1. Introduction
Advanced glycation end-products (AGEs) can be generated via a glycation reaction (known as the Maillard reaction) between the carbonyl group of a reducing sugar (glucose, fructose, or ribose) in blood and the free amino group of a protein [
1]. In the early stage, the condensation of glucose and amines produces an unstable Schiff base. The Schiff base is then arranged to form Amadori products. During the degradation of Amadori products, reactive carbonyl species are formed in the intermediate stage. At the advanced stage, a cross-link occurs between dicarbonyl intermediates and proteins (e.g., collagen), resulting in irreversible glycation end-products [
2]. These adducts accelerate the development and progression of diabetic complications by altering the normal physiological functions of a protein upon glycation [
3]. In addition, AGEs induce reactive oxygen species (ROS; e.g., superoxide (O
2−), hydrogen peroxide (H
2O
2), peroxynitrite (ONOO
-)), which cause intracellular oxidative stress and promote the mitochondrial apoptosis pathway by interacting with the receptor for AGEs (RAGE) [
4]. The increase in AGE formation in chronic hyperglycemic conditions is a major contributor on the pathogenesis of diabetic complications including retinopathy, neuropathy, and nephropathy [
5]. Therefore, inhibition of AGE formation or accumulation in the body is extensively regarded as the most effective ways for preventing or delaying AGE-related diabetic complications.
In hyperglycemic patients, there is an increase in the levels of methylglyoxal (MGO), which is one of the reactive glycation end-products and the major intermediate and precursor for AGE formation [
6]. MGO is more reactive than glucose, with a stronger ability to cross-link with the arginine or lysine residues of proteins, leading to the formation of stable end-products called AGEs and subsequent activation of RAGE, which then initiates various diabetic complications [
6,
7]. However, MGO can directly impair cellular function independently of the AGE-RAGE pathway through intracellular reactive oxygen species (ROS) generation, inducing oxidative damage to proteins, apoptosis by inducing oxidative stress, and increasing caspase activity [
5]. Several synthetic compounds, such as aminoguanidine (AG) and the thiazolium-derived compound ALT-711 (alagebrium) have been shown to inhibit the cross-linking of proteins and slow down the progression of diabetes-related complications. However, these agents were ultimately not approved for clinical use due to safety and efficacy issues during the clinical trial phase [
8,
9]. Therefore, the scavenging of AGEs may be a promising therapeutic approach for diabetes and its associated complications, and there is a need for the development of effective AGE-scavenging agents that are based on natural resources.
Ishige okamurae (
I. okamurae) is a brown seaweed that is distributed throughout the temperate coastal region, and is generally enrich in the coast of Jeju Island, Korea.
I. okamurae contains various natural antioxidant substances, among which phlorotannins are the most powerful water-soluble antioxidants [
10,
11]. Several previous studies have reported that
I. okamurae has a variety of biological benefits associated with phlorotannins, including anti-inflammatory, anti-hypertensive, anti-bacterial, anti-tumor, anti-obesity, and free radical-scavenging activities [
12,
13].
I. okamurae acts as an inhibitor of alpha-glucosidase, which is a key enzyme in the modulation of glucose absorption, and thus enhances glucose homeostasis [
14]. In addition, a phlorotannin-rich extract from
I. okamurae has been shown to have a positive effect on genetically diabetic mice. Among the many bioactive compounds isolated from
I. okamurae, phlorotannins have been extensively studied for their health benefits. In particular, diphlorethohydroxycarmalol (DPHC) has been considered to influence responses relevant to diabetes through the modulation of glucose-induced oxidative stress, as well as the inhibition of carbohydrate-digestive enzymes [
15]. Although many previous studies have been conducted on the various biological benefits of
I. okamurae, its preventive effects on AGE-related diabetic complications have not been investigated so far.
Brown seaweeds, which are rich in phlorotannins, are consumed for the prevention or improvement of chronic metabolic diseases due to their beneficial biological properties. On the basis of these investigations, we supposed that the phlorotannins contained in I. okamurae may have protective effects against AGE-induced renal damage. Therefore, in the present study, we evaluated the inhibitory effects of I. okamurae on AGE formation and AGE cross-links, as well as its nephroprotective effects against AGE-induced oxidative stress in mouse glomerular mesangial cells. In addition, the underlying mechanisms of action were partly elucidated using a protein expression analysis of key signaling pathways such as RAGE, MAPKs, and Nrf2/ARE.
2. Materials and Methods
2.1. Chemicals
Aminoguanidine (AG), glucose, fructose, bovine serum albumin (BSA), sodium azide, 3,3,5,5-tetramethylbenzidine (TMB) liquid substrate system for enzyme-linked immunosorbent assay (ELISA), MGO, sodium phosphate monobasic, and sodium phosphate dibasic were purchased from Sigma (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium/F-12 Nutrient Mixture (DMEM/F-12; Ham, 3:1 mixture) was obtained from Welgene Inc. (Daegu, Korea). Fetal bovine serum (FBS), hydroxyethyl-piperazineethane-sulfonic acid (HEPES), penicillin/streptomycin solution, and collagen I-coated plates were purchased from Gibco (Rockville, MD, USA). PRO-PREP™ Protein Extraction Solution, the OxiSelect™ Methylglyoxal (MG) Competitive ELISA Kit, and the RAGE antibody were purchased from iNtRON Biotechnology (Seongnam, Korea), Cell Biolabs Inc. (San Diego, CA, USA), and Merck Millipore (Billerica, MA, USA), respectively. Antibodies against catalase (CAT), heme oxygenase 1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), extracellular signal-regulated kinases (ERK), phospho-ERK (p-ERK), c-Jun N-terminal kinase (JNK), phosphor-JNK (p-JNK), p38, phosphor-p38 (p-p38), and superoxide dismutase 1 (SOD1) were purchased from Cell Signaling Technology (Danvers, MA, USA). β-actin and nuclear factor erythroid 2-related factor (Nrf2) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The receptor for advanced glycation end-products (RAGE) antibody was obtained from Merck Millipore (Billerica, MA, USA).
2.2. Sample Preparation
In brief, Ishige okamurae (I. okamurae) was collected from Jeju Island, South Korea. I. okamurae was washed with tap water to remove salt, sand, and epiphytes attached to the surface. Next, I. okamurae was lyophilized and ground to obtain a dry powder. Dried I. okamura powder was extracted in 50% ethanol under reflux conditions. The extract was concentrated and freeze-dried (final yield: 87 g). I. okamurae extracts (IOEs; kindly provided by Prof. Y.J. Jeon) were sealed and stored at −20 °C prior to use.
2.3. Identification of Phlorotannins in I. okamurae Using HPLC
Chromatographic analyses were conducted on an Acquity™ Arc equipped with a 2998 PDA detector and an Acquity™ QDa detector system (Waters Corporation, Beverly, MA, USA). The phlorotannin compounds in I. okamurae extract were analyzed using a Poroshell 120 EC-C18 column (4 µm, 4.6 mm × 100 mm; Agilent(Santa Clara, CA, USA)). The mobile phase consisted of (A) 0.1% formic acid in water and (B) acetonitrile containing 0.1% formic acid. The HPLC elution condition consisted of an isocratic system of 32% B at a flow rate of 0.4 mL/min and an injected volume of 10 µL.
2.4. Inhibitory Effect of I. okamurae on AGE Formation
The inhibiting ability of IOE on AGE formation via the Maillard reaction was evaluated according to the method described by Do et al. [
16], with slight modifications. BSA (10 mg/mL), glucose (100 mM) and fructose (100 mM) were incubated in phosphate-buffered saline (PBS, pH 7.4 with 0.02% sodium azide), in the presence or absence of AG (0.5 mM) or IOE (5, 20, and 100 μg/mL) at 37 °C for 7 day. AGE formation level was measured at an excitation wavelength of 350 nm and an emission wavelength of 450 nm, using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The AGE formation level was expressed as a percentage (%) decrease in the fluorescence intensity of the control (without test samples).
2.5. AGE Cross-Link Breaking Effect of I. okamurae
The effect of IOE on breaking AGE-BSA-induced cross-linking with collagen was evaluated according to the method of Kim et al. [
17] with slight modifications. To confirm the IOE-induced breaking activity of AGE-BSA-induced cross-linking of collagen, 1 mg/mL of AGE-BSA was added to collagen-coated 96-well plates and incubated at 37 °C for 4 h, following which the AGE-BSA complexes with collagen were incubated in either the presence or absence of ALT-711 or IOE at 37 °C for 18 h. The unattached AGE-BSA was washed with 0.05% PBST. Breaking levels were detected using TMB substrate. Breaking of AGE-induced cross-links was expressed in terms of percentage decrease in optical density.
2.6. AGE Cross-Link Inhibitory Effect of I. okamurae
The inhibitory effect of IOE on AGE-BSA-induced cross-linking to collagen was evaluated according to the method described by Do et al. [
18], with slight modifications. To confirm the inhibitory effect of IOE on AGE-induced cross-linking to collagen, 1 mg/mL of AGE-BSA was incubated with AG (0.5 mM) or IOE (5, 20, and 100 μg/mL) on collagen-coated 96-well plates at 37 °C for 18 h. The inhibitory ability of IOE was detected using TMB as a substrate. Inhibition of AGE-induced cross-linking was expressed in terms of percentage decrease in optical density.
2.7. Cell Culture
Mouse glomerular mesangial cells were cultured in a 3:1 mixture of DMEM/F-12 medium containing 5% FBS, 14mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in 5% CO2 and 95% air.
2.8. Nephroprotective Effect of I. okamurae
The nephroprotective effects of IOE were determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay. Mesangial cells were seeded at a density of 3.0 × 104 cells per well in a 96-well plate with 100 μL of DMEM/F12 medium containing 5% FBS and incubated for 6 h before sample treatment. The supernatant was removed and the cells were pretreated with various concentrations of IOE for 1 h, followed by treatment with MGO for 24 h. After removing the medium with samples, 0.5 mg/mL MTT reagent was added to the cells and incubated for 3 h at 37 °C. The resulting formazan product was dissolved in dimethyl sulfoxide. The number of viable cells was determined by measuring the absorbance at 570 nm (detection wavelength) and 630 nm (reference wavelength) using a microplate reader (Infinite M200; Tecan Austria GmbH, Grödig, Austria). Viability was expressed in terms of percentage (%) of absorbance of the control cells (without MGO and IOE).
2.9. Intracellular ROS Scavenging Activity of I. okamurae
The intracellular antioxidant capacity was determined using a fluorescent probe (2′,7′-dichlorofluorescin diacetate or DCFH-DA). Mouse glomerular mesangial cells were seeded at a density of 3.0 × 104 cells per well in a 96-well plate with 100 μL of DMEM/F12 medium containing 5% FBS and incubated for 6 h. The supernatant was subsequently removed, following which the cells were treated with various concentrations of IOE for 1 h, and then treated with MGO for 2 h. The cells were then washed twice with Hank’s balanced salt solution (HBSS) and incubated with 10 μM DCFH-DA in HBSS for 30 min. Fluorescence was measured using an Infinite M200 microplate reader. ROS production levels in the cells were measured at excitation and emission wavelengths of 485 and 530 nm, respectively. The degree of intracellular ROS levels was expressed as percentage (%) of the non-treated group (without MGO and IOE).
2.10. MGO-Derived AGE Concentration
The ability of IOE to inhibit intracellular AGE accumulation was evaluated by measuring the concentrations of MGO-cross-linked proteins using the OxiSelect™ Methylglyoxal (MG) Competitive ELISA Kit (Cell biolabs, San Diego, CA, USA). Mesangial cells were seeded at a density of 1.0 × 106 cells per 6-well plate and treated with MGO, with or without IOE, for 24 h. The cells were then collected and lysed to measure the MGO-derived AGE concentrations using an ELISA kit, by measuring the absorbance at 450 nm using an Infinite M200 microplate reader.
2.11. Apoptosis Analysis
Induction of apoptosis was measured using the Muse™ Annexin V & Dead Cell Kit (Luminex, Austin, TX, USA). Mesangial cells were seeded at a density of 1.0 × 106 cells per 6-well plate with 2 mL of DMEM/F12 medium containing 5% FBS and incubated for 6 h. Cells were pretreated with various concentrations of IOE for 1 h. Following that, MGO was added to the wells to induce apoptosis for 23 h. After removing the medium with samples and MGO, the cells were washed with cold HBSS and diluted to a density of 500 cells/μL prior to staining. Cells were resuspended in DMEM/F12 medium, added to the Muse™ Annexin V & Dead Cell Reagent, and then incubated for 20 min at room temperature in the dark. The assay results were measured using a Muse™ Cell Analyzer (Merck Millipore, Sydney, Australia). Results have been expressed in terms of percentage of total apoptotic cells (early and late apoptotic cells), percentage of live cells, and percentage of dead cells.
2.12. Western Blotting
The pretreated cells were scraped, and intracellular proteins were extracted using PRO-PREP™ Protein Extraction Solution for Cell/Tissue containing 1% protease and phosphatase (Thermo Fisher Scientific, Rockford, IL, USA). First, the protein contents were measured using a DC Protein Assay (Bio-Rad, Hercules, CA, USA), following which the protein content of each group was diluted to the same concentration. After being bathed in a metal bath at 100 °C for 5 min, the protein was cooled to room temperature and used as a backup. The remaining protein samples were stored at −80 °C until use. The diluted protein samples were subjected to electrophoresis at 200V using Any kD™ Mini-PROTEAN® TGX Stain-Free™ Gels, following which the separated proteins were transferred to the Trans-Blot® Turbo™ RTA Transfer Kit polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5% skim milk in Tris-buffered saline with 0.1% Tween-20 for 1 h. The membranes were then incubated with primary antibodies (used at a dilution of 1:1000) with weak shaking overnight at 4 °C. After incubation, the membranes were incubated with HRP-labeled goat anti-rabbit IgG or goat anti-mouse IgG (used at a dilution of 1:5000) for 1 h at room temperature. Finally, the membrane was exposed to WesternSure® Chemiluminescent Substrate (LI-COR, Lincoln, NE, USA), and luminescence was detected using ChemiDoc™ (Bio-Rad, Hercules, CA, USA).
2.13. Statistical Analysis
All experiments were independently performed in triplicate, and data are presented as mean ± standard deviation (n = 3). To assess the significant differences in the mean values, one-way analysis of variance was performed followed by the Tukey’s multiple comparisons test (p < 0.05) using GraphPad Prism ver. 9 (GraphPad Software, Inc., San Diego, CA, USA).
4. Discussion
Under hyperglycemic conditions, the carbonyl group of sugar and the free amino group of biological proteins become AGEs. During glycation, reducing sugars are dicarbonyl compounds, including MGO, glyoxal, and 3-deoxyclucosone. Of these, MGO is the most reactive compound, and well known for its glycation potency [
7]. MGO has been shown to induce aging, retinopathy, cardiovascular diseases, and renal dysfunction via dicarbonyl stress in vivo [
6]. In addition, elevated levels of MGO in Drosophila have been shown to induce fatty acid synthase, hyperglycemia, MGO-adducts, and insulin resistance [
21]. Moreover, accumulation of AGEs such as MGO in the tissues is implicated in AGE-related diabetes complications because it alters enzyme activity, reduces ligand binding, modifies protein half-life, and alters immunogenicity [
22]. Therefore, inhibiting the accumulation of AGEs, suppressing the production of AGEs, inhibiting/breaking cross-links between AGEs and proteins, or reducing AGE-induced oxidative stress is believed to be an effective strategy to prevent and delay AGE-related diabetic complications.
I. okamurae, an edible brown alga, is known to be effective in ameliorating blood glucose levels and insulin resistance, as well as, exhibiting strong antioxidant activity [
13,
23]. Several previous studies have reported that natural plant resources, especially those with potent antioxidant compounds appear to play a vital action in the improvement of disorders involving oxidative stress-related diabetes mellitus [
24]. Additionally, DPHC and IPA, which are representative compounds derived from
I. okamurae, are known to suppress elevated blood glucose levels after a meal and improve glucose homeostasis [
13,
15]. Therefore, we determined the DPHC and IPA contents of
I. okamurae using HPLC analysis (
Figure 1). In our study, IOE showed anti-glycation properties by inhibiting AGE formation and breaking/inhibiting AGEs-collagen cross-links (
Figure 2). These results indicated that
I. okamurae inhibits AGEs through a variety of mechanisms, suggesting that it is effective in preventing and delaying AGE-related diabetes complications.
Chronic hyperglycemic conditions further accelerate this reaction and increase the accumulation of AGEs in body tissues. AGE accumulation has been implicated in the development of insulin resistance and AGE-related diabetic complications. MGO treatment increases intracellular MGO concentrations and MGO-derived AGE concentrations, subsequently causing cellular damage and apoptotic cell death by oxidative stress in diverse cells [
25]. In addition, MGO, which is a reactive dicarbonyl compound of AGEs, increases intracellular oxidative stress and induces apoptosis [
7]. In the present study,
I. okamurae treatment exhibited protective effects against MGO-induced renal damage and intracellular ROS accumulation in mouse kidney mesangial cells (
Figure 3). In addition,
I. okamurae not only significantly reduced intracellular MGO accumulation, but also inhibited apoptotic cell death mediated by MGO-induced oxidative stress (
Figure 4). Therefore, our results demonstrated that IOE protects against MGO-induced cytotoxicity by downregulating MGO-induced ROS generation, MGO-cross-linked protein concentration, and apoptotic cell death.
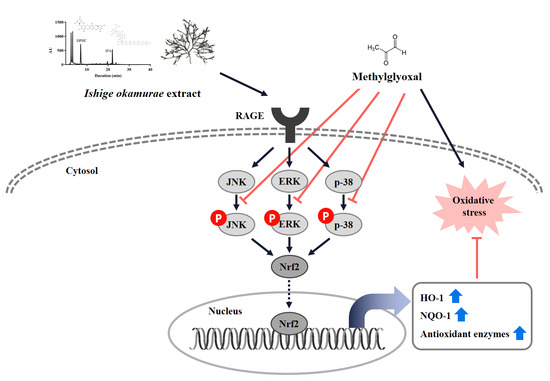
Our results indicated that IOE significantly reduced the level of apoptosis caused by MGO, but the mechanism of action was not clear. We hypothesized that certain signaling pathways activated by MGO may be involved in the mechanism of action. To determine whether RAGE plays an important role in MGO-induced cell dysfunction, we first measured the protein expression of the AGE receptor RAGE (
Figure 5). RAGE expression increased upon MGO treatment, but significantly decreased upon IOE treatment. The interaction of AGEs with RAGE enhances oxidative stress through ROS production by NADPH oxidases inside the mitochondria [
26].
We hypothesized that IOE would suppress MGO-induced toxicity by causing a change in the Nrf2 signaling pathway while reducing RAGE expression. Nrf2 is an essential regulator of ARE-mediated induction, including the regulation of antioxidant enzymes such as SOD, NQO1, and catalase. Several studies have reported that Nrf2 regulates AGE-induced inflammatory cytokine concentrations and oxidative stress [
27]. The transcription factor Nrf2 normally forms a complex with the cytoskeleton-associated protein Keap1 in the cytoplasm. When cells are exposed to oxidative stress, the Nrf2/Keap1 interaction is broken and free Nrf2 translocates into the nucleus to activate ARE-dependent genes [
28,
29]. To determine whether IOE can activate Nrf2 under MGO treatment, we performed a Western blot with MGO-treated samples. In our study, MGO treatment significantly reduced Nrf2 protein expression and its downstream pathway molecules (
Figure 6). However, IOE treatment significantly increased Nrf2 protein expression and the expression of antioxidant enzymes such as HO-1, NQO1, CAT, and SOD1. Therefore, our results suggest that IOE has an inhibitory effect on MGO-induced cytotoxicity by regulating the Nrf2 signaling pathway and reducing ROS production in renal cells.
MAPKs play an important role in cell differentiation and apoptosis. ERK, JNK, and p38 are major proteins in the MAPK group. ERK is associated with the proliferation and progression of certain cellular systems, but JNK, p38, and ERK are also associated with apoptosis [
30,
31]. In this study, we observed that pretreatment with IOE most dramatically inhibited the activation of ERK, JNK, and p38 (
Figure 7). Inhibition of apoptosis by IOE was accompanied by inhibition of MAPK activation, suggesting that IOE can regulate the MAPK signaling pathway in MGO-treated intermediate cells. MAPKs can be activated independently and are involved in cell death. In recent years, it has been suggested that MGO-induced cytotoxicity is associated with the activation of members of the MAPK family, including ERK, JNK, and p38 [
25]. Therefore, our results suggest that IOE has an inhibitory effect on MGO-induced cytotoxicity by regulating the Nrf2/ARE pathway and reducing ROS generation in renal cells.
An increase in the levels or accumulation of intracellular AGEs has been reported as one of the immediate causes of diabetes development and its related complications. Therefore, many researchers have attempted to prevent/improve/treat AGE-related diabetes and complications using natural resources or active compounds derived from them, as these present a low risk of side effects. Our results showed that I. okamurae, an edible brown alga, effectively inhibited various processes related to the production and accumulation of AGEs through actions such as inhibition of AGE formation, inhibition/breaking of AGE-protein cross-linking. In addition, it was demonstrated that I. okamurae exerts nephroprotective effects by reducing MGO-induced ROS generation and regulating the expression of proteins involved in the RAGE and Nrf2/ARE pathways in mesangial cells. Therefore, I. okamurae is considered a very useful natural resource with the potential to prevent and treat diabetic complications such as AGE-related diabetic nephropathy. However, since various physiologically active compounds exist in I. okamurae, additional studies using single phlorotannin compounds contained in I. okamurae are needed to clarify its efficacy. In addition, in order to verify the efficacy of the phlorotannin compound contained in I. okamurae for AGE-induced nephropathy, an in vivo experiment using an animal model is necessary.