Sensory Quality Evaluation of Superheated Steam-Treated Chicken Leg and Breast Meats with a Combination of Marination and Hot Smoking
Abstract
1. Introduction
2. Materials and Methods
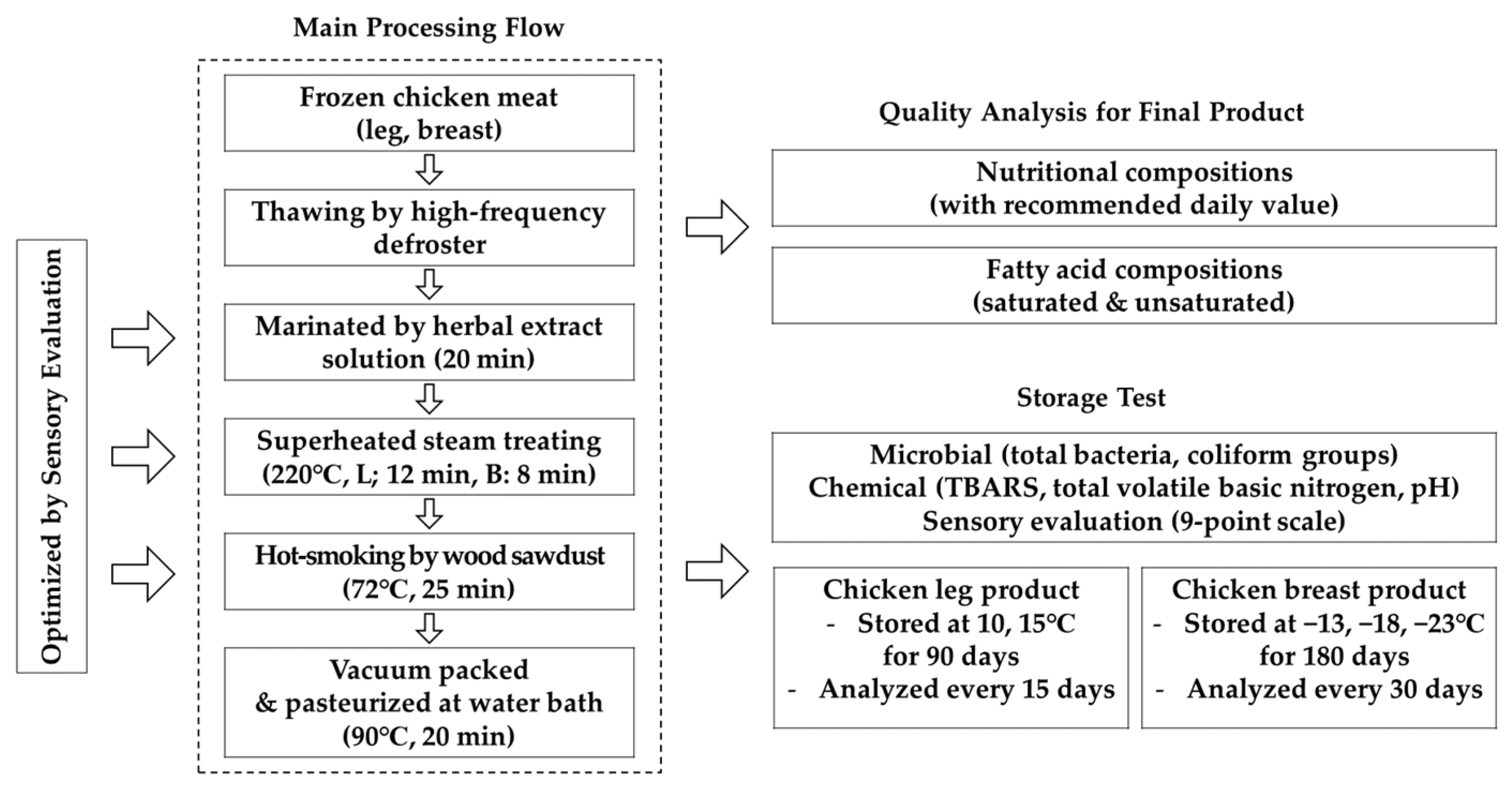
2.1. Sample Preparation
2.2. Drip Loss Measurement
2.3. Preparation for Treatment
2.4. Experiment for Optimization of Superheated Steam Treatment
2.5. Sensory Evaluation
2.6. Microbial Analysis
2.7. Thiobarbituric Acid Reactive Substances
2.8. Total Volatile Basic Nitrogen
2.9. Hydrogen Ion Concentration (pH)
2.10. Analysis of Nutritional Quality
2.11. Fatty Acid Analysis
2.12. Storage Quality Analysis
2.13. Statistical Analysis
3. Results and Discussion
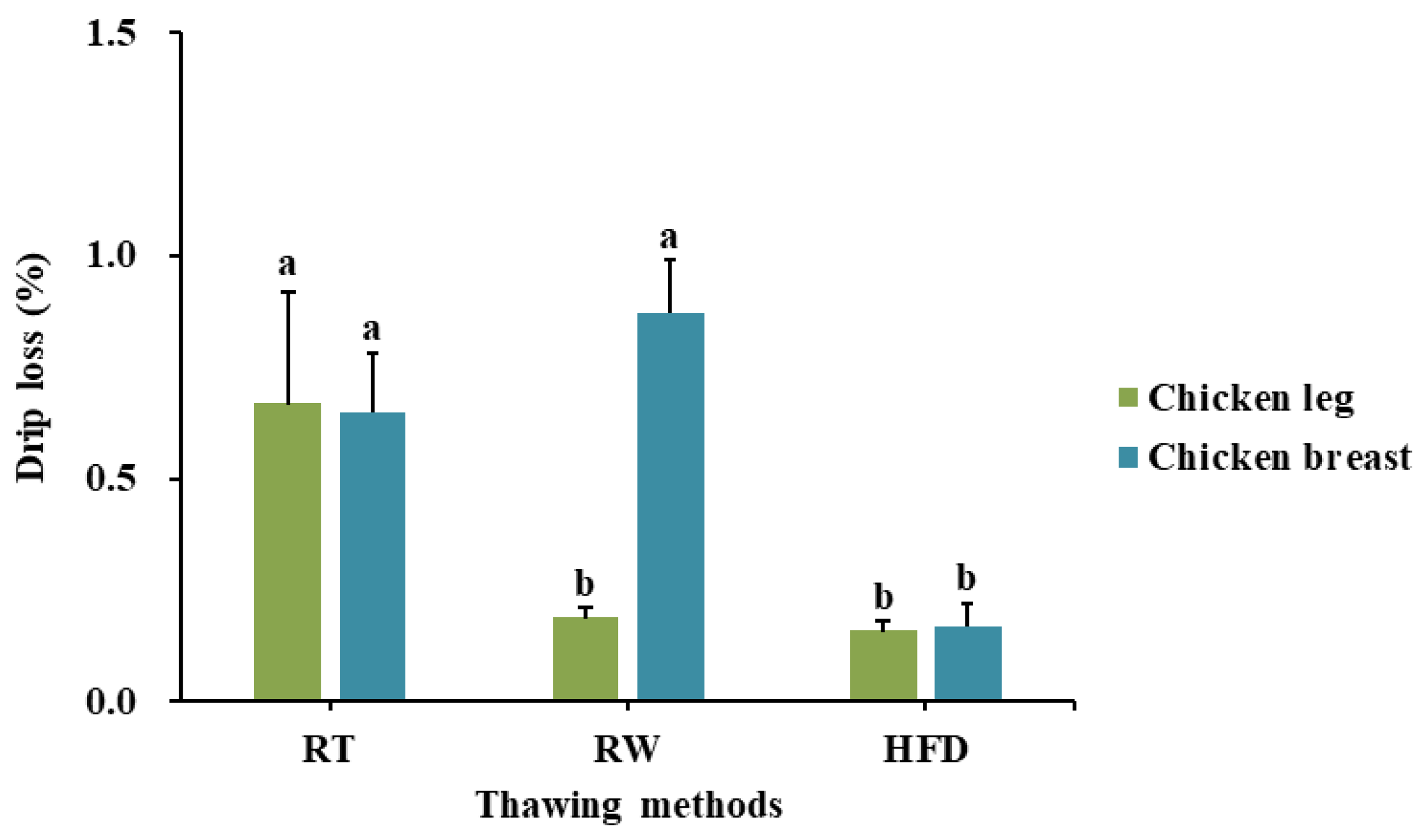
3.1. Effect of High-Frequency Defrosting on Variation in Drip Loss
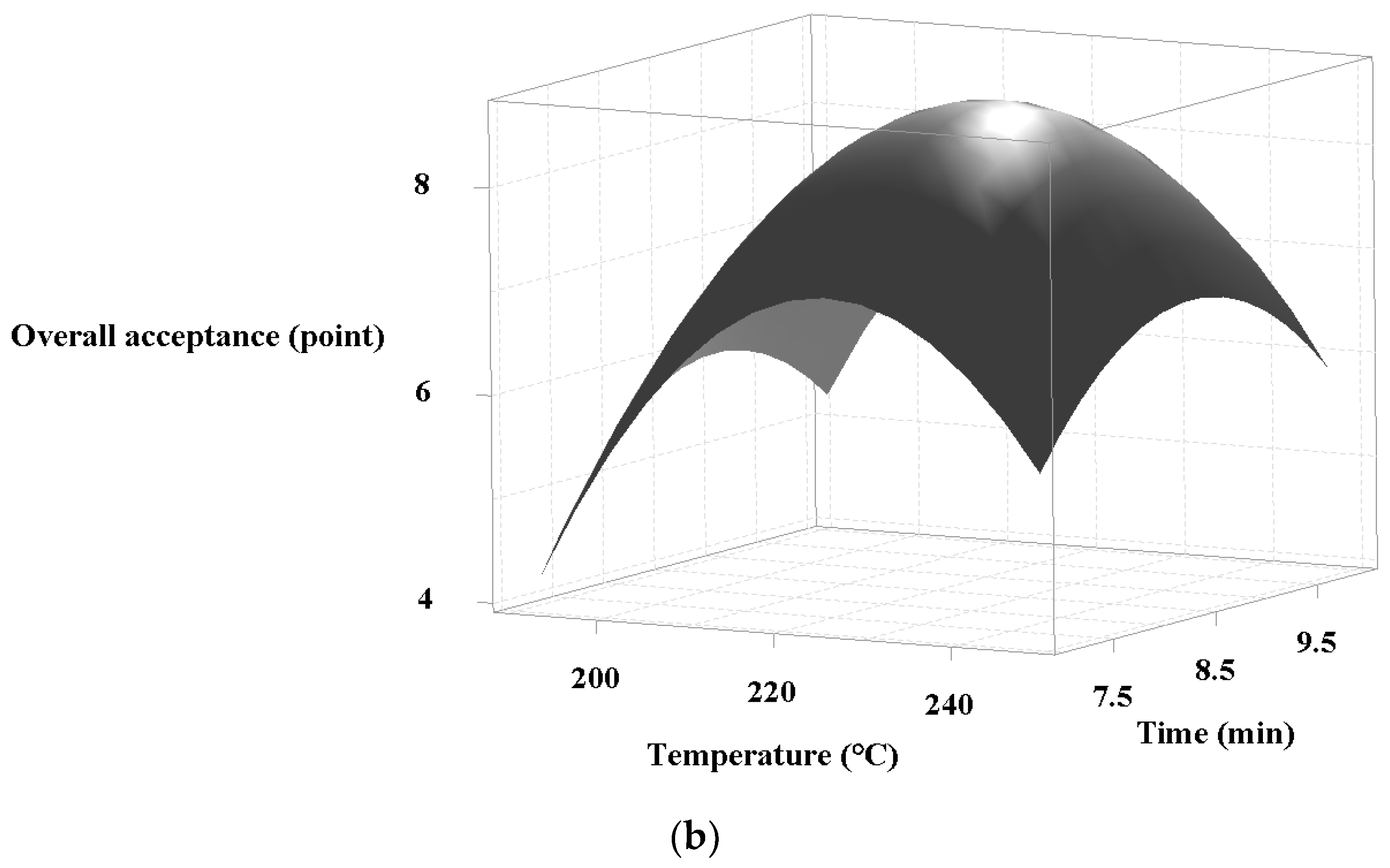
3.2. Optimization of Superheated Steam Treatment of Chicken Leg and Breast
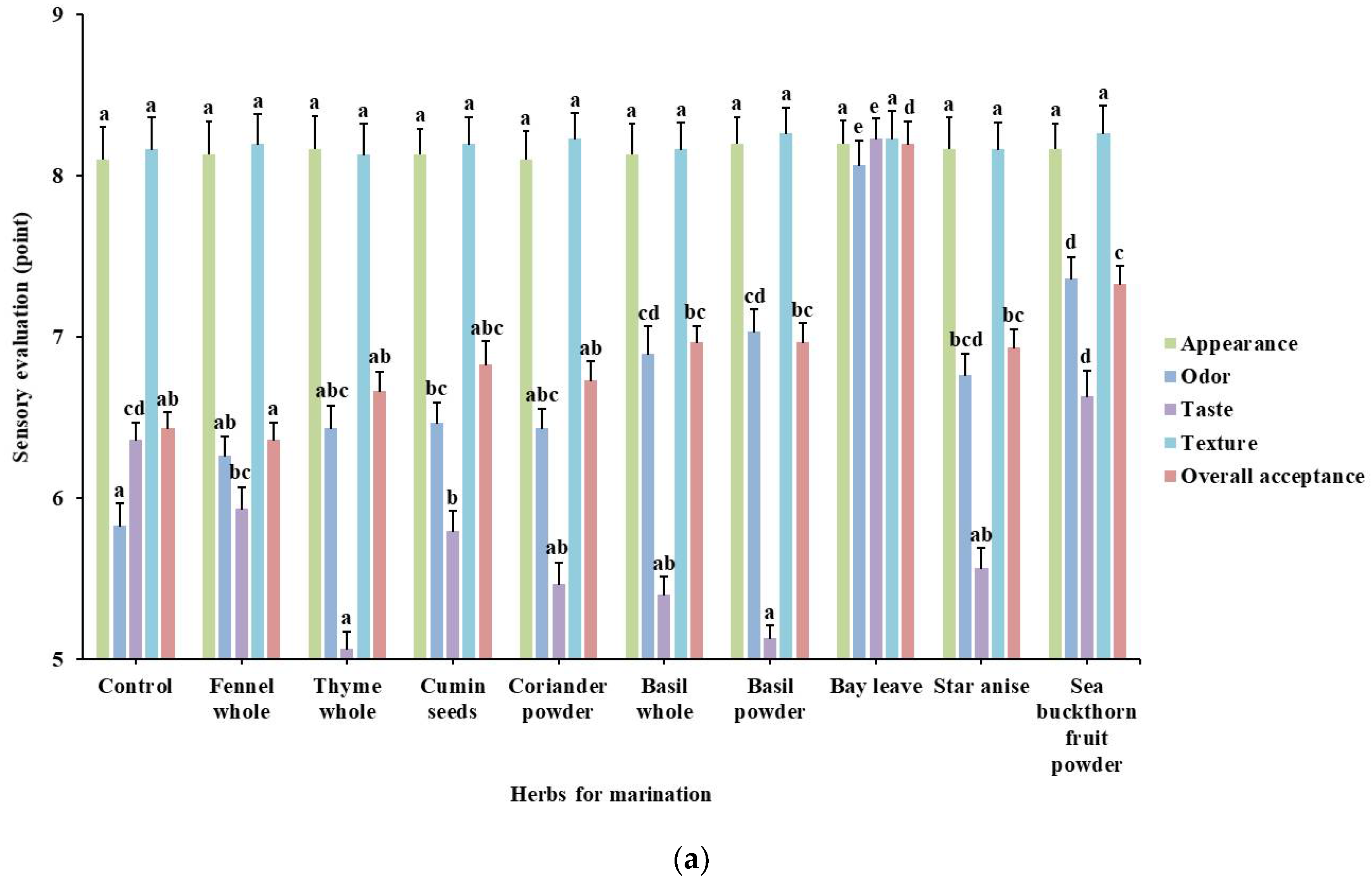
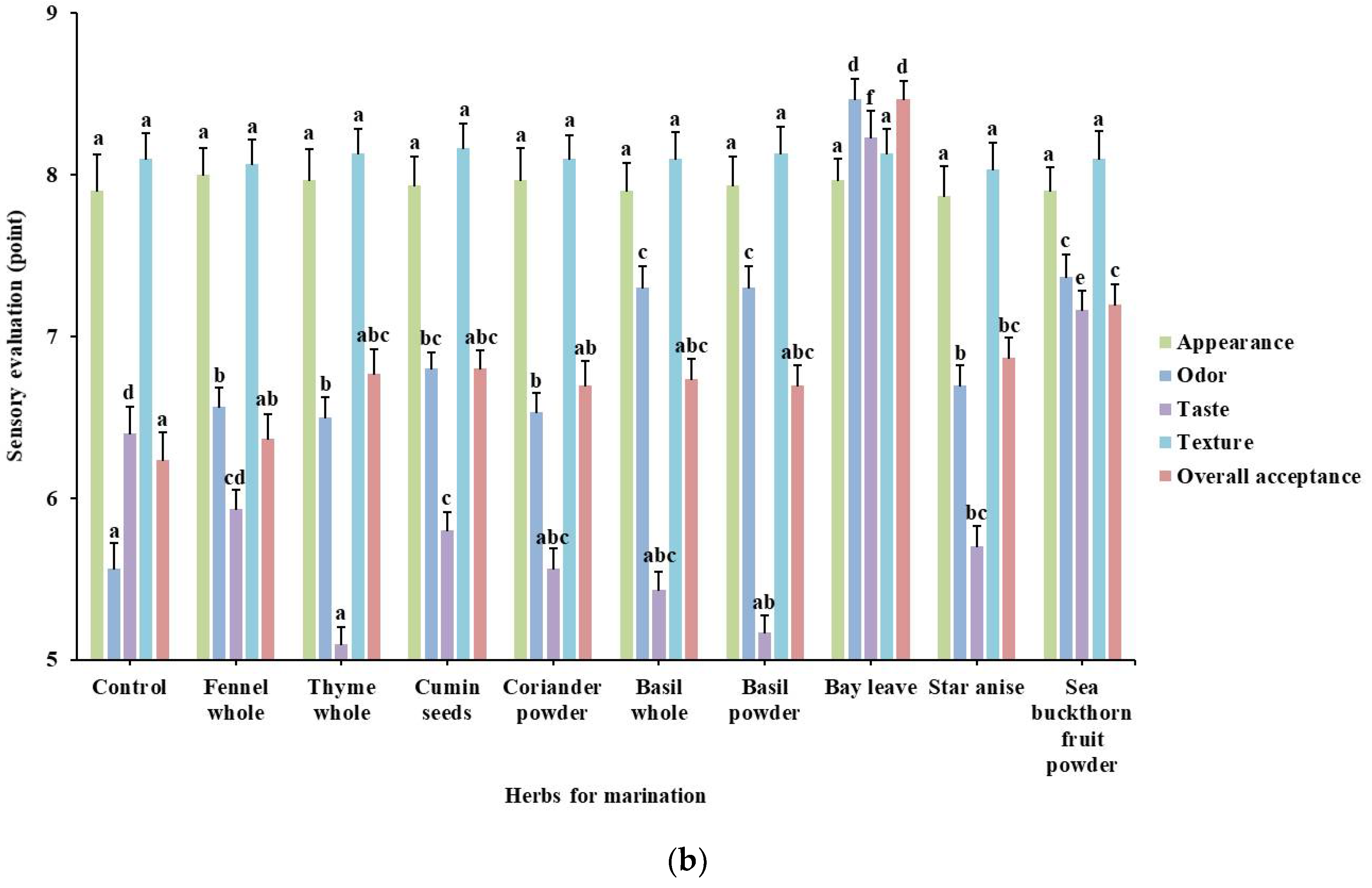
3.3. Effect of Marination with Herbal Extract Solutions on Sensory Evaluation
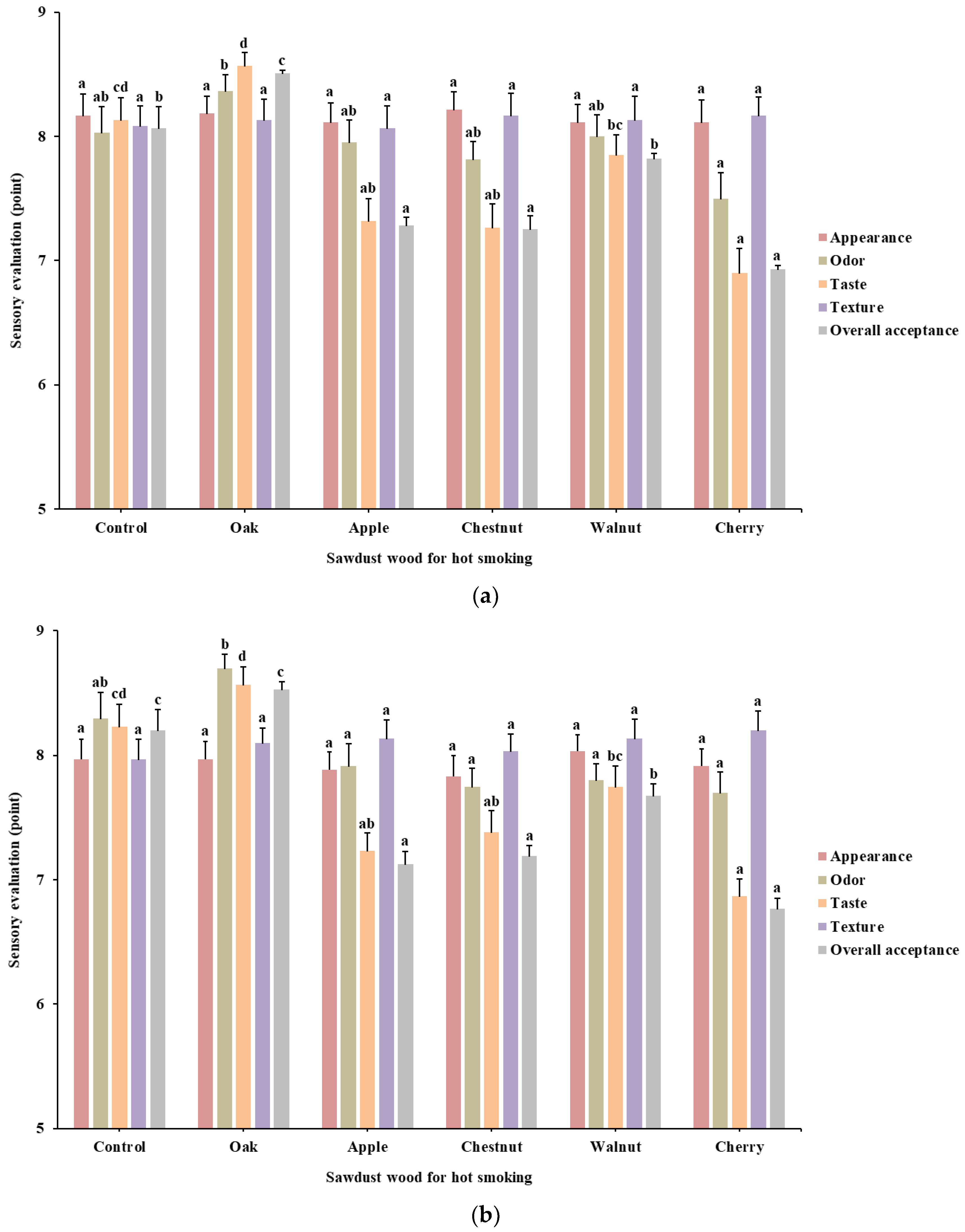
3.4. Hot Smoking Treatment
3.5. Fatty Acid Analysis
3.6. Nutritional Value
3.7. Effect of Storage Conditions on Processed Chicken Meats
3.7.1. Sensory Qualities
3.7.2. Microbial Qualities
3.7.3. Physicochemical Qualities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, I.; Joo, S.-T. Poultry Meat Quality in Relation to Muscle Growth and Muscle Fiber Characteristics. Food Sci. Anim. Resour. 2017, 37, 873–883. [Google Scholar] [CrossRef]
- Jung, S.B.; Kim, T.H.; Son, T.Y.; Yu, E.S.; Shin, J.Y.; Jung, J.Y.; Hwang, J.W.; Yang, J.Y. A Study on Development of the High Frequency Thawing Machine. J. Korean Soc. Ind. Converg. 2018, 21, 301–307. [Google Scholar] [CrossRef]
- Oh, J.-H.; Yoon, S.; Choi, Y. The Effect of Superheated Steam Cooking Condition on Physico-Chemical and Sensory Characteristics of Chicken Breast Fillets. Korean J. Food Cook. Sci. 2014, 30, 317–324. [Google Scholar] [CrossRef]
- Lunde, K.; Egelandsdal, B.; Choinski, J.; Mielnik, M.; Flåtten, A.; Kubberød, E. Marinating as a technology to shift sensory thresholds in ready-to-eat entire male pork meat. Meat Sci. 2008, 80, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Milly, P.J. Antimicrobial Properties of Liquid Smoke Fractions. Doctoral Dissertation, University of Georgia, Athens, GA, USA, December 2003. [Google Scholar]
- Asita, A.O.; Campbell, I.A. Anti-microbial activity of smoke from different woods. Lett. Appl. Microbiol. 1990, 10, 93–95. [Google Scholar] [CrossRef]
- Pereira, P.A.P.; De Salvia, M.A.; Carneiro, J.D.D.S. Sensory perception of Brazilian petit suisse cheese by a consumer panel using three-way internal and external preference maps. MOJ Food Process. Technol. 2020, 8, 109–112. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Park, Y.; Won, N.E.; Kim, H.; An, J.H.; Jeon, J.; Park, S.; Yoon, S.; Sohn, J.H.; Kim, J.; et al. Effect of steaming and hot smoking treatment combination on the quality characteristics of hagfish (Myxine glutinosa). J. Food Process. Preserv. 2020, 44, 14694. [Google Scholar] [CrossRef]
- Jaffe, T.R.; Wang, H.; Chambers, E. Determination of a lexicon for the sensory flavor attributes of smoked food products. J. Sens. Stud. 2017, 32, e12262. [Google Scholar] [CrossRef]
- AOAC International. Method of Analysis–Official methods 990.12. In International Official Methods of Analysis Official Methods, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2002. [Google Scholar]
- AOAC International. Method of Analysis–Official methods 991.14. In International Official Methods of Analysis Official Methods, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2002. [Google Scholar]
- Yildiz, P.O. Effect of Essential Oils and Packaging on Hot Smoked Rainbow Trout during Storage. J. Food Process. Preserv. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Mohibbullah; Won, N.E.; Jeon, J.-H.; An, J.H.; Park, Y.; Kim, H.; Bashir, K.M.I.; Park, S.-M.; Kim, Y.S.; Yoon, S.-J.; et al. Effect of superheated steam roasting with hot smoking treatment on improving physicochemical properties of the adductor muscle of pen shell (Atrina pectinate). Food Sci. Nutr. 2018, 6, 1317–1327. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). 8th General Analysis Method. Foodcode (Sik-Poom-Gong-Jeon); MFDS: Cheongju, Korea, 2020; Available online: https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=11142 (accessed on 23 June 2021).
- Ministry of Food and Drug Safety (MFDS). 4th General Analysis Method. Food additives code (Sik-Poom-Cheom-Ga-Mul-Gong-Jeon); MFDS: Cheongju, Korea, 2020; Available online: https://www.foodsafetykorea.go.kr/foodcode/04_03.jsp?idx=878 (accessed on 23 June 2021).
- AOAC International. Method of Analysis–Official methods 925.09, 923.03, 979.09, 962.09, and 923.05. In International Official Methods of Analysis Official Methods, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- AOAC International. Method of Analysis–Official methods 963.22. In International Official Methods of Analysis Official Methods, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). The Guidelines for Shelf-life Experiment of Food, Livestock, and Health & Functional Food, 5th ed.; MFDS: Cheongju, Korea, 2020; Available online: https://www.mfds.go.kr/brd/m_1060/view.do?seq=14285&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed on 12 August 2021).
- Yu, L.-H.; Lee, E.-S.; Chen, H.-S.; Jeong, J.-Y.; Choi, Y.-S.; Lim, D.-G.; Kim, C.-J. Comparison of Physicochemical Characteristics of Hot-boned Chicken Breast and Leg Muscles during Storage at 20 °C. Food Sci. Anim. Resour. 2011, 31, 676–683. [Google Scholar] [CrossRef]
- Jung, S.B.; Seo, T.R.; Jung, H.J.; Kim, B.K.; Cho, Y.J. Effect of High Frequency Thawing and General Thawing Methods on the Quality of Frozen Mackerel, Alaska pollack, Japanese Spanish mackerel, and Yellow croaker. J. FISHRIES Mar. Sci. Educ. 2016, 28, 1152–1158. [Google Scholar] [CrossRef][Green Version]
- Park, D.H.; Lee, S.; Kim, H.Y.; Seo, J.-H.; Lee, J.; Kim, S.; Shin, H.; Choi, M.-J. The Effect of Packaging, Freezing Method, and Thawing Method on the Quality Properties of Blanched Colocasia esculenta (L.) Schott Stem. Food Eng. Prog. 2016, 20, 135–142. [Google Scholar] [CrossRef]
- Joglekar, A.M.; May, A.T. Product excellence through design of experiments. Cereal Foods World 1987, 32, 857–868. [Google Scholar]
- Zimmermann, W.J. An Approach to Safe Microwave Cooking of Pork Roasts Containing Trichinella spiralis. J. Food Sci. 1983, 48, 1715–1718. [Google Scholar] [CrossRef]
- Kurup, A.H.; Deotale, S.; Rawson, A.; Patras, A. Thermal Processing of Herbs and Spices. Herbs, Spices Med. Plants. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119036685.ch1 (accessed on 1 June 2021). [CrossRef]
- Lee, J.H.; An, L.H. Effects of Herbs and Green Tea on the Sensory and the Antioxidative Qualities of Beef-Yukwonjeon. J. of the East Asian Soc. Diet. Life 2007, 17, 808–815. [Google Scholar]
- Migdał, W.; Zając, M.; Węsierska, E.; Tkaczewska, J.; Kulawik, P. PROCEEDINGS. Available online: http://istocar.bg.ac.rs/wp-content/uploads/2015/12/Proceedings-2015-2.pdf (accessed on 24 June 2021).
- Ratnani, R.D. Widiyanto A Review of Pyrolisis of Eceng Gondok (Water hyacinth) for Liquid Smoke. In Proceedings of the E3S Web of Conferences, Semarang, Indonesia, 14–15 August 2018; EDP Sciences: Les Ulis, France, 18 August 2018; Volume 73, p. 05010. [Google Scholar]
- Kralik, G.; Kralik, Z.; Grčević, M.; Hanžek, D. Quality of Chicken Meat. Anim. Husb. Nutr. 2018, 63. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Whittington, F.M.; Richardson, R.I. Effects of diets on fatty acids and meat quality. Options Méditerr. Ser. A 2003, 67, 133–141. [Google Scholar]
- Smith, S.B. Marbling and Its Nutritional Impact on Risk Factors for Cardiovascular Disease. Food Sci. Anim. Resour. 2016, 36, 435–444. [Google Scholar] [CrossRef]
- Kim, Y.H.; Min, J.S.; Hwang, G.S.; Lee, S.O.; Kim, I.S.; Bark, H.I.; Lee, M.H. Fatty acids composition and sensory characteristics of the commercial native chicken meat. Korean J. Food Sci. Technol. 1999, 31, 964–970. [Google Scholar]
- Northcutt, J.; A Foegeding, E.; Edens, F. Water-Holding Properties of Thermally Preconditioned Chicken Breast and Leg Meat. Poult. Sci. 1994, 73, 308–316. [Google Scholar] [CrossRef]
- Koh, H.-Y.; Yu, I.-J. Nutritional Analysis of Chicken Parts. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1028–1034. [Google Scholar] [CrossRef]
- Industry Resources on the Changes to the Nutrition Facts Label. Available online: https://www.fda.gov/food/food-labeling-nutrition/industry-resources-changes-nutrition-facts-label (accessed on 25 June 2021).
- Richter, S. Investigating Ice slurry’s Perceived Mechanical Abrasive Quality to Increase Pathogen Reduction on Poultry during Immersion Chilling. Doctoral Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 26 April 2018. [Google Scholar]
- Tompkin, R.B.; Christiansen, L.N.; Shaparis, A.B. Iron and the antibotulinal efficacy of nitrite. Appl. Environ. Microbiol. 1979, 37, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, J.; Li, C.; Ye, H.; Wang, Z.; Wu, X.; Xu, B. Evolution of Volatile Compounds and Spoilage Bacteria in Smoked Bacon during Refrigeration Using an E-Nose and GC-MS Combined with Partial Least Squares Regression. Molecules 2018, 23, 3286. [Google Scholar] [CrossRef] [PubMed]
- Katiyo, W.; De Kock, R.; Coorey, R.; Buys, E.M. Sensory implications of chicken meat spoilage in relation to microbial and physicochemical characteristics during refrigerated storage. LWT 2020, 128, 109468. [Google Scholar] [CrossRef]
- Kilcast, D.; Lewis, D.F. Structure and texture—their importance in food quality. Nutr. Bull. 1990, 15, 103–113. [Google Scholar] [CrossRef]
- Pande, G.; Akoh, C.C. Enzymatic modification of lipids fortrans-free margarine. Lipid Technol. 2013, 25, 31–33. [Google Scholar] [CrossRef]
- Olsson, G.B.; Ofstad, R.; Lødemel, J.B.; Olsen, R.L. Changes in water-holding capacity of halibut muscle during cold storage. LWT 2003, 36, 771–778. [Google Scholar] [CrossRef]
- Mathew, R.; Jaganathan, D.; Anandakumar, S. Effect of vacuum packaging method on shelf life of chicken. Imp. J. Interdiscip. Res. 2016, 2, 1859–1866. [Google Scholar]
- Kondjoyan, A.; Portanguen, S. Effect of superheated steam on the inactivation of Listeria innocua surface-inoculated onto chicken skin. J. Food Eng. 2008, 87, 162–171. [Google Scholar] [CrossRef]
- Heiszler, M.G.; Kraft, A.A.; Rey, C.R.; Rust, R.E. Effect of time and temperature of smoking on microorganisms on frankfurters. J. Food Sci. 1972, 37, 845–849. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yu, Z. Rapid evaluation of chicken meat freshness using gas sensor array and signal analysis considering total volatile basic nitrogen. Int. J. Food Prop. 2020, 23, 297–305. [Google Scholar] [CrossRef]
- Holman, B.W.; Bekhit, A.E.-D.A.; Waller, M.; Bailes, K.L.; Kerr, M.J.; Hopkins, D.L. The association between total volatile basic nitrogen (TVB-N) concentration and other biomarkers of quality and spoilage for vacuum packaged beef. Meat Sci. 2021, 179, 108551. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety (MFDS). 5th Standards and Specifications by Food. Foodcode (Sik-Poom-Gong-Jeon); MFDS: Cheongju, Korea, 2020; Available online: https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=54 (accessed on 3 July 2021).
- Effect of Methodology on the Determination of Total Volatile Basic Nitrogen as an Index of Quality of Meat and Fish. Egypt. J. Food Saf. 2013, 1, 23–34. [CrossRef]
- Altissimi, S.; Mercuri, M.L.; Framboas, M.; Tommasino, M.; Pelli, S.; Benedetti, F.; Di Bella, S.; Haouet, N. Indicators of protein spoilage in fresh and defrosted crustaceans and cephalopods stored in domestic condition. Ital. J. Food Saf. 2017, 6, 6921. [Google Scholar] [CrossRef]
- Choe, J.; Kim, H.-Y. Physicochemical characteristics of breast and thigh meats from old broiler breeder hen and old laying hen and their effects on quality properties of pressed ham. Poult. Sci. 2020, 99, 2230–2235. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Hwang, K.-E.; Jeong, T.-J.; Kim, Y.-B.; Jeon, K.-H.; Kim, E.-M.; Sung, J.-M.; Kim, H.-W.; Kim, C.-J. Comparative Study on the Effects of Boiling, Steaming, Grilling, Microwaving and Superheated Steaming on Quality Characteristics of Marinated Chicken Steak. Food Sci. Anim. Resour. 2016, 36, 1–7. [Google Scholar] [CrossRef]
- Chun, J.-Y.; Kwon, B.-G.; Lee, S.-H.; Min, S.-G.; Hong, G.-P. Studies on Physico-chemical Properties of Chicken Meat Cooked in Electric Oven Combined with Superheated Steam. Food Sci. Anim. Resour. 2013, 33, 103–108. [Google Scholar] [CrossRef]


| Independent Variables | Symbol | Unit | Meats | Range Level | ||||
|---|---|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | +1 | +1.414 | ||||
| Temperature | X1 | °C | Leg | 192 | 200 | 220 | 240 | 248 |
| Breast | 192 | 200 | 220 | 240 | 248 | |||
| Time | X2 | min | Leg | 11.1 | 11.5 | 12.5 | 13.5 | 13.9 |
| Breast | 7.1 | 7.5 | 8.5 | 9.5 | 9.9 | |||
| Run No. | Coded Values | Chicken Leg | Chicken Breast | |||||
|---|---|---|---|---|---|---|---|---|
| Actual Values | Responses | Actual Values | Responses | |||||
| X1 | X2 | X1 | X2 | YL | X1 | X2 | YB | |
| 1 | −1 | −1 | 200 | 11.5 | 6.95 | 200 | 7.5 | 6.10 |
| 2 | 1 | −1 | 240 | 11.5 | 7.90 | 240 | 7.5 | 6.57 |
| 3 | −1 | 1 | 200 | 13.5 | 7.67 | 200 | 9.5 | 7.14 |
| 4 | 1 | 1 | 240 | 13.5 | 7.10 | 240 | 9.5 | 7.24 |
| 5 | −1.41 | 0 | 192 | 12.5 | 7.14 | 192 | 8.5 | 5.71 |
| 6 | +1.41 | 0 | 248 | 12.5 | 8.05 | 248 | 8.5 | 7.29 |
| 7 | 0 | −1.41 | 220 | 11.1 | 8.00 | 220 | 7.1 | 7.48 |
| 8 | 0 | +1.41 | 220 | 13.9 | 7.38 | 220 | 9.9 | 7.57 |
| 9 | 0 | 0 | 220 | 12.5 | 8.38 | 220 | 8.5 | 8.81 |
| 10 | 0 | 0 | 220 | 12.5 | 8.67 | 220 | 8.5 | 8.33 |
| 11 | 0 | 0 | 220 | 12.5 | 8.67 | 220 | 8.5 | 8.48 |
| Responses | p-Value | ||||
|---|---|---|---|---|---|
| Model | Linear (X1, X2) | Quadratic (X1X1, X2X2) | Interaction (X1X2) | Lack of Fit | |
| YL (R2 = 0.908) | >0.013 | 0.123 | >0.006 | >0.035 | 0.23 |
| YB (R2 = 0.903) | >0.014 | 0.096 | >0.004 | 0.682 | 0.193 |
| Responses | Optimum Conditions | Predicted Value (p) | Experimental Value (E) | E/P | Desirability | |
|---|---|---|---|---|---|---|
| X1 | X2 | |||||
| YL | +0.27 (225.43 °C) | −0.21 (12.29 min) | 8.61 | 8.86 ± 0.14 | 1.03 | 0.97 |
| YB | +0.16 (223.14 °C) | +0.19 (8.69 min) | 8.59 | 8.71 ± 0.18 | 1.01 | 0.93 |
| Fatty Acids | Shorthand | Chicken Leg (100 g) | Chicken Breast (100 g) |
|---|---|---|---|
| Caprylic acid | C8:0 | 0.00 | - |
| Capric acid | C10:0 | 0.00 | 0.00 |
| Lauric acid | C12:0 | 0.00 | 0.00 |
| Myristic acid | C14:0 | 0.07 | 0.03 |
| Pentadecanoic acid | C15:0 | 0.02 | 0.03 |
| Palmitic acid | C16:0 | 3.04 | 0.88 |
| Magaric acid | C17:0 | 0.01 | 0.01 |
| Stearic acid | C18:0 | 0.76 | 0.23 |
| Arachidic acid | C20:0 | 0.01 | 0.00 |
| Heneicosylic acid | C21:0 | 0.01 | 0.00 |
| Behenic acid | C22:0 | 0.01 | 0.00 |
| ∑ SFA 1 | 3.94 | 1.18 | |
| Myristoleic acid | C14:1 | 0.02 | 0.01 |
| Palmitoleic acid | C16:1 | 0.93 | 0.24 |
| Magaoleic acid | C17:1 | 0.01 | 0.01 |
| Oleic acid | C18:1 | 5.30 | 1.44 |
| Eicosenoic acid | C20:1 | 0.01 | 0.00 |
| Eicosadienoic acid | C20:2 | 0.02 | 0.01 |
| Erucic acid | C22:1 | 0.02 | 0.01 |
| ∑ MUFA 2 | 6.33 | 1.73 | |
| Linoleic acid | C18:2 n-6 | 1.87 | 0.52 |
| γ-Linolenic acid | C18:3 n-6 | 0.02 | 0.01 |
| Dihomo γ-Linolenic acid | C20:3 n-6 | 0.00 | 0.00 |
| Arachidonic acid | C20:4 n-6 | 0.07 | 0.04 |
| ∑ n-6 | 1.96 | 0.57 | |
| Linolenic acid | C18:3 n-3 | 0.13 | 0.04 |
| Eicosatrienoic acid | C20:3 n-3 | 0.00 | 0.00 |
| Eicosapentaenoic acid (EPA) | C20:5 n-3 | 0.00 | 0.01 |
| Docosapentaenoic acid (DPA) | C22:5 n-3 | 0.01 | 0.01 |
| Docosahexaenoic acid (DHA) | C22:6 n-3 | 0.00 | 0.03 |
| ∑ n-3 | 0.15 | 0.09 | |
| ∑ PUFA 3 | 2.10 | 0.66 | |
| Total fatty acid (g) | 12.37 | 3.57 | |
| PUFA/SFA | 0.53 | 0.56 | |
| n-6/n-3 | 13.07 | 6.33 |
| Parameters | Chicken Leg (100 g) | Chicken Breast (100 g) | Daily Values 1 | |||
|---|---|---|---|---|---|---|
| Amount | % DV 2 | Amount | % DV 2 | |||
| Ash | g | 1.31 | - | 1.50 | - | - |
| Calories | cal | 241.46 | - | 149.26 | - | - |
| Sodium | g | 0.22 | 9.6 | 0.24 | 10.4 | 2.3 |
| Carbohydrate | g | 0.65 | 0.2 | 1.18 | 0.4 | 275 |
| Sugar | g | 0.62 | 1.2 | 1.00 | 2.0 | 50 |
| Dietary fiber | g | 1.46 | 5.2 | 2.16 | 7.7 | 28 |
| Crude fat | g | 12.46 | 16.0 | 3.58 | 4.6 | 78 |
| Trans fat | g | - | - | - | - | 2 |
| Saturated fat | g | 3.94 | 19.7 | 1.18 | 5.9 | 20 |
| Cholesterol | mg | 138.63 | 46.2 | 75.53 | 25.2 | 300 |
| Crude protein | g | 25.83 | 51.7 | 28.08 | 56.2 | 50 |
| Vitamin D | μg | - | - | - | - | 20 |
| Potassium | g | 0.28 | 6.0 | 0.32 | 6.8 | 4.7 |
| Iron | mg | 5.27 | 29.3 | 3.18 | 17.7 | 18 |
| Calcium | g | 0.15 | 11.5 | 0.08 | 6.2 | 1.3 |
| Temp | Day | Appearance | Odor | Taste | Texture | Overall Acceptance |
|---|---|---|---|---|---|---|
| 10 °C | 0 | 8.48 a ± 0.15 | 8.29 a ± 0.16 | 8.52 a ± 0.13 | 8.24 a ± 0.15 | 8.52 a ± 0.11 |
| 15 | 8.43 a ± 0.13 | 8.19 a,b ± 0.15 | 8.48 a ± 0.13 | 8.24 a ± 0.14 | 8.62 a ± 0.13 | |
| 30 | 8.33 a ± 0.16 | 8.05 a,b ± 0.20 | 7.95 a,b ± 0.15 | 8.43 a ± 0.16 | 8.38 a ± 0.15 | |
| 45 | 8.24 a ± 0.14 | 7.86 a,b,c ± 0.16 | 7.90 a,b ± 0.14 | 8.05 a,b ± 0.18 | 8.14 a ± 0.14 | |
| 60 | 8.10 a ± 0.12 | 7.48 b,c ± 0.18 | 7.86 a,b ± 0.26 | 8.00 a,b ± 0.20 | 8.10 a ± 0.19 | |
| 75 | 7.19 b ± 0.13 | 6.71 d ± 0.20 | 7.33 b ± 0.21 | 7.29 c ± 0.16 | 7.33 b ± 0.14 | |
| 90 | 7.19 b ± 0.16 | 7.24 c,d ± 0.21 | 7.48 b ± 0.18 | 7.48 b,c ± 0.19 | 7.48 b ± 0.13 | |
| 15 °C | 0 | 8.48 a ± 0.15 | 8.29 a ± 0.16 | 8.52 a ± 0.13 | 8.24 a ± 0.15 | 8.52 a ± 0.11 |
| 15 | 8.29 a ± 0.12 | 8.14 a ± 0.13 | 8.10 a,b ± 0.10 | 8.29 a ± 0.14 | 8.29 a ± 0.12 | |
| 30 | 8.19 a ± 0.11 | 8.05 a ± 0.19 | 7.90 a,b ± 0.15 | 7.81 ab ± 0.16 | 8.19 a ± 0.13 | |
| 45 | 8.10 a ± 0.12 | 8.05 a ± 0.15 | 7.71 b,c ± 0.14 | 8.00 a ± 0.15 | 8.05 a ± 0.13 | |
| 60 | 7.33 b ± 0.17 | 6.86 b ± 0.16 | 7.14 c ± 0.19 | 7.24 b ± 0.14 | 7.29 b ± 0.14 | |
| 75 | 7.14 b ± 0.13 | 6.90 b ± 0.17 | 7.10 c ± 0.17 | 7.24 b ± 0.15 | 7.05 b ± 0.13 | |
| 90 | 7.05 b ± 0.15 | 6.90 b ± 0.18 | 7.19 c ± 0.19 | 7.24 b ± 0.12 | 7.19 b ± 0.15 |
| Temp | Day | Appearance | Odor | Taste | Texture | Overall Acceptance |
|---|---|---|---|---|---|---|
| −13 °C | 0 | 8.48 a ± 0.13 | 8.14 a ± 0.13 | 8.33 a ± 0.14 | 8.29 a ± 0.16 | 8.48 a ± 0.13 |
| 30 | 8.43 a,b ± 0.11 | 8.10 a ± 0.12 | 8.19 a,b ± 0.13 | 8.05 a ± 0.15 | 8.10 a,b ± 0.15 | |
| 60 | 8.10 a,b,c ± 0.18 | 7.71 a,b ± 0.17 | 7.95 a,b ± 0.16 | 8.14 a ± 0.17 | 8.24 a,b ± 0.15 | |
| 90 | 8.10 a,b,c ± 0.18 | 7.90 a,b ± 0.14 | 8.05 a,b ± 0.19 | 7.86 a ± 0.17 | 8.19 a,b ± 0.15 | |
| 120 | 7.76 a,b,c ± 0.19 | 7.43 b ± 0.11 | 7.76 a,b ± 0.15 | 7.76 a ± 0.15 | 8.05 a,b ± 0.13 | |
| 150 | 7.67 b,c ± 0.21 | 7.67 a,b ± 0.19 | 7.90 a,b ± 0.17 | 7.57 a ± 0.20 | 7.81 b ± 0.16 | |
| 180 | 7.62 c ± 0.22 | 7.57 a,b ± 0.16 | 7.57 b ± 0.13 | 7.57 a ± 0.20 | 7.76 b ± 0.15 | |
| −18 °C | 0 | 8.48 a ± 0.13 | 8.14 a ± 0.13 | 8.33 a ± 0.14 | 8.29 a ± 0.16 | 8.48 a ± 0.13 |
| 30 | 8.24 a,b ± 0.14 | 8.10 a ± 0.12 | 8.14 a ± 0.16 | 8.24 a ± 0.14 | 8.43 a ± 0.15 | |
| 60 | 8.48 a ± 0.11 | 8.00 a ± 0.14 | 8.10 a ± 0.18 | 8.05 a ± 0.16 | 8.33 a ± 0.13 | |
| 90 | 8.29 a,b ± 0.17 | 7.81 a,b ± 0.15 | 8.10 a ± 0.18 | 8.19 a ± 0.18 | 8.38 a ± 0.15 | |
| 120 | 7.62 b ± 0.19 | 7.29 b ± 0.17 | 7.62 a ± 0.16 | 7.62 a ± 0.18 | 7.90 a ± 0.15 | |
| 150 | 8.05 a,b ± 0.20 | 8.00 a ± 0.18 | 7.62 a ± 0.18 | 7.81 a ± 0.21 | 8.14 a ± 0.19 | |
| 180 | 8.10 a,b ± 0.17 | 7.90 a,b ± 0.15 | 7.95 a ± 0.21 | 7.90 a ± 0.18 | 8.05 a ± 0.16 | |
| −23 °C | 0 | 8.48 a ± 0.13 | 8.14 a ± 0.13 | 8.33 a ± 0.14 | 8.29 a ± 0.16 | 8.48 a ± 0.13 |
| 30 | 8.24 a ± 0.15 | 8.10 a ± 0.17 | 8.10 a,b ± 0.14 | 8.19 a ± 0.18 | 8.38 a ± 0.15 | |
| 60 | 8.19 a ± 0.18 | 8.00 a ± 0.17 | 8.00 a,b ± 0.18 | 7.86 a ± 0.16 | 7.95 a ± 0.15 | |
| 90 | 7.76 a ± 0.19 | 7.71 a ± 0.20 | 8.00 a,b ± 0.15 | 7.81 a ± 0.20 | 8.14 a ± 0.19 | |
| 120 | 7.90 a ± 0.17 | 7.76 a ± 0.19 | 7.52 b ± 0.19 | 7.57 a ± 0.20 | 7.86 a ± 0.16 | |
| 150 | 7.76 a ± 0.17 | 7.67 a ± 0.16 | 7.76 a,b ± 0.19 | 7.71 a ± 0.14 | 7.90 a ± 0.15 | |
| 180 | 7.86 a ± 0.19 | 7.71 a ± 0.20 | 8.00 a,b ± 0.18 | 7.57 a ± 0.16 | 7.95 a ± 0.18 |
| Day | Chicken Leg | Day | Chicken Breast | |||
|---|---|---|---|---|---|---|
| 10 °C | 15 °C | −13 °C | −18 °C | −23 °C | ||
| 0 | 2.53 a ± 0.04 | 2.53 a,b ± 0.04 | 0 | ND | ND | ND |
| 15 | 1.78 a ± 0.36 | 2.29 a ± 0.02 | 30 | 1.48 a ± 0.44 | 1.00 ± 0.00 | ND |
| 30 | 2.02 a ± 0.30 | 2.43 a ± 0.08 | 60 | 1.30 ± 0.00 | 1.00 ± 0.00 | 1.40 ± 0.00 |
| 45 | 1.84 a ± 0.45 | 2.73 b,c ± 0.04 | 90 | 1.09 a ± 0.12 | 1.15 a ± 0.21 | 1.20 a ± 0.28 |
| 60 | 2.11 a ± 0.25 | 2.77 b,c ± 0.10 | 120 | 1.39 a ± 0.30 | 1.35 a ± 0.07 | 1.24 a ± 0.34 |
| 75 | 2.14 a ± 0.47 | 2.88 c ± 0.12 | 150 | 1.36 a ± 0.22 | 1.15 a ± 0.21 | 1.16 a ± 0.28 |
| 90 | 2.84 a ± 0.17 | 3.43 d ± 0.04 | 180 | 1.62 a ± 0.28 | 1.30 a ± 0.30 | 1.24 a ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, W.-H.; Choi, J.-S. Sensory Quality Evaluation of Superheated Steam-Treated Chicken Leg and Breast Meats with a Combination of Marination and Hot Smoking. Foods 2021, 10, 1924. https://doi.org/10.3390/foods10081924
Cho W-H, Choi J-S. Sensory Quality Evaluation of Superheated Steam-Treated Chicken Leg and Breast Meats with a Combination of Marination and Hot Smoking. Foods. 2021; 10(8):1924. https://doi.org/10.3390/foods10081924
Chicago/Turabian StyleCho, Woo-Hee, and Jae-Suk Choi. 2021. "Sensory Quality Evaluation of Superheated Steam-Treated Chicken Leg and Breast Meats with a Combination of Marination and Hot Smoking" Foods 10, no. 8: 1924. https://doi.org/10.3390/foods10081924
APA StyleCho, W.-H., & Choi, J.-S. (2021). Sensory Quality Evaluation of Superheated Steam-Treated Chicken Leg and Breast Meats with a Combination of Marination and Hot Smoking. Foods, 10(8), 1924. https://doi.org/10.3390/foods10081924







