Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits
Abstract
:1. Introduction
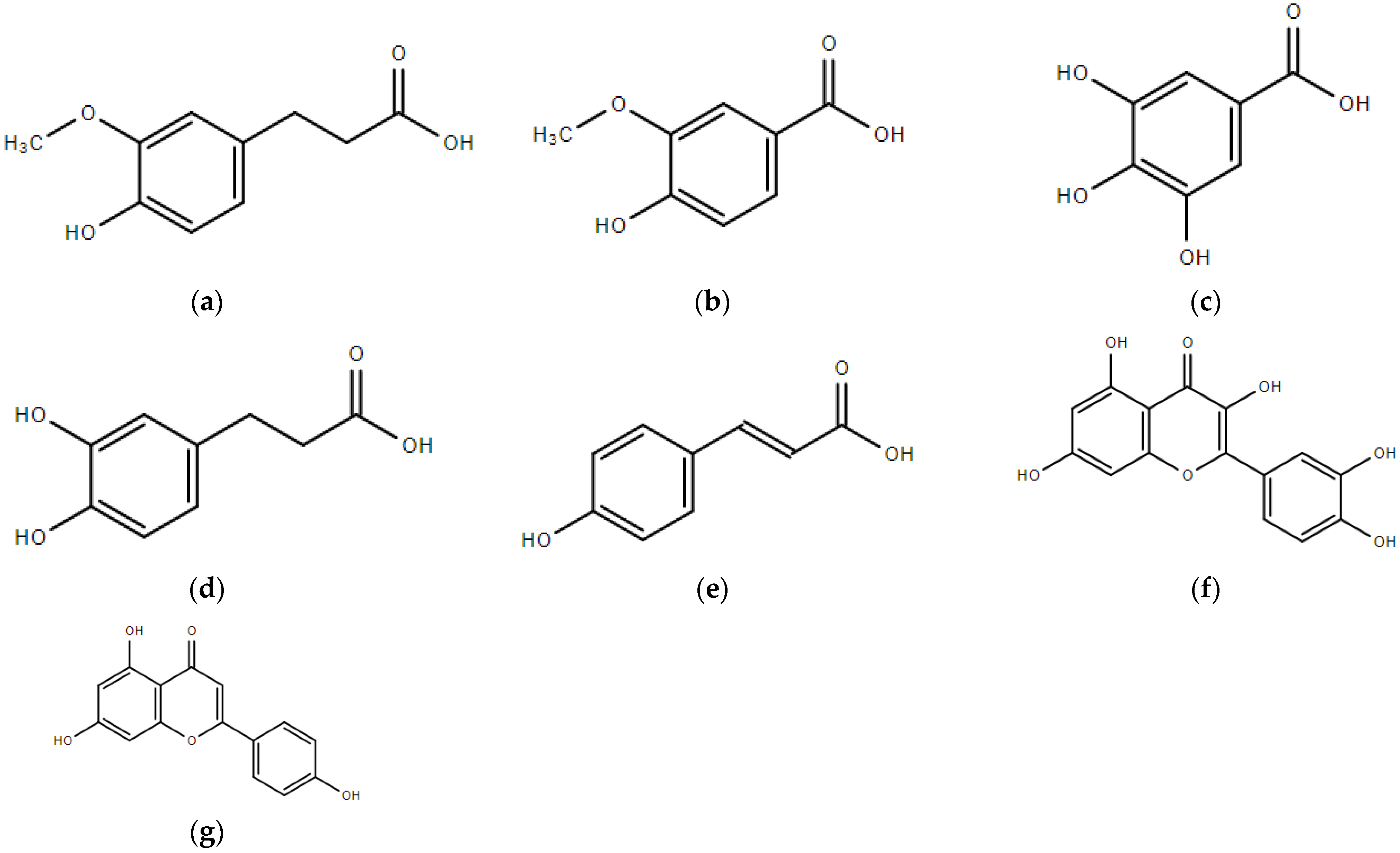
2. Phenolic Compounds in Sorghum Grain
3. Health Benefits and Potential Molecular Mechanisms of Phenolic Compounds in Sorghum Grain
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Effect
3.3. Anti-Proliferative Effect
3.4. Anti-Diabetic Effect
3.5. Anti-Atherogenic Effect
4. Effect of Processing on Phenolic Compounds in Sorghum
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 March 2019).
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic compositions and antioxidant activities differ significantly among sorghum grains with different applications. Molecules 2018, 23, 1023. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.K.; Umakanth, A.V.; Narsaiah, T.B.; Uma, A. Exploring anthocyanins, antioxidant capacity and a-glucosidase inhibition in bran and flour extracts of selected sorghum genotypes. Food Biosci. 2021, 41, 100979. [Google Scholar] [CrossRef]
- Awika, J.M.; Yang, L.; Browning, J.D.; Faraj, A. Comparative Antioxidant, Antiproliferative and Phase II Enzyme Inducing Potential of Sorghum (Sorghum bicolor) Varieties. LWT-Food Sci. Tech. 2009, 42, 1041–1046. [Google Scholar] [CrossRef]
- Dykes, L.; Ronney, W.L.; Rooney, L.W. Evaluation of phenolics and antioxidantactivity of black sorghum hybrids. J. Cereal Sci. 2013, 58, 278–283. [Google Scholar] [CrossRef]
- Arbex, P.M.; de Moreira, M.E.C.; Toledo, R.C.L.; de Morais Cardoso, L.; Pinheiro-Sant’ana, H.M.; dos Benjamin, L.A.; Licursi, L.; Carvalho, C.W.P.; Queiroz, V.A.V.; Martino, H.S.D. Extruded Sorghum Flour (Sorghum bicolor L.) Modulate Adiposity and Inflammation in High Fat Diet-Induced Obese Rats. J. Funct. Foods 2018, 42, 346–355. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Xu, J.; Herald, T.; Smolensky, D.; Perumal, R.; Wang, W. Sorghum phenolic compounds are associated with cell growth inhibition through cell cycle arrest and apoptosis in human hepatocarcinoma and colorectal adenocarcinoma cells. Foods 2021, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Earp, C.F.; McDonough, C.M.; Rooney, L.W. Microscopy of pericarp development in the caryopsis of Sorghum bicolor (L.) Moench. J. Cereal Sci. 2004, 39, 21–27. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W.; Waniska, R.D.; Rooney, W.L. Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J. Agric. Food Chem. 2005, 53, 6813–6818. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Seitz, M.L.; Rooney, L.W.; Rooney, L.W. Flavonoid composition of red sorghum genotypes. Food Chem. 2009, 116, 313–317. [Google Scholar] [CrossRef]
- de Cardoso, L.M.; Pinheiro, S.S.; de Carvalho, C.W.P.; Queiroz, V.A.V.; de Menezes, C.B.; Moreira, A.V.B.; de Barros, F.A.R.; Awika, J.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Phenolic Compounds Profile in Sorghum Processed by Extrusion Cooking and Dry Heat in a Conventional Oven. J. Cereal Sci. 2015, 65, 220–226. [Google Scholar] [CrossRef]
- Dykes, L.; Peterson, G.C.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem. 2011, 127, 173–179. [Google Scholar] [CrossRef]
- Su, X.; Rhodes, D.; Xu, J.; Chen, X.; Davis, H.; Wang, D.; Herald, T.J.; Wang, W. Phenotypic diversity of anthocyanins in sorghum accessions with various pericarp pigments. J. Nutr. Food Sci. 2017, 7. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Shen, S.; Johnson, S.; Fang, Z. Comprehensive Profiling of Phenolic Compounds by HPLC-DAD-ESI-QTOF-MS/MS to Reveal Their Location and Form of Presence in Different Sorghum Grain Genotypes. Food Res. Int. 2020, 137, 109671. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Damasceno Teixeira, T.V.; Zhang, P.; Warner, R.D.; Shen, S.; Fang, Z. Cellular Antioxidant Activities of Phenolic Extracts from Five Sorghum Grain Genotypes. Food Biosci. 2021, 41, 101068. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Sorghum Polyphenols and Other Bioactive Components as Functional and Health Promoting Food Ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Yones, H.A.; Karim, N.; Taha, E.M.; Chen, W. Potential Processing Technologies for Developing Sorghum-Based Food Products: An Update and Comprehensive Review. Trends Food Sci. Tech. 2021, 110, 168–182. [Google Scholar] [CrossRef]
- Billa, E.; Koullas, D.P.; Monties, B.; Koubios, E.G. Structure and composition of sweet sorghum stalk components. Ind. Crops Prod. 1997, 6, 297–302. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Waniska, R.D. Anthocyanins from Black Sorghum and Their Antioxidant Properties. Food Chem. 2004, 90, 293–301. [Google Scholar] [CrossRef]
- Bröhan, M.; Jerković, V.; Collin, S. Potentiality of red sorghum for producing stilbenoid-enriched beers with high antioxidant activity. J. Agric. Food Chem. 2011, 59, 4088–4094. [Google Scholar] [CrossRef]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Wu, G.; Johnson, S.K.; Blanchard, C.L. Characterization of Phenolic Compounds and Antioxidant Activity in Sorghum Grains. J. Cereal Sci. 2018, 84, 103–111. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Chen, Y.; Li, L.; Sun, J.; Huang, C.; Ou, S.; Zhang, H. Effects of sorghum, purple rice and rhubarb rice on lipids status and antioxidant capacity in mice fed a high-fat diet. J. Funct. Foods 2017, 37, 103–111. [Google Scholar] [CrossRef]
- Devi, P.S.; Kumar, M.S.; Das, S.M. DNA damage protecting activity and free radical scavenging activity of anthocyanins from red sorghum (Sorghum bicolor) bran. Biotech. Res. Int. 2012, 2012, 258787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y.; Zhang, D.; Cai, M.; Chen, G. Ultrasound Assisted Extraction of Polyphenolic Compounds from Red Sorghum (Sorghum bicolor L.) Bran and Their Biological Activities and Polyphenolic Compositions. Ind. Crop. Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Barros, F.; Dykes, L.; Awika, J.M.; Rooney, L.W. Accelerated Solvent Extraction of Phenolic Compounds from Sorghum Brans. J. Cereal Sci. 2013, 58, 305–312. [Google Scholar] [CrossRef]
- Herrman, D.A.; Brantsen, J.F.; Ravisankar, S.; Lee, K.M.; Awika, J.M. Stability of 3-deoxyanthocynin pigement structure relative to anthocyanins from grains under microwave assisted extraction. Food Chem. 2020, 333, 127494. [Google Scholar] [CrossRef]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y. Subcritical Water Extraction of Polyphenolic Compounds from Sorghum (Sorghum bicolor L.) Bran and Their Biological Activities. Food Chem. 2018, 262, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Tyagi, A.; Chen, X.Q.; Chelliah, R.; Kim, J.-H.; Han, S.-I.; Oh, D.-H. UHPLC-ESI-QTOF-MS/MS Characterization, Antioxidant and Antidiabetic Properties of Sorghum Grains. Food Chem. 2021, 337, 127788. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Wu, G.; Shen, Y.; Qi, Y.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Li, Y.; Johnson, S.K. Improvement of in vitro and cellular antioxidant properties of Chinese steamed bread through sorghum addition. LWT-Food Sci. Tech. 2018, 91, 77–83. [Google Scholar] [CrossRef]
- González-Montilla, F.M.; Chávez-Santoscoy, R.A.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Isolation and Identification of Phase II Enzyme Inductors Obtained from Black Shawaya Sorghum [Sorghum bicolor (L.) Moench] Bran. J. Cereal Sci. 2012, 55, 126–131. [Google Scholar] [CrossRef]
- Lewis, J.B. Effects of Bran from Sorghum Grains Containing Different Classes and Levels of Bioactive Compounds in Colon Carcinogenesis. Master’s Thesis, Texas A&M University, College Station, TA, USA, 2008. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2017, 54, 287–293. [Google Scholar]
- Moraes, E.A.; Natal, D.I.G.; Queiroz, V.A.V.; Schaffert, R.E.; Cecon, P.R.; de Paula, S.O.; dos Anjos Benjamim, L.; Reibeiro, S.M.R.; Martino, H.S.D. Sorghum genotype may reduce low-grade inflammatory response and oxidative stress and maintains jejunum morphology of rats fed a hyperlipidic diet. Food Res. Int. 2012, 49, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Ajiboye, T.O.; Komolafe, Y.O.; Oloyede, O.B.; Ogunbode, S.M.; Adeoye, M.D.; Abdulsalami, I.O.; Nurudeen, Q.Q. Polyphenolic extract of Sorghum bicolor grains reactive oxygen species detoxification in N-nitrosodiethylamine-treated rats. Food Sci. Hum. Wellness 2013, 2, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Shim, T.J.; Kim, T.M.; Jang, K.C.; Ko, J.Y.; Kim, D.J. Toxicological evaluation and anti-inflammatory activity of a golden gelatinous sorghum bran extract. Biosci. Biotech. Biochem. 2013, 77, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, T.; Zheng, W.; Gao, B.; Zhu, H.; Xu, R.; Deng, H.; Wang, B.; Wu, Y.; Sun, X.; et al. Triacylglycerols compositions, soluble and bound phenolics of red sorghums, and their radical scavenging and anti-inflammatory activities. Food Chem. 2021, 340, 128123. [Google Scholar] [CrossRef] [PubMed]
- Burdette, A.; Garner, P.L.; Mayer, E.P.; Hargrove, J.L.; Hartle, D.K.; Greenspan, P. Anti-inflammatory activity of select sorghum (Sorghum bicolor ) brans. J. Med. Food 2010, 13, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D.A. Comparative study on phenolic content, antioxidant activity and anti-inflammatory capacity of aqueous and ethanolic extracts of sorghum in lipopolyshaccaride-induced RAW264.7 macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, L.; Taddeo, S.S.; Weeks, B.R.; Carroll, R.J.; Dykes, L.; Rooney, L.W.; Turner, N.D. Impact of novel sorghum bran diets on DSS-induced colitis. Nutrients 2017, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, A.R.; de Castro Moreira, M.E.; Grancieri, M.; Toledo, R.C.L.; de Oliveira Araújo, F.; Mantovani, H.C.; Queiroz, V.A.V.; Martino, H.S.D. Extruded Sorghum (Sorghum bicolor L.) Improves Gut Microbiota, Reduces Inflammation, and Oxidative Stress in Obese Rats Fed a High-Fat Diet. J. Funct. Foods 2019, 58, 282–291. [Google Scholar] [CrossRef]
- Gilchrist, A.K.; Smolensky, D.; Ngwaga, T.; Chauhan, D.; Cox, S.; Perumal, R.; Noronha, L.E.; Rhames, S.R. High-polyphenol extracts from Sorghum bicolor attenuate replication of Legionella pneumophila within RW 264.7 macrophages. FEMS Microb. Lett. 2020, 367. [Google Scholar] [CrossRef] [PubMed]
- Dia, P.; Pangloli, P.; Jones, L.; McClure, A.; Patel, A. Phytochemical concentrations and biological activities of Sorghum bicolor alcoholic extracts. Food Funct. 2016, 7, 3410–3420. [Google Scholar] [CrossRef] [PubMed]
- Carbonneau, M.-A.; Cisse, M.; Mora-Soumille, N.; Dairi, S.; Rosa, M.; Michel, F.; Lauret, C.; Cristol, J.-P.; Dangles, O. Antioxidant Properties of 3-Deoxyanthocyanidins and Polyphenolic Extracts from Côte d’Ivoire’s Red and White Sorghums Assessed by ORAC and in vitro LDL Oxidisability Tests. Food Chem. 2014, 145, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Browning, J.D.; Awika, J.M. Sorghum 3-deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J. Agric. Food Chem. 2009, 57, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Kunyanga, C.N.; Imungi, J.K.; Okoh, M.W.; Biesalki, H.K. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT-Food Sci. Tech. 2012, 45, 269–276. [Google Scholar] [CrossRef]
- Smolensky, D.; Rhodes, D.; McVey, D.S.; Fawver, Z.; Perumal, R.; Herald, T.; Noronha, L. High-Polyphenol Sorghum Bran Extract Inhibits Cancer Cell Growth Through ROS Induction, Cell Cycle Arrest, and Apoptosis. J. Med. Food 2018, 21, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Chang, J.Y.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. Quercetin, a Potent Inhibitor against b-Catenin/Tcf Signaling in SW480 Colon Cancer Cells. Biochem. Biophys. Res. Commun. 2005, 328, 227–234. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Preto, A.; Seruca, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Luteolin, Quercetin and Ursolic Acid Are Potent Inhibitors of Proliferation and Inducers of Apoptosis in Both KRAS and BRAF Mutated Human Colorectal Cancer Cells. Cancer Lett. 2009, 281, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Hargrove, J.L.; Greenspan, P.; Hartle, D.K.; Dowd, C. Inhibition of Aromatase and α-Amylase by Flavonoids and Proanthocyanidins from Sorghum bicolor Bran Extracts. J. Med. Food 2011, 14, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Suganyadevi, P.; Saravanakumar, K.M.; Mohandas, S. The Antiproliferative Activity of 3-Deoxyanthocyanins Extracted from Red Sorghum (Sorghum bicolor) Bran through P53-Dependent and Bcl-2 Gene Expression in Breast Cancer Cell Line. Life Sci. 2013, 92, 379–382. [Google Scholar] [CrossRef]
- Massey, A.R.; Reddivari, L.; Vanamala, J. The Dermal Layer of Sweet Sorghum (Sorghum bicolor) Stalk, a Byproduct of Biofuel Production and Source of Unique 3-Deoxyanthocyanidins, Has More Antiproliferative and Proapoptotic Activity than the Pith in P53 Variants of HCT116 and Colon Cancer Stem Cells. J. Agric. Food Chem. 2014, 62, 3150–3159. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, Z.; Qin, P.; Yao, Y.; Meng, X.; Zou, J.; Zhu, K.; Ren, G. Chemical characterization of a procyanidin rich extract from sorghum bran and its effect on oxidative stress and tumor inhibition in vivo. J. Agric. Food Chem. 2011, 59, 8609–8615. [Google Scholar] [CrossRef]
- Park, J.H.; Darvin, P.; Lim, E.J.; Joung, Y.H.; Hong, D.Y.; Park, E.U.; Park, S.H.; Choi, S.K.; Moon, E.-S.; Cho, B.W.; et al. Hwanggeumchal Sorghum Induces Cell Cycle Arrest, and Suppresses Tumor Growth and Metastasis through Jak2/STAT Pathways in Breast Cancer Xenografts. PLoS ONE 2012, 7, e40531. [Google Scholar] [CrossRef]
- Cox, S.; Noronba, L.; Herald, T.; Bean, S.; Lee, S.H.; Perumal, E.; Wang, W.; Smolensky, D. Evaluation of ethanol-based extraction conditions of sorghum bran bioactive compounds with downstream anti-proliferative properties in human cancer cells. Heliyon 2019, 5, e01589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.-M.; Kim, E.-H.; Yeo, M.-A.; Kim, S.-J.; Seo, M.; Moon, H.-I. Antidiabetic Effects of Three Korean Sorghum Phenolic Extracts in Normal and Streptozotocin-Induced Diabetic Rats. Food Res. Int. 2011, 44, 127–132. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, S.H.; Chung, I.-M.; Park, Y. Sorghum Extract Exerts an Anti-Diabetic Effect by Improving Insulin Sensitivity via PPAR-γ in Mice Fed a High-Fat Diet. Nutr. Res. Pract. 2012, 6, 322. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, Y. Anti-diabetic effect of sorghum extract on hepatic gluconeogenesis of streptozotoxin-induced diabetic rats. Nutr. Met. 2012, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irondi, E.A.; Adegoke, B.M.; Effion, E.S.; Oyewo, S.O.; Alamu, E.O.; Boligon, A.A. Enzymes inhibitory property, antioxidant activity and phenolics profile of raw and roasted red sorghum grains in vitro. Food Sci. Hum. Wellness 2019, 8, 142–148. [Google Scholar] [CrossRef]
- Gobbo, L.C.D.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Gaytán-Martínez, M.; Cabrera-Ramírez, Á.H.; Morales-Sánchez, E.; Ramírez-Jiménez, A.K.; Cruz-Ramírez, J.; Campos-Vega, R.; Velazquez, G.; Loarca-Piña, G.; Mendoza, S. Effect of Nixtamalization Process on the Content and Composition of Phenolic Compounds and Antioxidant Activity of Two Sorghums Varieties. J. Cereal Sci. 2017, 77, 1–8. [Google Scholar] [CrossRef]
- Hamad, S.A.A.; Mustafa, I.; Magboul, B.I.; Qasem, A.A.; Ahmed, I.A.M. Nutritional quality of raw and cooked flours of a high b-glucan sorghum inbred line. J. Cereal Sci. 2019, 90, 102857. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Luo, J.; Johnson, S.; Fang, Z. Effect of Processing on the Phenolic Contents, Antioxidant Activity and Volatile Compounds of Sorghum Grain Tea. J. Cereal Sci. 2019, 85, 6–14. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Cabrera-Ramírez, Á.H.; Rodríguez-Castillo, N.; Campos-Vega, R.; Loarca-Piña, G.; Gaytán-Martínez, M. Impact of Cooking and Nixtamalization on the Bioaccessibility and Antioxidant Capacity of Phenolic Compounds from Two Sorghum Varieties. Food Chem. 2020, 309, 125684. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ashton, J.; Simic, A.; Fang, Z.; Johnson, S.K. Mineral availability is modified by tannin and phytate content in sorghum flaked breakfast cereals. Food Res. Int. 2017, 103, 509–514. [Google Scholar] [CrossRef]
- Randhir, R.; Kwon, Y.-I.; Shetty, K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts ad seedlings. Innov. Food Sci. Emerg. Technol. 2008, 9, 355–364. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.; Qin, P.; Ren, G. Effects of processing on phytochemical profiles and biological activities for production of sorghum tea. Food Res. Int. 2013, 53, 685–687. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor I.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2015, 57, 372–390. [Google Scholar] [CrossRef]
| Sorghum Source | TPC (mg/g) | TFC (mg/g) | TAC (mg/g) | Reference |
|---|---|---|---|---|
| White pericarp | 0.24–34.78 GAE | 0.06–0.38 RE | 0.02 CCE; 0.09 GAE | [2,21,22] |
| Yellow pericarp | - | - | Bran: 0.26–0.81 AE; Flour: 0.10–0.35 AE | [3] |
| Black pericarp | 4.13–11.50 GAE | 0–0.20 RE | 3.02 GAE, 0.18 CCE | [2,21] |
| Brown pericarp | 3.58 GAE; 1.74 FAE | 1.39 CE | 5.55 GAE; | [21] |
| Red pericarp | 0.66–47.86 GAE | 0–0.60 RE | 0.41–0.60 GAE; 2.66–8.93 CCE | [21,22] |
| Sorghum Source | Bioactive Extracts | Antioxidant Activity | Reference |
|---|---|---|---|
| Hongyingzi, Hongzhenzhu, Dongbei sorghum, Jiangsu sorghum, Jiliang 2 sorghum, Longza 11, black grain sorghum, white Longmi sorghum. | Caffeic acid, p-coumaric acid, ferulic acid, protocatechuic acid, luteolindin, apigeninidin, luteolin, apigenin, taxifolin, naringenin. | Antioxidant activities against DPPH and FRAP assays. | [2] |
| Tannin-containing sorghum varieties (Sumac, Hi-Tannin, Seredo, CR 35:5 × 2), non-tannin varieties (white variety, KARI-Mtama, red variety, ICSV-III), Mizzou, Tx430. | Condensed tannins, 3-DXA, phenolics. | Induced phase II detoxifying enzymes; anti-proliferative effect on esophageal, OE33, colon cancer cells. | [4] |
| Liberty, Mr-Buster, Cracker, IS131C, Shawaya Short Black 1. | Phenolic extracts. | Antioxidant activities against DPPH and FRAP assays; Anti-proliferative effect on Caco-2 cells. | [15] |
| Tx3362, Shawaya Black, Black PI Tall, Hyb 107, Hyb 115, Hyb 116, Hyb 117, Hyb 118. | Total phenolics, condensed tannins, flavan-4-ols, 3-DXA. | Antioxidant activities against DPPH and ABTS assays. | [5] |
| Sorghum Source | Bioactive Extracts | Anti-Inflammatory Effect | Reference |
|---|---|---|---|
| Red sorghum. | Phenolics, flavonoids, anthocyanins. | Antioxidant activities against DPPH, FRAP, superoxide radical scavenging, hydroxyl radical scavenging assays, metal chelating, hydrogen peroxide. | [23] |
| Tong Za 117, Tong Za 141, Tong Za 142, Tong Za 143, Chi Za 109, Chi Za 101. | Ferulic acid, p-coumaric acid, caffeic acid, 3,4-dihydroxybenzoic acid, luteolinidin, apigeninidin, 5-methoxyluteolinidin, 7-methoxy apigeninidin. | Antioxidant activities against DPPH and ABTS assays; inhibitory effect on IL-6 and IL-1β. | [37] |
| Sumac, Mycogen 726, black sorghum, white sorghums. | Total phenolic extracts. | Inhibitory effect on IL-1β and TNF-α. | [38] |
| SC84MX, SC84KS, PI57048. | Phenolics, flavonoids, tannins, 3-DXA, anthocyanins. | Antioxidant activities against DPPH, ORAC and nitric oxide assays; inhibited cellular production of NO, IL-6, ROS. | [39] |
| PUI570481 | Polyphenol extracts. | Inhibitory effect on IL-6 and TNF-α. | [42] |
| 1-Terral REV 9924, 2-Pioneer 84P8D, 3-Dekalb DK-54-00, 4-FFR353, 5-DynaGro DG765B, 6-Pioneer 83P99, 7-Dekalb DK-51-01, 8-Terral REV 9782, 9-Terral REV 9562, 10-Terral REV9883. | Naringenin, eriodicytol, apigenin, luteolin, apigeninidin, luteolinidin. | Antioxidant activities against DPPH and NO assays; Inhibitory effect on OVCA cells. | [43] |
| White sorghum, red sorghum. | Gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, luteolinidin, apigeninidin, p-coumaric acid, flavanols, quercetin, hydroxycinnamic acid, 5-methoxy luteolinidin, 7-methoxy-luteolinidin, 5,7-dimethoxy-luteolinidin, 7-methoxy-apigeninidin, 5,7-dimethoxy-apigeninidin. | Antioxidant activities against ORAC and nitric oxide assays; inhibited cellular production of NO, IL-6, ROS. | [44] |
| Sorghum Source | Bioactive Extracts | Anti-Proliferative Effect | Reference |
|---|---|---|---|
| Black sorghum varieties (Macia, Sumac, PI152653, PI152687, PI193073, PI1329694, PI1559733, PI1559855, PI1568282, PI1570366, PI1570481, PI1570484, PI1570819, PI1570889, PI1570993). | Total phenolic extracts. | Anti-proliferative effect on HepG2 and Caco-2 cells: induction G1/S cell cycle arrest, activation of p53. | [48] |
| Red sorghum | 3-DXA extracts. | Inhibitory effect on MCF7 cancer cells through up-regulating p53 and down-regulating Bcl-2 genes. | [52] |
| Dale, M81E | Vanillic acid, p-coumaric acid, ferulic acid, caffeic acid, apigeninidin, luteolinidin, malvidin-3-O-glucoside, apigenin, luteolin, trans-resveratrol, luteoferol. | Inhibitory effect on HCT116 and colon cancer stem cells through activating p53 gene. | [53] |
| Hwanggeumchal sorghum. | Total polyphenol extracts. | Anti-proliferative effect on MDA-MB 231 and MC7 cells: down-regulating VEGF, VEGF-R2, cyclin D, cyclin E, pRb and up-regulating p53. | [55] |
| TX430, Sumac. | Total phenolic extracts. | Anti-proliferative effect on HepG2 and HCT15 cells. | [56] |
| Sorghum Source | Bioactive Extracts | Anti-Diabetic and Anti-Atherogenic Effects | Reference |
|---|---|---|---|
| Brown sorghum varieties (SOR 01, SOR 03, SOR 08, SOR 11, SOR 17, SOR 21, SOR 24, SOR 33) | Gallic acid, chlorogenic acid, caffeic acid, ellagic acid, p-coumaric acid, quercetin, luteolin, apigenin. | Inhibitory effect on α-amylase and α-glucosidase activities. | [28] |
| Hwanggeumchal sorghum. | Phenolic extracts. | Reduced the serum glucose, total cholesterol, triglycerides, urea, uric acid, creatinine. | [57] |
| KNICS-579 | Polyphenol extracts. | Reduced the concentration of triglycerides, total LDL-cholesterol and glucose. | [60] |
| Red sorghum | Total phenolic extracts. | Antioxidant activity against ABTS, DPPH, FRAP assays; Inhibitory effect on pancreatic lipase, α-amylase and α-glucosidase activities. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, W.; Zhao, Y. Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits. Foods 2021, 10, 1921. https://doi.org/10.3390/foods10081921
Xu J, Wang W, Zhao Y. Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits. Foods. 2021; 10(8):1921. https://doi.org/10.3390/foods10081921
Chicago/Turabian StyleXu, Jingwen, Weiqun Wang, and Yong Zhao. 2021. "Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits" Foods 10, no. 8: 1921. https://doi.org/10.3390/foods10081921
APA StyleXu, J., Wang, W., & Zhao, Y. (2021). Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits. Foods, 10(8), 1921. https://doi.org/10.3390/foods10081921







