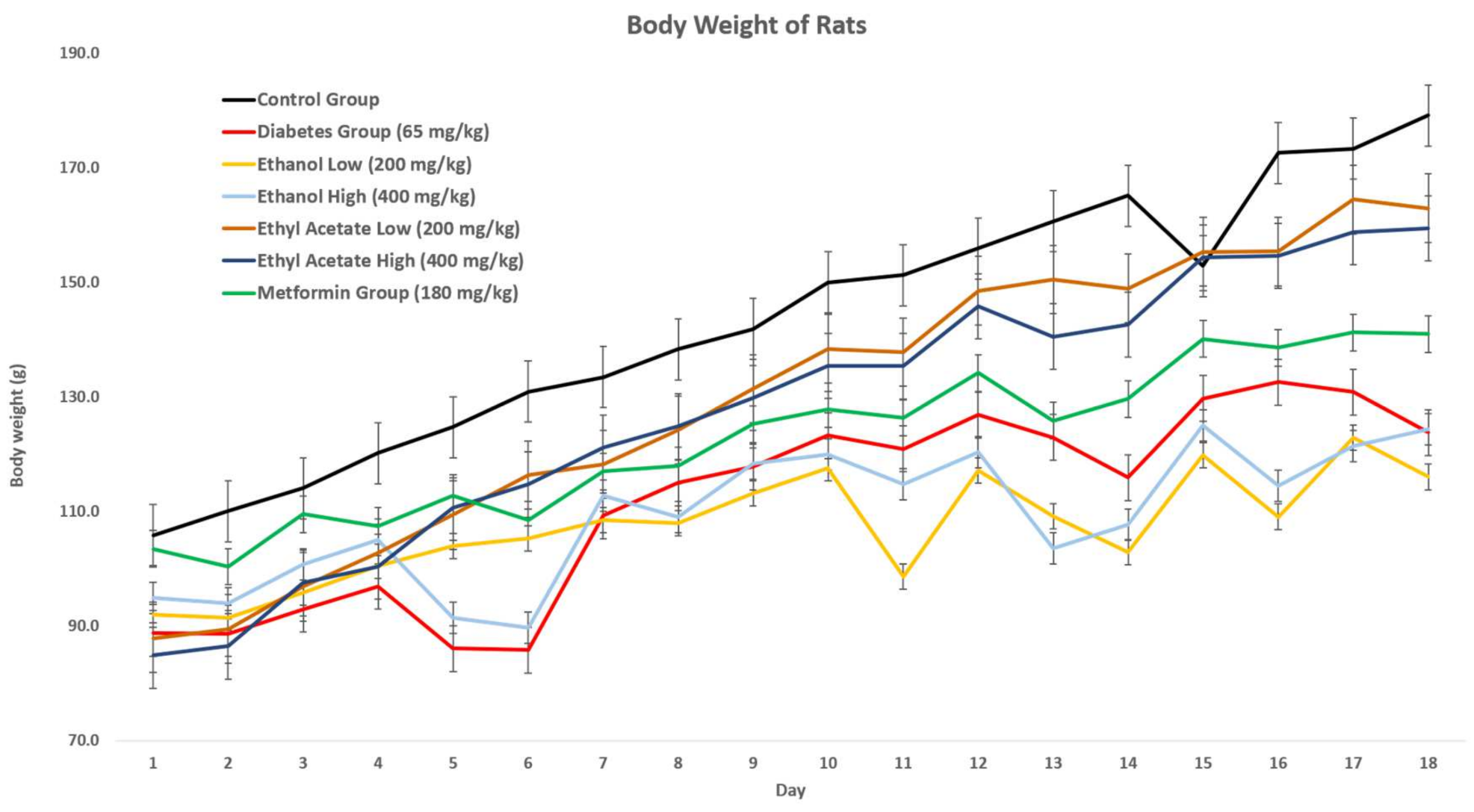
3.7.1. Effect of P. tetrastromatica Extracts on the Body Weight of Rats
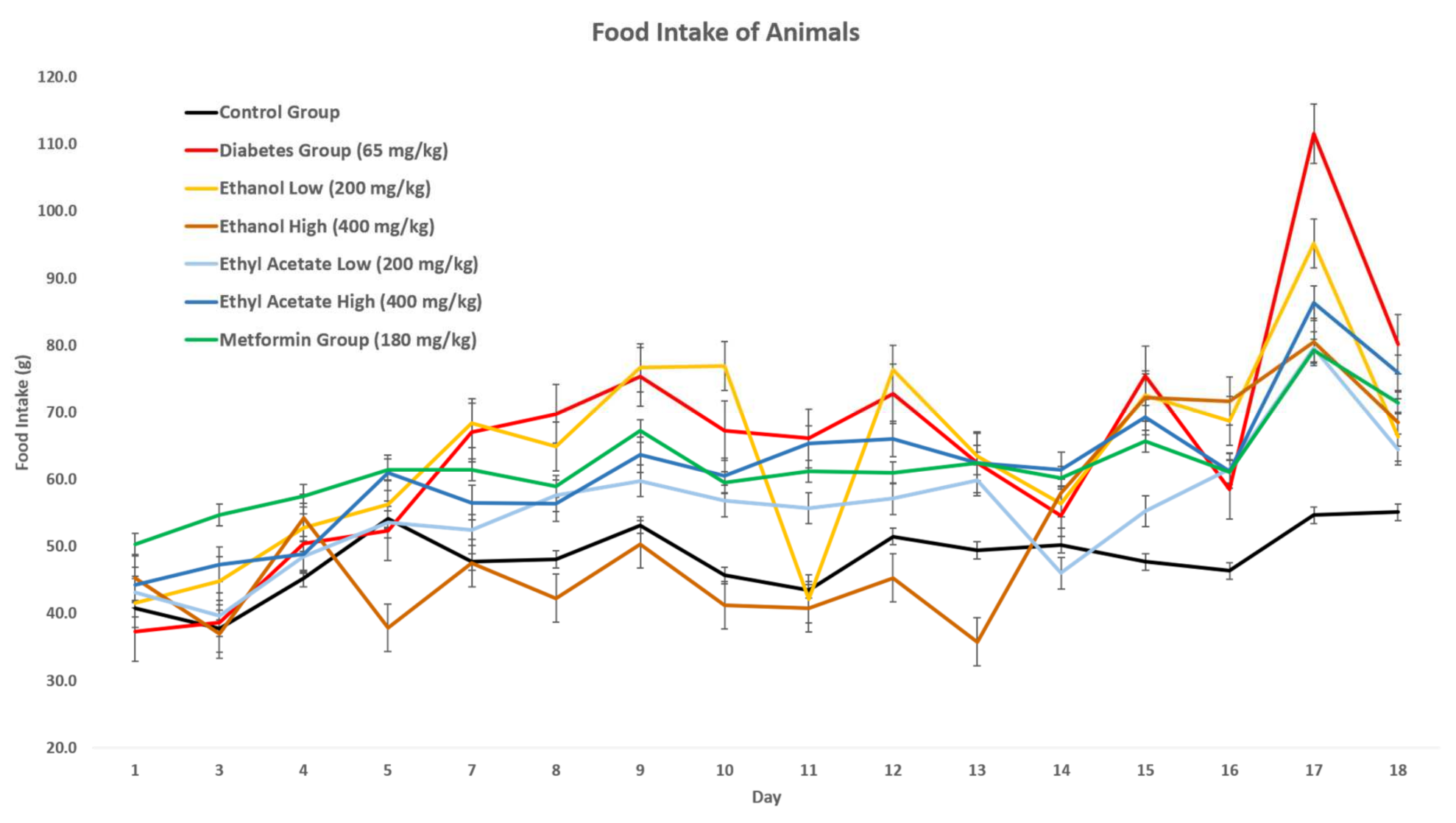
3.7.2. Effect of P. tetrastromatica Extracts on Food Intake
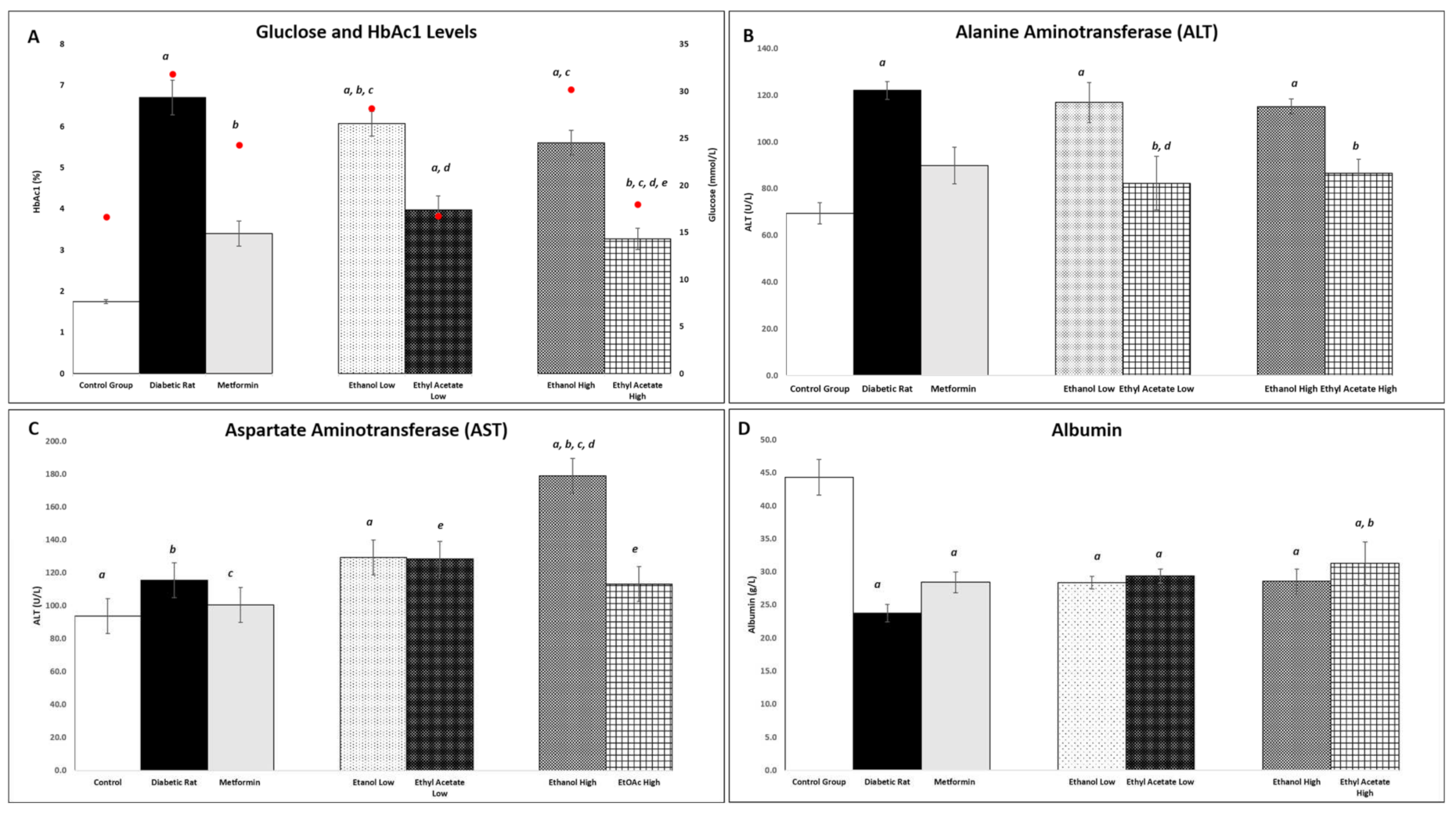
3.7.3. Effect of P. tetrastromatica Extracts on Plasma Glucose and Glycated Haemoglobin Levels
3.7.4. Effect of P. tetrastromatica Extract on Plasma ALT and AST Levels
3.7.5. Effect of P. tetrastromatica Extracts on Plasma Albumin Levels
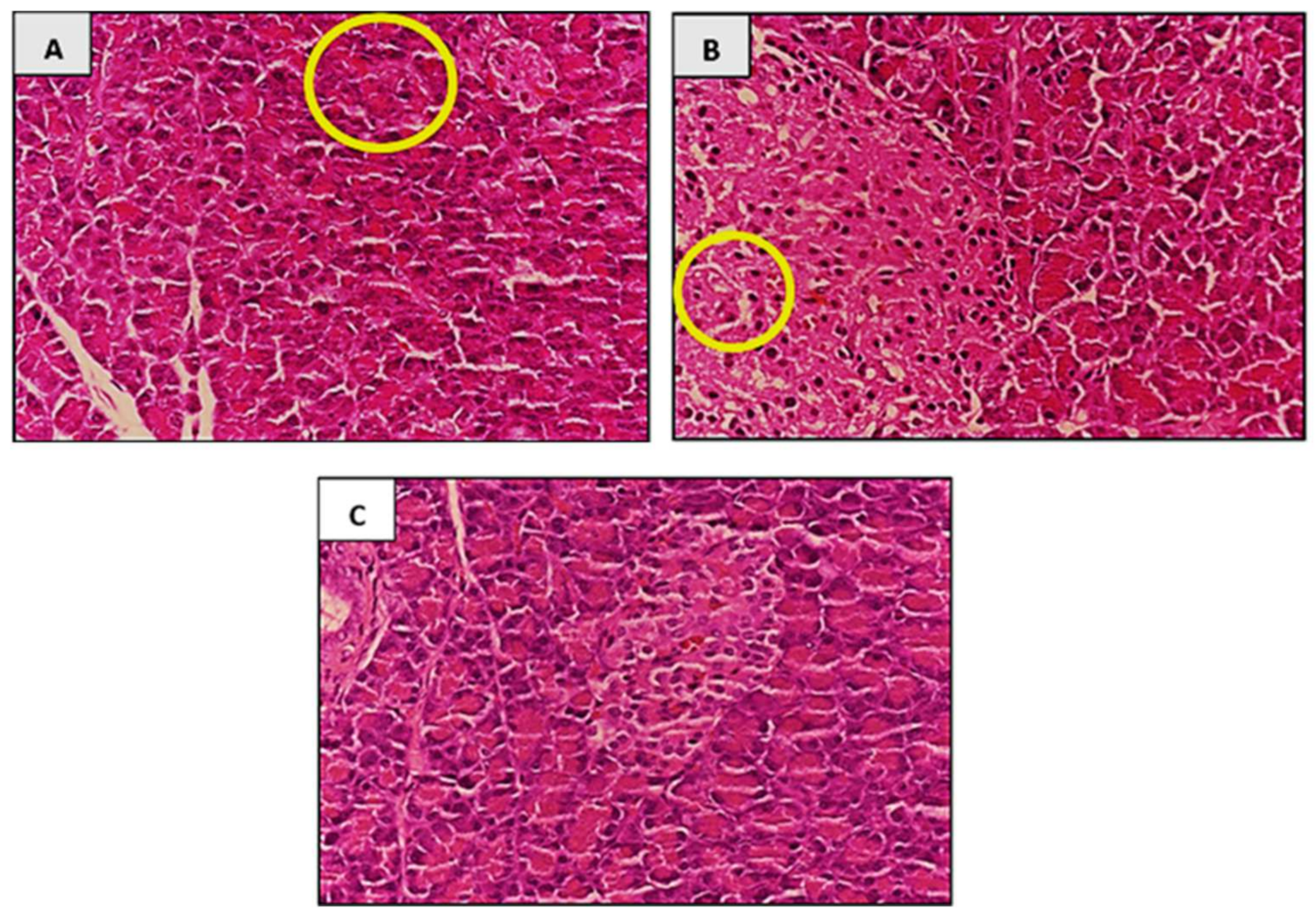
3.7.6. Histopathological Analysis
3.8. Presence of Bioactive Components in P. tetrastromatica
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WWF | World Wildlife Fund |
| SPS | Sulphated polysaccharide |
| WHO | World Health Organisation |
| STZ | Streptozotocin |
| DNA | Deoxyribonucleic Acid |
| PCR | Polymerase chain reaction |
| AOAC | Association of Official Analytical Chemists |
| v/v | volume/volume |
| h | Hour |
| DW | Dry weight |
| Hex | Hexane |
| EtOAc | Ethyl Acetate |
| GC | Gas chromatography |
| FAME | Fatty acid methyl ester |
| FA | Fatty acid |
| HCl | Hydrochloric acid |
| HPLC | High-Performance Liquid Chromatography |
| HNO3 | Nitric acid |
| H2O2 | Hydrogen peroxide |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| FC | Folin–Ciocalteau |
| GAE | Gallic acid equivalent |
| QE | Quercetin equivalent |
| TAA | Total Antioxidant Activity |
| AAE | Ascorbic acid equivalent |
| OGTT | Oral Glucose Tolerance Test |
| AST | Aspartate Transaminase |
| ALT | Alanine Transaminase |
| SD | Sprague Dawley |
| ACUC | Animal Care and Use Committee |
| EAA | Essential amino acid |
| NEAA | Nonessential amino aid |
| TNEAA | Total Nonessential amino aid |
| TAA | Total Amino acid |
| PUFA | Polyunsaturated fatty acids |
| MUFA | Monounsaturated fatty acids |
| SFA | Saturated fatty acids |
References
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidant 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Nutritional and Functional Bioactivity Value of Selected Azorean Macroalgae: Ulva Compressa, Ulva Rigida, Gelidium Microdon, and Pterocladiella Capillacea. J. Food Sci. 2017, 82, 1757–1764. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Goncalves, A.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Aroyehun, A.Q.; Palaniveloo, K.; Ghazali, F.; Rizman-Idid, M.; Abdul Razak, S. Effects of Seasonal Variability on The Physico- Chemical, Biochemical, and Nutritional Composition of Western Peninsular Malaysia Gracilaria Manilaensis. Molecules 2019, 24, 3298. [Google Scholar] [CrossRef]
- Aroyehun, Q.B.; Abdul Razak, S.; Palaniveloo, K.; Nagappan, T.; Suraiza Nabila Rahmah, N.; Wee Jin, G.; Chellappan, D.K.; Chellian, J.; Kunnath, A.P. Bioprospecting Cultivated Tropical Green Algae, Caulerpa Racemosa: A Perspective on Nutritional Properties, Antioxidative Capacity and Anti-Diabetic Potential. Foods 2020, 9, 1313. [Google Scholar] [CrossRef]
- Karmakar, P.; Ghosh, T.; Sinha, S.; Saha, S.; Mandal, P.; Ghosal, P.K.; Ray, B. Polysaccharides from The Brown Seaweed Padina Tetrastromatica: Characterization of a Sulfated Fucan. Carbohydr. Polym. 2009, 78, 416–421. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Sharifuddin, Y.; Chin, Y.X.; Lim, P.E.; Phang, S.M. Potential Bioactive Compounds from Seaweed for Diabetes Management. Mar. Drugs 2015, 13, 5447–5491. [Google Scholar] [CrossRef] [Green Version]
- Win, N.N.; Hanyuda, T.; Arai, S. Three New Records of Padina in Japan based on Morphological and Molecular Markers. Phycol. Res. 2008, 56, 288–300. [Google Scholar] [CrossRef]
- Nagappan, T.; Vairappan, C.S. Nutritional and Bioactive Properties of Three Edible Species of Green Algae, Genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in Food Products: Biochemical and Nutritional Aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient Content of Tropical Edible Seaweeds, Eucheuma Cottonii, Caulerpa Lentillifera and Sargassum Polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Lim, K.K.; Lim, K.H.; Teh, C.H.; Kee, C.C.; Cheong, S.M.; Khoo, Y.Y.; Baharudin, A.; Ling, M.Y.; Omar, M.A.; et al. Physical Activity and Overweight/Obesity among Malaysian Adults: Findings from The 2015 National Health and Morbidity Survey (NHMS). BMC Public Health 2017, 17, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Seasonal Variability of The Biochemical Composition and Antioxidant Properties of Fucus Spiralis at Two Azorean Islands. Mar. Drugs 2018, 16, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherry, P.; O‘Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Agregan, R.; Munekata, P.E.; Franco, D.; Carballo, J.; Sahin, S.; Lacomba, R.; Barba, F.J. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum Nodosum, Fucus Vesiculosus and Bifurcaria Bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [Green Version]
- Maehre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of Protein, Lipid and Mineral Contents in common Norwegian Seaweeds and Evaluation of their Potential as Food and Feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Rosemary, T.; Arulkumar, A.; Paramasivam, S.; Mondragon-Portocarrero, A.; Miranda, J.M. Biochemical, Micronutrient and Physicochemical Properties of The Dried Red Seaweeds Gracilaria Edulis and Gracilaria Corticata. Molecules 2019, 24, 2225. [Google Scholar] [CrossRef] [Green Version]
- Khairy, H.M.; El-Shafay, S.M. Seasonal Variations in The Biochemical Composition of Some Common Seaweed Species from the Coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 2013, 55, 435–452. [Google Scholar] [CrossRef] [Green Version]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal Variation in The Chemical Composition of Two Tropical Seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queiros, A.S.; Abreu, H.; Silva, A.; Cardoso, S.M. Screening of Ulva Rigida, Gracilaria sp., Fucus Vesiculosus and Saccharina Latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, J.L. Dietary Fiber and Body Weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Netzel, M.E.; Fletcher, M.T.; Tinggi, U.; Sultanbawa, Y. Chemical and Nutritional Composition of Terminalia Ferdinandiana (kakadu plum) Kernels: A Novel Nutrition Source. Foods 2018, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med Sci. Off. J. Isfahan Univ. Med Sci. 2014, 19, 164. [Google Scholar]
- Misurcova, L.; Ambrozova, J.; Samek, D. Seaweed Lipids as Nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 339–355. [Google Scholar]
- Sakthivel, R.; Devi, K.P. Evaluation of Physicochemical Properties, Proximate and Nutritional Composition of Gracilaria Edulis Collected from Palk Bay. Food Chem. 2015, 174, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Morgano, M.A.; Rabonato, L.C.; Milani, R.F.; Miyagusku, L.; Balian, S.C. Assessment of Trace Elements In Fishes of Japanese Foods Marketed in Sao Paulo (Brazil). Food Control. 2011, 22, 778–785. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Nus, M.; Sanchez-Montero, J.M.; Sanchez-Muniz, F.J. Effect of Seaweed and Cholesterol- Enriched Diets on Postprandial Lipoproteinaemia in Rats. Br. J. Nutr. 2009, 102, 1728–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Circuncisao, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, Flavonoid, and Amino Acid Compositions Reveal that Selected Tropical Seaweeds have The Potential to be Functional Food Ingredients. J. Food Process. Preserv. 2019, 43, e14266. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant Activities and Phenolic Contents of Three Red Seaweeds (Division: Rhodophyta) Harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Chakraborty, K.; Praveen, N.K.; Vijayan, K.K.; Rao, G.S. Evaluation of Phenolic Contents and Antioxidant Activities of Brown Seaweeds belonging to Turbinaria spp. (Phaeophyta, Sargassaceae) Collected from Gulf of Mannar. Asian Pac. J. Trop. Biomed. 2013, 3, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Caccetta, R.; Al Salami, H. Screening for Antidiabetic Activities. In Metabolomics Tools for Natural Product Discovery; Roessner, U.T.E., Dias, D.A., Eds.; Springer Nature: Cham, Switzerland, 2013; pp. 207–218. [Google Scholar]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponnanikajamideen, M.; Suneetha, V.; Rajeshkumar, S. Antidiabetic, Antihyperlipedimic and Antioxidant Activity of Marine Brown Seaweed Padina Tetrastromatica. J. Chem. Pharm. Sci. 2017, 10, 379–384. [Google Scholar]
- Granneman, J.G.; Stricker, M. Food Intake and Gastric Emptying in Rats with Streptozotocin-Induced Diabetes. Am. J. Physiol. 1984, 247, 1054–1061. [Google Scholar] [CrossRef]
- Petchi, R.R.; Vijaya, C.; Parasuraman, S. Antidiabetic Activity of Polyherbal Formulation in Streptozotocin–Nicotinamide Induced Diabetic Wistar Rats. J. Tradit. Complement. Med. 2014, 4, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbs, R.J.; Mazlan, N.; Whybrow, S. Carbohydrates, Appetite and Feeding Behavior in Humans. J. Nutr. 2011, 131, 2775–2781. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Guzman, M.; Rodriguez-Nogales, A.; Algieri, F.; Galvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular- Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Cui, J.; Kang, I.; Zhang, G.; Lee, Y. Potential Antidiabetic Effects of Seaweed Extracts by Upregulating Glucose Utilization and Alleviating Inflammation in C2C12 Myotubes. Int. J. Environ. Res. Public Health 2021, 18, 1367. [Google Scholar] [CrossRef]
- Mohan, D.S.; Saraswathy, M.; Kurup, M.K.G. Attenuation of Hyperglycemia and Hyipidemia in High Calorie Fed/Streptozotocin- Treated Rats by hydromethanolic extract of Padina Tetrastromatica. Bangladesh J. Pharmacol. 2014, 9, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, J.; Nafizah, A.N.; Zariyantey, A.H.; Budin, S. Mechanisms of Diabetes-Induced Liver Damage: The Role of Oxidative Stress and Inflammation. Sultan Qaboos Univ. Med J. 2016, 16, e132. [Google Scholar] [CrossRef] [PubMed]
- Turner, C. Practicing Alcohol Moderation: A Comprehensive Workbook; Turner, C., Ed.; Routledge: New York, NY, USA, 2020; p. 118. [Google Scholar]
- Cho, N.H.; Jang, H.C.; Choi, S.H.; Kim, H.R.; Lee, H.K.; Chan, J.C.; Lim, S. Abnormal Liver Function Test Predicts Type 2 Diabetes: A Community-Based Prospective Study. Diabetes Care 2007, 30, 2566–2568. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.K. Diabetic Nephropathy-Complications and Treatment. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef] [Green Version]
- Like, A.A.; Appel, M.C.; Williams, R.M.; Rossini, A.A. Streptozotocin-Induced Pancreatic Insulitis in Mice. Morphologic and Physiologic Studies. Lab. Investig. J. Tech. Methods Pathol. 1978, 38, 470–486. [Google Scholar]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Antidiabetic Potential of Marine Algae by Inhibiting Key Metabolic Enzymes. Front. Life Sci. 2015, 8, 148–159. [Google Scholar] [CrossRef]
- Lee, S.H.; Li, Y.; Karadeniz, F.; Kim, M.M.; Kim, S.K. α-Glucosidase and α-Amylase Inhibitory Activities of Phloroglucinol Derivatives from Edible Marine Brown Alga, Ecklonia Cava. J. Sci. Food Agric. 2008, 89, 1552–1558. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C. Metformin: Its Botanical Background. Pract. Diabetes Int 2004, 21, 115–117. [Google Scholar] [CrossRef]
- He, J.H.; Chen, L.X.; Li, H. Progress in the Discovery of Naturally Occurring Anti-Diabetic Drugs and in the Identification of their Molecular Targets. Fitoterapia 2019, 134, 270–289. [Google Scholar] [CrossRef]
- Parameswaran, P.S.; Naik, C.G.; Das, B.; Kamat, S.Y.; Bose, A.K.; Nair, M.S.R. Constituents of the Brown Alga Padina Tetrastromatica (Hauck)-II. Indian J. Chem. Sect. B 2016, 35, 463–467. [Google Scholar]
- Chia, P.Y. Antioxidant and Antimicrobial Compounds from the Marine Algae Padina Antillarum. Master’s Thesis, Universiti Tunku Abdul Rahman, Kampar, Malaysia, 2010. [Google Scholar]

| Group | Group Description |
|---|---|
| Group I | Normal control group |
| Group II | Diabetes-induced group (65 mg kg−1) |
| Group III | Ethanol extract-treated group (200 mg kg−1) |
| Group IV | Ethanol extract-treated group (400 mg kg−1) |
| Group V | Ethyl acetate extract-treated group (200 mg kg−1) |
| Group VI | Ethyl acetate extract-treated group (400 mg kg−1) |
| Group VII | Metformin treated group (180 mg kg−1) |
| Essential Amino Acids (EAAs) | Composition (mg g−1) |
| Threonine | 1.319 ± 0.009 |
| Valine | 1.155 ± 0.003 |
| Isoleucine | 1.049 ± 0.106 |
| Leucine | 3.659 ± 0.187 |
| Lysine | 2.997 ± 0.102 |
| Sum TEAAs | 10.179 ± 0.06 |
| Nonessential Amino acids (NEAAs) | Composition (mg g−1) |
| Aspartic acids | 24.704 ± 0.218 |
| Glutamic acid | 6.829 ± 0.086 |
| Serine | 0.47 ± 0.001 |
| Glutamine | 10.307 ± 0.086 |
| Glycine | 4973.57 ± 0.129 |
| Alanine | 0.150 ± 0.003 |
| Cysteine | 0.247 ± 0.003 |
| Tyrosine | 2.307 ± 0.02 |
| Arginine | 1.233 ± 0.06 |
| Sum TNEAAs | 50.838 ± 0.19 |
| EAAs/TAAs (%) | 16.68 ± 0.05 |
| NEAAs/TAAs (%) | 83.32 ± 0.05 |
| EAAs/NEAAs (%) | 20.02 ± 0.07 |
| Chemical score | 0.478 |
| Fatty Acid | Content (g 100 g Oil−1) |
|---|---|
| SFAs | |
| Myristic acid (C14:0)) | 0.1 ± 0.00 |
| Palmitic acid (C16:0) | 0.83 ± 0.06 |
| Stearic acid (C18:0) | 0.3 ± 0.00 |
| Arachidic acid (C20:0) | 0.10 ± 0.00 |
| Total (%) | 1.37 ± 0.07 (73.20 ± 2.88) |
| MUFA | |
| Oleic acid(C18:1ω9) | 0.40 ± 0.10 |
| Total (%) | 0.4 ± 0.05 (21.44 ± 2.83) |
| PUFA | |
| Linoleic acid (C18:2 ω6) cis | 0.10 ± 0.00 |
| Total | 0.1 ± 0.00 (5.36 ± 0.1) |
| Total FA | 1.87 ± 0.03 |
| Minerals | Content (mg 100 g−1) |
|---|---|
| Macro Metal | |
| Calcium | 4388.3 |
| Magnesium | 3.47 |
| Potassium | 171.3 |
| Sodium | 148.2 |
| Na/K | 0.87 |
| Trace Mineral | |
| Copper | 0.60 |
| Iron | 37.3 |
| Manganese | 326.34 |
| Molybdenum | 0.53 |
| Selenium | 0.068 |
| Zinc | 1.34 |
| Chromium | 0.65 |
| Cobalt | 0.14 |
| Heavy metal/TWIs | |
| Total Arsenic | 0.49 |
| Cadmium | 0.01 |
| Aluminium | 530.64 |
| Lead | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaniveloo, K.; Yee-Yinn, L.; Jia-Qi, L.; Chelliah, A.; Sze-Looi, S.; Nagappan, T.; Razak, S.A.; Dua, K.; Chellian, J.; Chellappan, D.K.; et al. Nutritional Profile, Antioxidative and Antihyperglycemic Properties of Padina tetrastromatica from Tioman Island, Malaysia. Foods 2021, 10, 1932. https://doi.org/10.3390/foods10081932
Palaniveloo K, Yee-Yinn L, Jia-Qi L, Chelliah A, Sze-Looi S, Nagappan T, Razak SA, Dua K, Chellian J, Chellappan DK, et al. Nutritional Profile, Antioxidative and Antihyperglycemic Properties of Padina tetrastromatica from Tioman Island, Malaysia. Foods. 2021; 10(8):1932. https://doi.org/10.3390/foods10081932
Chicago/Turabian StylePalaniveloo, Kishneth, Liaw Yee-Yinn, Leong Jia-Qi, Alvin Chelliah, Song Sze-Looi, Thilahgavani Nagappan, Shariza Abdul Razak, Kamal Dua, Jestin Chellian, Dinesh Kumar Chellappan, and et al. 2021. "Nutritional Profile, Antioxidative and Antihyperglycemic Properties of Padina tetrastromatica from Tioman Island, Malaysia" Foods 10, no. 8: 1932. https://doi.org/10.3390/foods10081932
APA StylePalaniveloo, K., Yee-Yinn, L., Jia-Qi, L., Chelliah, A., Sze-Looi, S., Nagappan, T., Razak, S. A., Dua, K., Chellian, J., Chellappan, D. K., & Kunnath, A. P. (2021). Nutritional Profile, Antioxidative and Antihyperglycemic Properties of Padina tetrastromatica from Tioman Island, Malaysia. Foods, 10(8), 1932. https://doi.org/10.3390/foods10081932







