The 10,000-Year Success Story of Wheat!
Abstract
1. Introduction
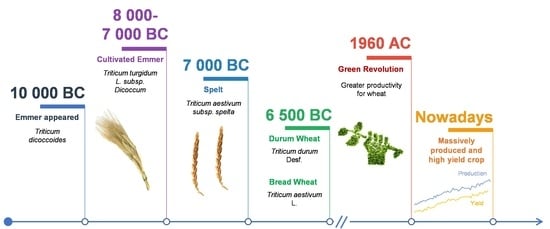
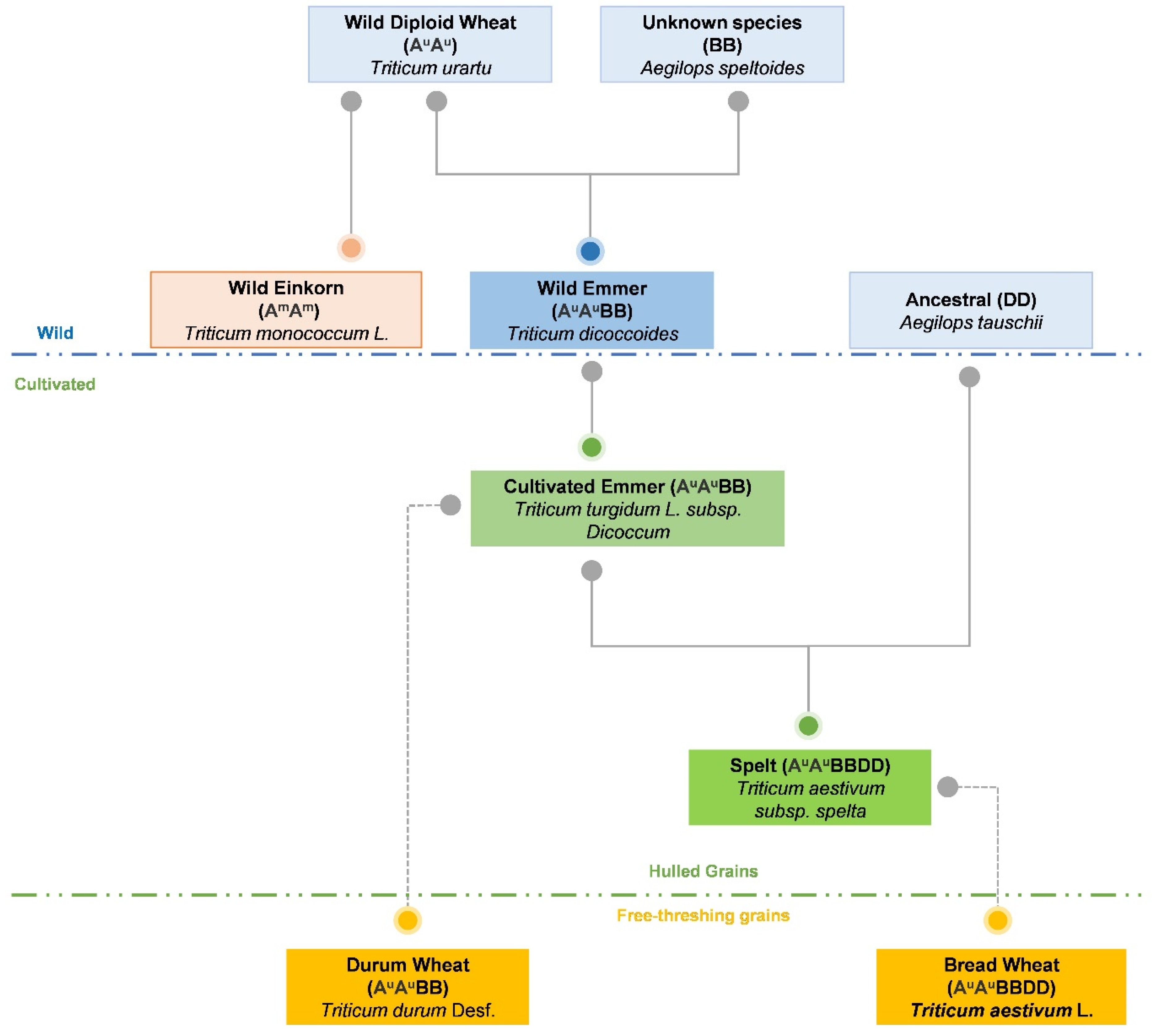
2. Origin and Evolution
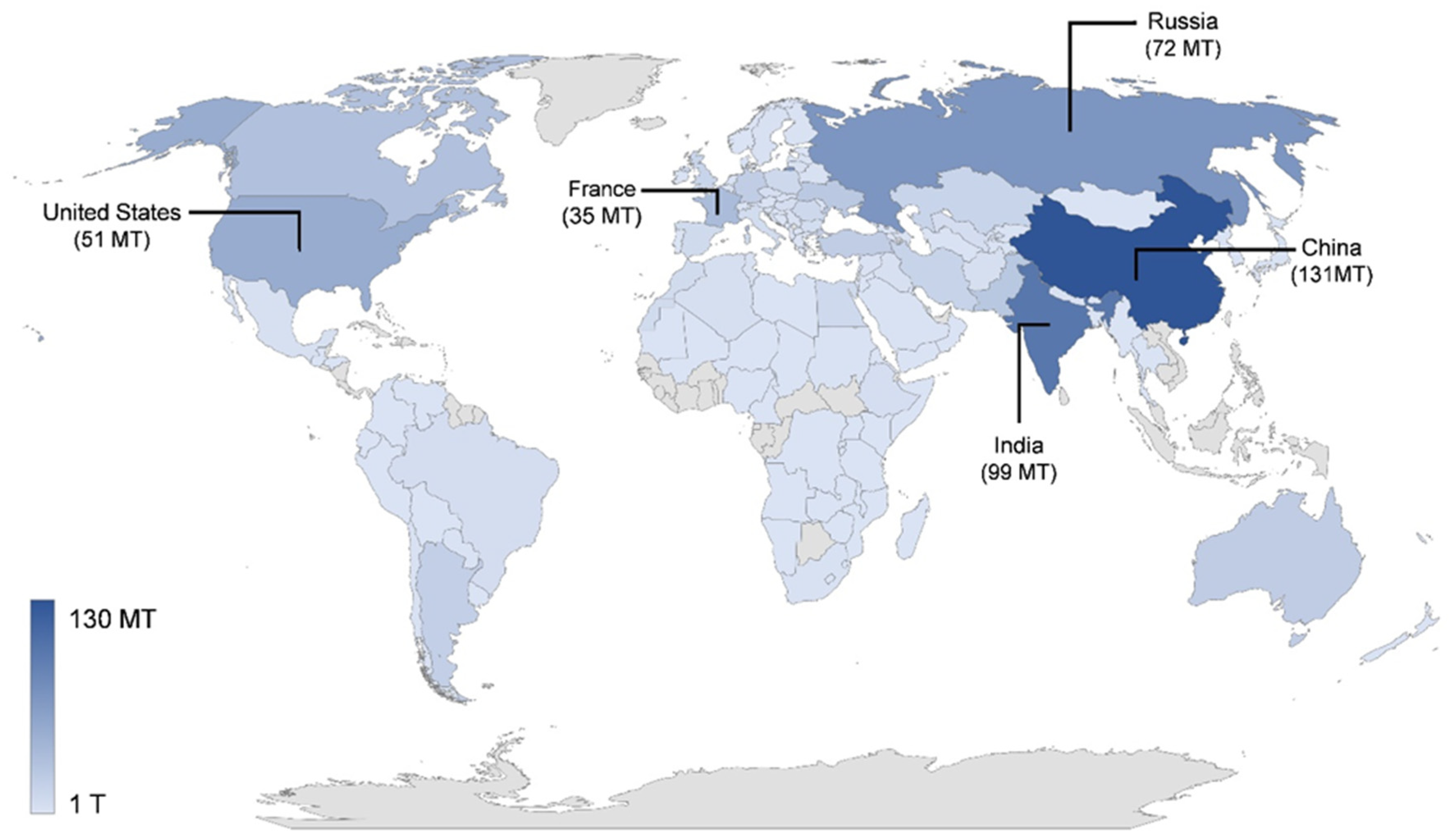
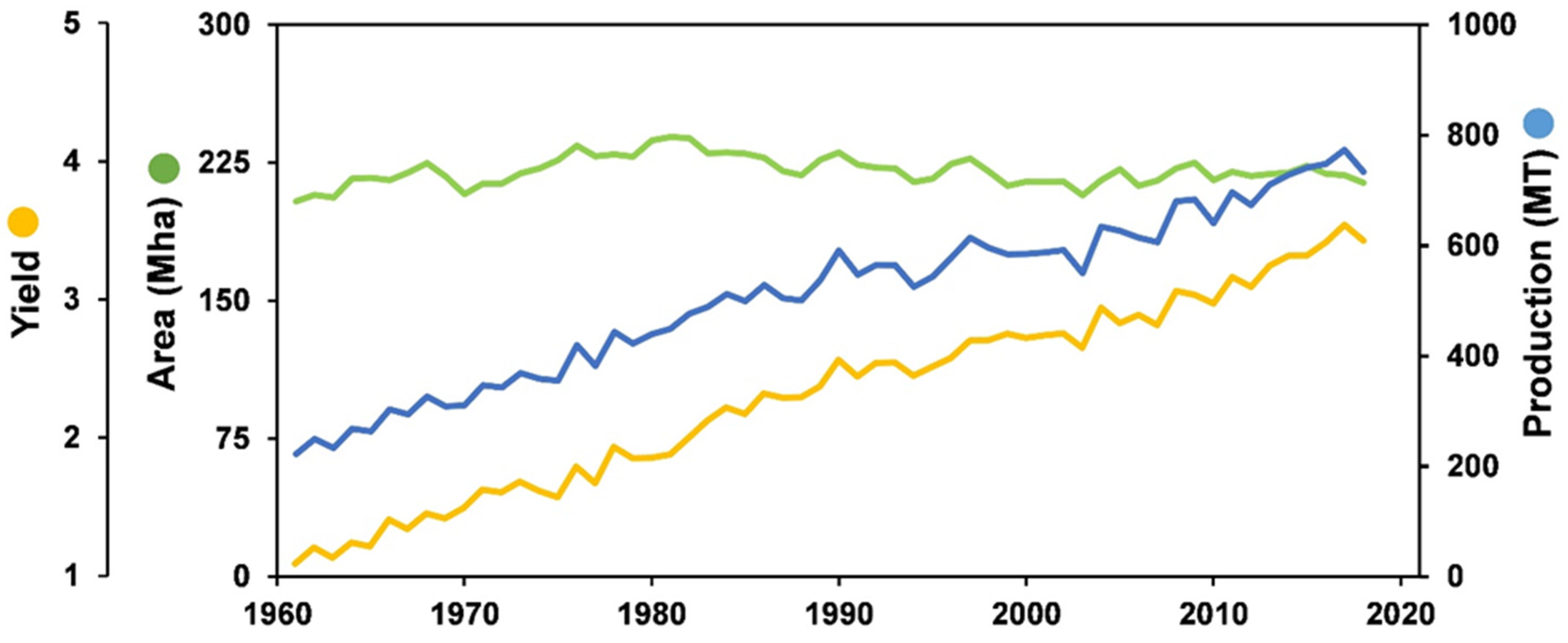
3. Production
4. Wheat Consumption Habits
5. Nutritional Value and Health Impact
6. Functional and Technological Properties
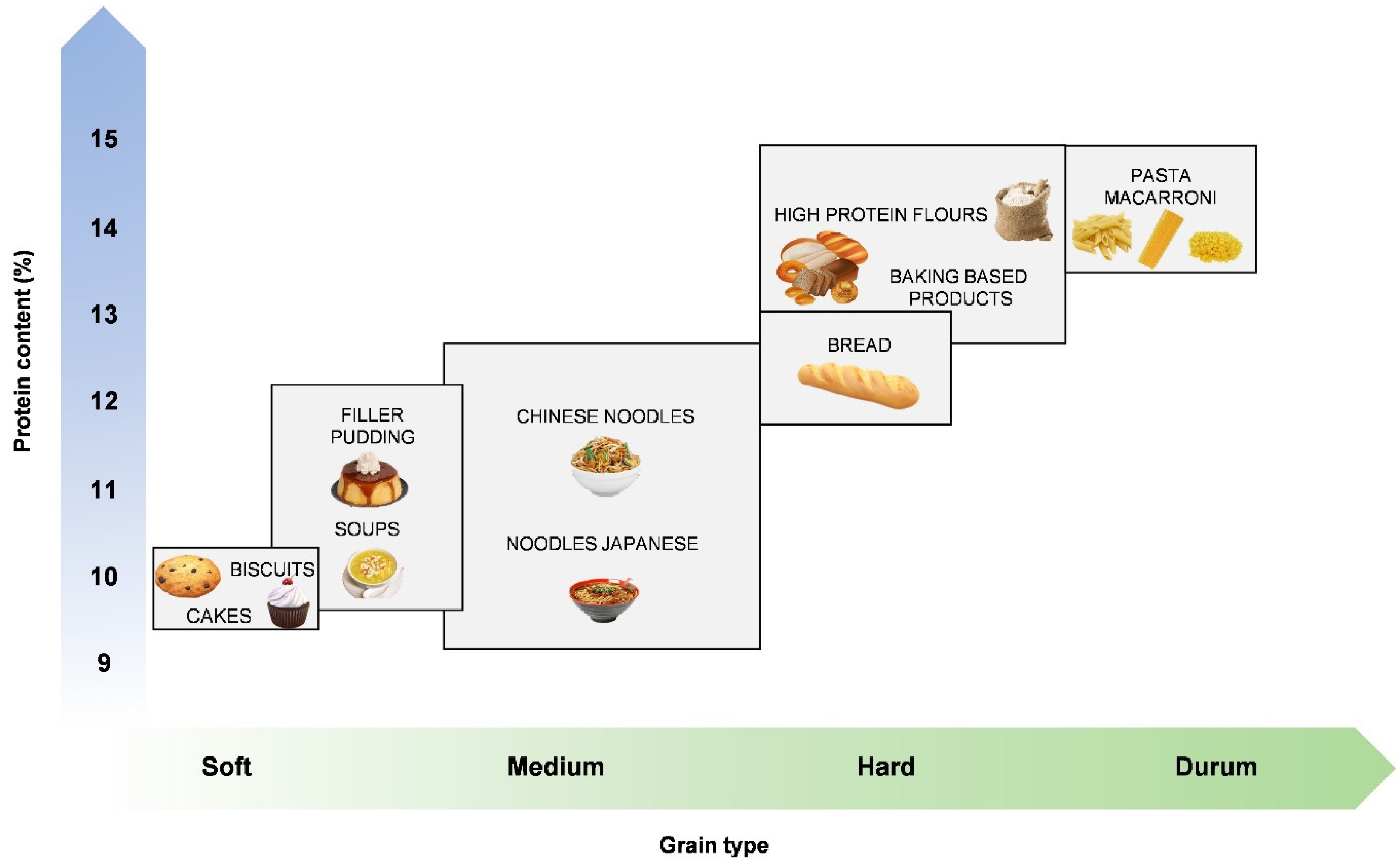
6.1. Grain Hardness
6.2. Wheat Proteins
6.2.1. Non-Gluten Proteins
6.2.2. Gluten
6.3. Effect of Gluten Proteins on Wheat Functionality
6.4. Models of Gluten Structure and Function in Dough
6.4.1. Pom-Pom Model
6.4.2. Loop-Train Model
6.4.3. Particle-Gel Model
6.4.4. Linear Glutenin Hypothesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization. Available online: http://www.fao.org/faostat/en/#home (accessed on 14 October 2020).
- Venske, E.; Dos Santos, R.S.; Busanello, C.; Gustafson, P.; Costa de Oliveira, A. Bread wheat: A role model for plant domestication and breeding. Hereditas 2019, 156, 16. [Google Scholar] [CrossRef] [PubMed]
- William, A.; Alain, B.; Maarten, V.G. The World Wheat Book: A History of Wheat Breeding; Lavoisier: Paris, France, 2011; Volume 2. [Google Scholar]
- Shewry, P.R. Do ancient types of wheat have health benefits compared with modern bread wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef]
- Curtis, B. Wheat in the World. Bread Wheat: Improvement and Production; Food & Agriculture Organization of the UN: Rome, Italy, 2002. [Google Scholar]
- Dubcovsky, J.; Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 2007, 316, 1862–1866. [Google Scholar] [CrossRef]
- Goncharov, N. Comparative-genetic analysis–a base for wheat taxonomy revision. Czech J. Genet. Plant Breed. 2005, 41, 52–55. [Google Scholar] [CrossRef]
- Peng, J.H.; Sun, D.; Nevo, E. Domestication evolution, genetics and genomics in wheat. Mol. Breed. 2011, 28, 281. [Google Scholar] [CrossRef]
- Dhaka, V.; Khatkar, B.J. Effects of gliadin/glutenin and HMW-GS/LMW-GS ratio on dough rheological properties and bread-making potential of wheat varieties. J. Food Qual. 2015, 38, 71–82. [Google Scholar] [CrossRef]
- Peña, R.; Trethowan, R.; Pfeiffer, W.; Ginkel, M.V. Quality (end-use) improvement in wheat: Compositional, genetic, and environmental factors. J. Crops Prod. 2002, 5, 1–37. [Google Scholar] [CrossRef]
- Peña, R. Wheat for bread and other foods. In Bread Wheat Improvement and Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; pp. 483–542. [Google Scholar]
- Callejo, M.J.; Vargas-Kostiuk, M.-E.; Ribeiro, M.; Rodríguez-Quijano, M. Triticum aestivum ssp. vulgare and ssp. spelta cultivars: 2. Bread-making optimisation. Eur. Food Res. Technol. 2019, 245, 1399–1408. [Google Scholar] [CrossRef]
- Rodríguez-Quijano, M.; Vargas-Kostiuk, M.-E.; Ribeiro, M.; Callejo, M.J. Triticum aestivum ssp. vulgare and ssp. spelta cultivars. 1. Functional evaluation. Eur. Food Res. Technol. 2019, 245, 1561–1570. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated ancient wheats (Triticum spp.): A potential source of health-beneficial food products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef]
- Harlan, J.R.; Zohary, D. Distribution of wild wheats and barley. Science 1966, 153, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.-J.; Atlin, G.; Payne, T. Multi-location testing as a tool to identify plant response to global climate change. Clim. Chang. Crop Prod. 2010, 1, 115–138. [Google Scholar]
- Hawkesford, M.J.; Araus, J.L.; Park, R.; Calderini, D.; Miralles, D.; Shen, T.; Zhang, J.; Parry, M.A. Prospects of doubling global wheat yields. Food Energy Secur. 2013, 2, 34–48. [Google Scholar] [CrossRef]
- Sakamoto, S. Patterns of phylogenetic differentiation in the tribe Triticeae. Seiken Ziho 1973, 24, 11–31. [Google Scholar]
- Consortium, I.W. A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar]
- Petersen, G.; Seberg, O.; Yde, M.; Berthelsen, K. Phylogenetic relationships of Triticum and Aegilops and evidence for the origin of the A, B, and D genomes of common wheat (Triticum aestivum). Mol. Phylogenet. Evol. 2006, 39, 70–82. [Google Scholar] [CrossRef]
- Feuillet, C.; Langridge, P.; Waugh, R. Cereal breeding takes a walk on the wild side. Trends Genet. 2008, 24, 24–32. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin; Oxford University Press on Demand: Oxford, UK, 2012. [Google Scholar]
- Feldman, M.; Sears, E.R. The wild gene resources of wheat. Sci. Am. 1981, 244, 102–113. [Google Scholar] [CrossRef]
- Ling, H.-Q.; Zhao, S.; Liu, D.; Wang, J.; Sun, H.; Zhang, C.; Fan, H.; Li, D.; Dong, L.; Tao, Y. Draft genome of the wheat A-genome progenitor Triticum urartu. Nature 2013, 496, 87–90. [Google Scholar] [CrossRef]
- Gustafson, P.; Raskina, O.; Ma, X.; Nevo, E. Wheat evolution, domestication, and improvement. In Wheat: Science and Trade; Wiley: Danvers, MA, USA, 2009; pp. 5–30. [Google Scholar]
- Feldman, M.; Levy, A. Allopolyploidy—A shaping force in the evolution of wheat genomes. Cytogenet. Genome Res. 2005, 109, 250–258. [Google Scholar] [CrossRef]
- Chantret, N.; Salse, J.; Sabot, F.; Rahman, S.; Bellec, A.; Laubin, B.; Dubois, I.; Dossat, C.; Sourdille, P.; Joudrier, P.; et al. Molecular basis of evolutionary events that shaped the hardness locus in diploid and polyploid wheat species (Triticum and Aegilops). Plant Cell 2005, 17, 1033–1045. [Google Scholar] [CrossRef]
- Ikanović, J.; Popović, V.; Janković, S.; Živanović, L.; Rakić, S.; Dončić, D.J.G. Khorasan wheat population researching (triticum turgidum, ssp turanicum (mckey) in the minimum tillage conditions. Genetika 2014, 46, 105–115. [Google Scholar] [CrossRef]
- Brown, A.H. Variation under domestication in plants: 1859 and today. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2523–2530. [Google Scholar] [CrossRef][Green Version]
- Levy, A.A.; Feldman, M. The impact of polyploidy on grass genome evolution. Plant Physiol. 2002, 130, 1587–1593. [Google Scholar] [CrossRef]
- Sabot, F.; Guyot, R.; Wicker, T.; Chantret, N.; Laubin, B.; Chalhoub, B.; Leroy, P.; Sourdille, P.; Bernard, M. Updating of transposable element annotations from large wheat genomic sequences reveals diverse activities and gene associations. Mol. Genet. Genom. 2005, 274, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Nalam, V.J.; Vales, M.I.; Watson, C.J.; Kianian, S.F.; Riera-Lizarazu, O. Map-based analysis of genes affecting the brittle rachis character in tetraploid wheat (Triticum turgidum L.). Theor. Appl. Genet. 2006, 112, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.; Belford, R.; Perry, M.; Tennant, D. Growth, development and light interception of old and modern wheat cultivars in a Mediterranean-type environment. Aust. J. Agric. Res. 1989, 40, 473–487. [Google Scholar]
- Poland, J.; Endelman, J.; Dawson, J.; Rutkoski, J.; Wu, S.; Manes, Y.; Dreisigacker, S.; Crossa, J.; Sánchez-Villeda, H.; Sorrells, M. Genomic selection in wheat breeding using genotyping-by-sequencing. Plant Genome 2012, 5, 103–113. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawson, T. From green to gold: Agricultural revolution for food security. J. Exp. Bot. 2020, 71, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Sun, J.; Jia, J.; Ren, Z. Haplotype variation of Green Revolution gene Rht-D1 during wheat domestication and improvement. J. Integr. Plant Biol. 2014, 56, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; van Ginkel, M. Increasing the Yield Potential in Wheat: Breaking the Barriers; Cimmyt: Mex, Mexico, 1996. [Google Scholar]
- Börner, A.; Plaschke, J.; Korzun, V.; Worland, A. The relationships between the dwarfing genes of wheat and rye. Euphytica 1996, 89, 69–75. [Google Scholar] [CrossRef]
- Na, T.; Jiang, Y.; He, B.-R.; Hu, Y.-G. The effects of dwarfing genes (Rht-B1b, Rht-D1b, and Rht8) with different sensitivity to GA3 on the coleoptile length and plant height of wheat. Agric. Sci. China 2009, 8, 1028–1038. [Google Scholar]
- Hooley, R. Gibberellins: Perception, transduction and responses. Plant Mol. Biol. 1994, 26, 1529–1555. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.E.; Hartley, N.M.; Murphy, G.P.; Devos, K.M.; Flintham, J.E.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F. ‘Green revolution’genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef]
- Allan, R. Agronomic comparisons between Rht1 and Rht2 semidwarf genes in winter wheat. Crop Sci. 1989, 29, 1103–1108. [Google Scholar] [CrossRef]
- Rebetzke, G.; Richards, R.; Fettell, N.; Long, M.; Condon, A.G.; Forrester, R.; Botwright, T. Genotypic increases in coleoptile length improves stand establishment, vigour and grain yield of deep-sown wheat. Field Crops Res. 2007, 100, 10–23. [Google Scholar] [CrossRef]
- Khan, K. Wheat: Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Indexmundi. Available online: https://www.indexmundi.com/ (accessed on 23 July 2021).
- Cauvain, S.P. Breadmaking: Improving Quality; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Vansteelandt, J.; Delcour, J. Physical behavior of durum wheat starch (Triticum durum) during industrial pasta processing. J. Agric. Food Chem. 1998, 46, 2499–2503. [Google Scholar] [CrossRef]
- Kaup, S.; Walker, C. Couscous in North Africa; Cereal Foods World: St. Paul, MN, USA, 1986. [Google Scholar]
- Elias, E. Durum wheat products. In Durum Wheat Improvement in the Mediterranean Region: New Challenges, Serie A: Séminaires Méditerranéennes; CIHEAM: Paris, France, 1995; Volume 40, pp. 23–31. [Google Scholar]
- Pauly, A.; Pareyt, B.; Fierens, E.; Delcour, J.A. Wheat (Triticum aestivum L. and T. turgidum L. ssp. durum) kernel hardness: II. Implications for end-product quality and role of puroindolines therein. Compr. Rev. Food Sci. Food Saf. 2013, 12, 427–438. [Google Scholar] [CrossRef]
- Qarooni, J. Wheat characteristics for flat breads: Hard or soft, white or red? Cereal Foods World 1996, 41, 391–395. [Google Scholar]
- Nagao, S. Quality characteristics of soft wheats and their utilization in Japan. II. Evaluation of wheats from the United States, Australia, France and Japan. Cereal Chem. 1977, 54, 198–204. [Google Scholar]
- Lee, M.R.; Swanson, B.G.; Baik, B.K.J. Influence of amylose content on properties of wheat starch and breadmaking quality of starch and gluten blends. Cereal Chem. 2001, 78, 701–706. [Google Scholar] [CrossRef]
- Peng, Q.; Wu, R.-L.; Kong, Z.-Y.; Zhang, B.-Q.J. Effect of waxy wheat flour blends on the quality of fresh and stale bread. Agric. Sci. China 2009, 8, 401–409. [Google Scholar]
- Graybosch, R.A.J. Technology. Waxy wheats: Origin, properties, and prospects. Trends Food Sci. Technol. 1998, 9, 135–142. [Google Scholar] [CrossRef]
- Arzani, A. Emmer (Triticum turgidum spp. dicoccum) flour and breads. In Flour and Breads and Their Fortification in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 69–78. [Google Scholar]
- Hidalgo, A.; Brandolini, A.J. Agriculture. Nutritional properties of einkorn wheat (Triticum monococcum L.). J. Sci. Food Agric. 2014, 94, 601–612. [Google Scholar] [CrossRef]
- Konvalina, P.; Moudrý, J., Jr.; Moudrý, J.J. Quality parametres of emmer wheat landraces. J. Cent. Eur. Agric. 2008, 9, 539–545. [Google Scholar]
- Geisslitz, S.; Scherf, K.A. Rediscovering ancient wheats. J. Cereal Sci. 2020, 65, 236–243. [Google Scholar]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F.J. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Folloni, S.; Sforza, S.; Vittadini, E.; Prandi, B. Current Trends in Ancient Grains-Based Foodstuffs: Insights into Nutritional Aspects and Technological Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Piergiovanni, A.; Caponio, F.; Paradiso, V.M.; Summo, C.; Simeone, R. Evaluation of the technological characteristics and bread-making quality of alternative wheat cereals in comparison with common and durum wheat. Food Sci. Technol. Int. 2011, 17, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Frakolaki, G.; Giannou, V.; Topakas, E.; Tzia, C. Chemical characterization and breadmaking potential of spelt versus wheat flour. J. Cereal Sci. 2018, 79, 50–56. [Google Scholar] [CrossRef]
- Gomez-Becerra, H.F.; Erdem, H.; Yazici, A.; Tutus, Y.; Torun, B.; Ozturk, L.; Cakmak, I. Grain concentrations of protein and mineral nutrients in a large collection of spelt wheat grown under different environments. J. Cereal Sci. 2010, 52, 342–349. [Google Scholar] [CrossRef]
- Koenig, A.; Konitzer, K.; Wieser, H.; Koehler, P. Classification of spelt cultivars based on differences in storage protein compositions from wheat. Food Chem. 2015, 168, 176–182. [Google Scholar] [CrossRef]
- Sabença, C.; Ribeiro, M.; Sousa, T.d.; Poeta, P.; Bagulho, A.S.; Igrejas, G.J.F. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar] [CrossRef]
- Bakery Products Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026); Mordor Intelligence: Telangana, India, 2021.
- Di Cagno, R.; Barbato, M.; Di Camillo, C.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; De Vincenzi, M.; Gobbetti, M.; Cucchiara, S.J. Gluten-free sourdough wheat baked goods appear safe for young celiac patients: A pilot study. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 777–783. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; Giovannini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.J.A.; et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.-M.; Loponen, J.; Poussa, T.; Huang, X.; Sontag-Strohm, T.; Salmenkari, H.; Korpela, R.J.N. Pilot study: Comparison of sourdough wheat bread and yeast-fermented wheat bread in individuals with wheat sensitivity and irritable bowel syndrome. Nutrition 2017, 9, 1215. [Google Scholar] [CrossRef]
- Meziani, S.; Jasniewski, J.; Gaiani, C.; Ioannou, I.; Muller, J.-M.; Ghoul, M.; Desobry, S.J. Effects of freezing treatments on viscoelastic and structural behavior of frozen sweet dough. J. Food Eng. 2011, 107, 358–365. [Google Scholar] [CrossRef]
- Wang, P.; Xu, L.; Nikoo, M.; Ocen, D.; Wu, F.; Yang, N.; Jin, Z.; Xu, X.J. Effect of frozen storage on the conformational, thermal and microscopic properties of gluten: Comparative studies on gluten-, glutenin-and gliadin-rich fractions. Food Hydrocoll. 2014, 35, 238–246. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Mohanad, B.; Xu, L.; Ning, Y.; Xu, J.; Wu, F.; Yang, N.; Jin, Z.; Xu, X. Effect of frozen storage on physico-chemistry of wheat gluten proteins: Studies on gluten-, glutenin-and gliadin-rich fractions. Food Hydrocoll. 2014, 39, 187–194. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Hu, Z.; Li, L.; Li, B. Molecular structure evaluation of wheat gluten during frozen storage. Food Biophys. 2017, 12, 60–68. [Google Scholar] [CrossRef]
- Phimolsiripol, Y.; Siripatrawan, U.; Tulyathan, V.; Cleland, D.J. Effects of freezing and temperature fluctuations during frozen storage on frozen dough and bread quality. J. Food Eng. 2008, 84, 48–56. [Google Scholar] [CrossRef]
- Loveday, S.M.; Huang, V.T.; Reid, D.S.; Winger, R.J. Water dynamics in fresh and frozen yeasted dough. Crit. Rev. Food Sci. Nutr. 2012, 52, 390–409. [Google Scholar] [CrossRef]
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A plant-based high-carbohydrate, low-fat diet in overweight individuals in a 16-week randomized clinical trial: The role of carbohydrates. Nutrition 2018, 10, 1302. [Google Scholar] [CrossRef]
- Guragain, Y.N.; Ganesh, K.; Bansal, S.; Sathish, R.S.; Rao, N.; Vadlani, P.V. Low-lignin mutant biomass resources: Effect of compositional changes on ethanol yield. Ind. Crops Prod. 2014, 61, 1–8. [Google Scholar] [CrossRef]
- Khan, K.; Shewry, P.R. Wheat: Chemistry and Technology; AACC International: Eagan, MN, USA, 2009. [Google Scholar]
- Bushuk, W.; Rasper, V.F. Wheat: Production, Properties and Quality; Springer Science & Business Media: Berlin, Germany, 1994. [Google Scholar]
- Poutanen, K.J. Past and future of cereal grains as food for health. Trends Food Sci. Technol. 2012, 25, 58–62. [Google Scholar] [CrossRef]
- Escarnot, E.; Dornez, E.; Verspreet, J.; Agneessens, R.; Courtin, C.M. Quantification and visualization of dietary fibre components in spelt and wheat kernels. J. Cereal Sci. 2015, 62, 124–133. [Google Scholar] [CrossRef]
- Finnie, S.; Jeannotte, R.; Faubion, J.M. Quantitative characterization of polar lipids from wheat whole meal, flour, and starch. Cereal Chem. J. 2009, 86, 637–645. [Google Scholar] [CrossRef]
- Uthayakumaran, S.; Wrigley, C. Wheat: Grain-quality characteristics and management of quality requirements. In Cereal Grains; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–134. [Google Scholar]
- Righetti, L.; Rubert, J.; Galaverna, G.; Folloni, S.; Ranieri, R.; Stranska-Zachariasova, M.; Hajslova, J.; Dall’Asta, C. Characterization and Discrimination of Ancient Grains: A Metabolomics Approach. Int. J. Mol. Sci. 2016, 17, 1217. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Pompei, C. Carotenoids evolution during pasta, bread and water biscuit preparation from wheat flours. Food Chem. 2010, 121, 746–751. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Ratti, S.J. Influence of genetic and environmental factors on selected nutritional traits of Triticum monococcum. J. Agric. Food Chem. 2009, 57, 6342–6348. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Ribeiro, M.; Sousa, T.d.; Sabença, C.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Advances in quantification and analysis of the celiac-related immunogenic potential of gluten. Compr. Rev. Food Sci. Food Saf. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Korner, R.; Ren, O.K. The intes-final T cell response to a gliadin in adult celiac disease is fo-cused on a single deamidated glutamine targeted by tissue transglutaminase. JEM 2000, 191, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Molberg, Ø.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Laviano, L.; Catellani, M.; Milc, J.; Prandi, B.; Boukid, F.; Sforza, S.; Dossena, A.; Graziano, S.; Gullì, M.; et al. Influence of environmental and genetic factors on content of toxic and immunogenic wheat gluten peptides. Eur. J. Agron. 2020, 118, 126091. [Google Scholar] [CrossRef]
- Ribeiro, M.; Nunes, F.M.; Guedes, S.; Domingues, P.; Silva, A.M.; Carrillo, J.M.; Rodriguez-Quijano, M.; Branlard, G.; Igrejas, G. Efficient chemo-enzymatic gluten detoxification: Reducing toxic epitopes for celiac patients improving functional properties. Sci. Rep. 2015, 5, 1–17. [Google Scholar] [CrossRef]
- Van Herpen, T.W.; Goryunova, S.V.; Van Der Schoot, J.; Mitreva, M.; Salentijn, E.; Vorst, O.; Schenk, M.F.; Van Veelen, P.A.; Koning, F.; Van Soest, L.J.; et al. Alpha-gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC Genom. 2006, 7, 1–13. [Google Scholar] [CrossRef]
- Malalgoda, M.; Simsek, S. Celiac disease and cereal proteins. Food Hydrocoll. 2017, 68, 108–113. [Google Scholar] [CrossRef]
- Ribeiro, M.; Nunes, F.M. We might have got it wrong: Modern wheat is not more toxic for celiac patients. Food Chem. 2019, 278, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Rodriguez-Quijano, M.; Nunes, F.M.; Carrillo, J.M.; Branlard, G.; Igrejas, G. New insights into wheat toxicity: Breeding did not seem to contribute to a prevalence of potential celiac disease’s immunostimulatory epitopes. Food Chem. 2016, 213, 8–18. [Google Scholar] [CrossRef]
- Prandi, B.; Tedeschi, T.; Folloni, S.; Galaverna, G.; Sforza, S. Peptides from gluten digestion: A comparison between old and modern wheat varieties. Food Res. Int. 2017, 91, 92–102. [Google Scholar] [CrossRef]
- Malalgoda, M.; Meinhardt, S.W.; Simsek, S. Detection and quantitation of immunogenic epitopes related to celiac disease in historical and modern hard red spring wheat cultivars. Food Chem. 2018, 264, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Spaenij–Dekking, L.; Kooy–Winkelaar, Y.; van Veelen, P.; Drijfhout, J.W.; Jonker, H.; van Soest, L.; Smulders, M.J.; Bosch, D.; Gilissen, L.J.; Koning, F. Natural variation in toxicity of wheat: Potential for selection of nontoxic varieties for celiac disease patients. Gastroenterology 2005, 129, 797–806. [Google Scholar] [CrossRef]
- Pasha, I.; Anjum, F.; Morris, C. Grain hardness: A major determinant of wheat quality. Food Sci. Technol. Int. 2010, 16, 511–522. [Google Scholar] [CrossRef]
- Gazza, L.; Nocente, F.; Ng, P.; Pogna, N. Genetic and biochemical analysis of common wheat cultivars lacking puroindoline a. Theor. Appl. Genet. 2005, 110, 470–478. [Google Scholar] [CrossRef]
- Ribeiro, M.; Rodríguez-Quijano, M.; Giraldo, P.; Pinto, L.; Vázquez, J.F.; Carrillo, J.M.; Igrejas, G. Effect of allelic variation at glutenin and puroindoline loci on bread-making quality: Favorable combinations occur in less toxic varieties of wheat for celiac patients. Eur. Food Res. Technol. 2017, 243, 743–752. [Google Scholar] [CrossRef]
- Greenblatt, G.; Bettge, A.; Morris, C. Relationship between Endosperm Texture and the Occurrence of Friabilin and Bound Polar Lipids on Wheat Starch; American Association of Cereal Chemists: St. Paul, MN, USA, 1995. [Google Scholar]
- Pomeranz, Y. Composition and functionality of wheat-flour components. Wheat Chem. Technol. 1971, 3, 385–674. [Google Scholar]
- Guerrieri, N.; Cavaletto, M. Cereals proteins. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 223–244. [Google Scholar]
- Gao, L.; Wang, A.; Li, X.; Dong, K.; Wang, K.; Appels, R.; Ma, W.; Yan, Y. Wheat quality related differential expressions of albumins and globulins revealed by two-dimensional difference gel electrophoresis (2-D DIGE). J. Proteom. 2009, 73, 279–296. [Google Scholar] [CrossRef]
- Boukid, F.; Prandi, B.; Faccini, A.; Sforza, S.J. A complete mass spectrometry (MS)-based peptidomic description of gluten peptides generated during in vitro gastrointestinal digestion of durum wheat: Implication for celiac disease. J. Am. Soc. Mass Spectrom. 2019, 30, 1481–1490. [Google Scholar] [CrossRef]
- Platzer, B.; Baker, K.; Vera, M.P.; Singer, K.; Panduro, M.; Lexmond, W.S.; Turner, D.; Vargas, S.O.; Kinet, J.P.; Maurer, D.; et al. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol. 2015, 8, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Veraverbeke, W.S.; Delcour, J.A. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. Crit. Rev. Food Sci. Nutr. 2002, 42, 179–208. [Google Scholar] [CrossRef] [PubMed]
- Tomić, J.; Torbica, A.; Popović, L.; Strelec, I.; Vaštag, Ž.; Pojić, M.; Rakita, S.J. Albumins characterization in relation to rheological properties and enzymatic activity of wheat flour dough. JAST 2015, 17, 805–816. [Google Scholar]
- Chen, X.; Cao, X.; Zhang, Y.; Islam, S.; Zhang, J.; Yang, R.; Liu, J.; Li, G.; Appels, R.; Keeble-Gagnere, G.; et al. Genetic characterization of cysteine-rich type-b avenin-like protein coding genes in common wheat. Sci. Rep. 2016, 6, 30692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Hu, X.; Islam, S.; She, M.; Peng, Y.; Yu, Z.; Wylie, S.; Juhasz, A.; Dowla, M.; Yang, R.; et al. New insights into the evolution of wheat avenin-like proteins in wild emmer wheat (Triticum dicoccoides). Proc. Natl. Acad. Sci. USA 2018, 115, 13312–13317. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Xiao, Y.; Crossa, J.; González-Santoyo, H.; Huerta, J.; Singh, R.; Dreisigacker, S. Sources of the highly expressed wheat bread making (wbm) gene in CIMMYT spring wheat germplasm and its effect on processing and bread-making quality. Euphytica 2016, 209, 689–692. [Google Scholar] [CrossRef]
- Beccari, J.B. De Bononiensi Scientiarum et Artium Atque Academia Commentarii; Part I; Nabu Press: Charleston, SC, USA, 1745; pp. 122–127. [Google Scholar]
- Osborne, T.B. The Vegetable Proteins; Longmans, Green and Company: London, UK, 1924. [Google Scholar]
- Shewry, P.R.; Tatham, A.S. The characteristics, structures and evolutionary relationships of prolamins. In Seed Proteins; Springer: Berlin/Heidelberg, Germany, 1999; pp. 11–33. [Google Scholar]
- Osborne, T.B. The Proteins of the Wheat Kernel; Carnegie Institution: Washington, DC, USA, 1907. [Google Scholar]
- Ribeiro, M.; Nunes-Miranda, J.D.; Branlard, G.; Carrillo, J.M.; Rodriguez-Quijano, M.; Igrejas, G. One hundred years of grain omics: Identifying the glutens that feed the world. J. Proteome Res. 2013, 12, 4702–4716. [Google Scholar] [CrossRef]
- Ribeiro, M.; Nunes, F.M.; Rodriguez-Quijano, M.; Carrillo, J.M.; Branlard, G.; Igrejas, G. Next-generation therapies for celiac disease: The gluten-targeted approaches. Trends Food Sci. Technol. 2018, 75, 56–71. [Google Scholar] [CrossRef]
- Tatham, A.S.; Shewry, P.R. The S-poor prolamins of wheat, barley and rye: Revisited. J. Cereal Sci. 2012, 55, 79–99. [Google Scholar] [CrossRef]
- Payne, P.; Holt, L.M.; Jackson, E.A. Wheat storage proteins: Their genetics and their potential for manipulation by plant breeding. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 359–371. [Google Scholar]
- Payne, P.I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu. Rev. Plant Physiol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Shewry, P.; Tatham, A. Disulphide bonds in wheat gluten proteins. J. Cereal Sci. 1997, 25, 207–227. [Google Scholar] [CrossRef]
- Branlard, G.; Dardevet, M.; Saccomano, R.; Lagoutte, F.; Gourdon, J. Genetic diversity of wheat storage proteins and bread wheat quality. Euphytica 2001, 119, 59–67. [Google Scholar] [CrossRef]
- Agarwal, M.; Shrivastava, N.; Padh, H. Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep. 2008, 27, 617–631. [Google Scholar] [CrossRef]
- Payne, P.I.; Lawrence, G.J. Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1, and Glu-D1 which code for high-molecular-weight subunits of glutenin in hexaploid wheat. Cereal Res. Commun. 1983, 11, 29–35. [Google Scholar]
- Payne, P.I.; Holt, L.M.; Lawrence, G.J.; Law, C.N. The genetics of gliadin and glutenin, the major storage proteins of the wheat endosperm. Plant Foods Hum. Nutr. 1982, 31, 229–241. [Google Scholar] [CrossRef]
- Jackson, E.A.; Holt, L.M.; Payne, P.I. Characterisation of high molecular weight gliadin and low-molecular-weight glutenin subunits of wheat endosperm by two-dimensional electrophoresis and the chromosomal localisation of their controlling genes. Theor. Appl. Genet. 1983, 66, 29–37. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Yan, Y.; Appels, R.; Mahmood, T.; He, Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef]
- Ribeiro, M.; Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Review of Structural Features and Binding Capacity of Polyphenols to Gluten Proteins and Peptides In Vitro: Relevance to Celiac Disease. Antioxidants 2020, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 78–81. [Google Scholar] [CrossRef]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Dupont, F.M.; Vensel, W.H.; Tanaka, C.K.; Hurkman, W.J.; Altenbach, S.B. Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci. 2011, 9, 1–29. [Google Scholar] [CrossRef]
- Payne, P.I.; Corfield, K.G.; Holt, L.M.; Blackman, J.A. Correlations between the inheritance of certain high-molecular weight subunits of glutenin and bread-making quality in progenies of six crosses of bread wheat. J. Sci. Food Agric. 1981, 32, 51–60. [Google Scholar] [CrossRef]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Igrejas, G.; Guedes-Pinto, H.; Carnide, V.; Clement, J.; Branlard, G. Genetical, biochemical and technological parameters associated with biscuit quality. II. Prediction using storage proteins and quality characteristics in a soft wheat population. J. Cereal Sci. 2002, 36, 187–197. [Google Scholar] [CrossRef]
- Metakovsky, E.; Branlard, G.; Chernakov, V.; Upelniek, V.; Redaelli, R.; Pogna, N. Recombination mapping of some chromosome 1A-, 1B-, 1D-and 6B-controlled gliadins and low-molecular-weight glutenin subunits in common wheat. Theor. Appl. Genet. 1997, 94, 788–795. [Google Scholar] [CrossRef]
- Masci, S.; Rovelli, L.; Kasarda, D.; Vensel, W.; Lafiandra, D. Characterisation and chromosomal localisation of C-type low-molecular-weight glutenin subunits in the bread wheat cultivar Chinese Spring. Theor. Appl. Genet. 2002, 104, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhen, S.; Luo, N.; Han, C.; Lu, X.; Li, X.; Xia, X.; He, Z.; Yan, Y. Low molecular weight glutenin subunit gene Glu-B3h confers superior dough strength and breadmaking quality in wheat (Triticum aestivum L.). Sci. Rep. 2016, 6, 27182. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Q.; Cloutier, S. Molecular characterization and genomic organization of low molecular weight glutenin subunit genes at the Glu-3 loci in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 2008, 116, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, C.; Békés, F.; Bushuk, W. Gluten: A balance of gliadin and glutenin. In Gliadin and Glutenin: The Unique Balance of Wheat Quality; Amer Assn of Cereal Chemists: St. Paul, MN, USA, 2006; pp. 3–32. [Google Scholar]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative Study on Gluten Protein Composition of Ancient (Einkorn, Emmer and Spelt) and Modern Wheat Species (Durum and Common Wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef]
- Dobraszczyk, B.; Morgenstern, M. Rheology and the breadmaking process. J. Cereal Sci. 2003, 38, 229–245. [Google Scholar] [CrossRef]
- McLeish, T.; Larson, R. Molecular constitutive equations for a class of branched polymers: The pom-pom polymer. J. Rheol. 1998, 42, 81–110. [Google Scholar] [CrossRef]
- Belton, P. Mini review: On the elasticity of wheat gluten. J. Cereal Sci. 1999, 29, 103–107. [Google Scholar] [CrossRef]
- Don, C.; Lichtendonk, W.; Plijter, J.; Hamer, R. Glutenin macropolymer: A gel formed by glutenin particles. J. Cereal Sci. 2003, 37, 1–7. [Google Scholar] [CrossRef]
- Hamer, R.; Vliet, T.v. Understanding the structure and properties of gluten: An overview. In Proceedings of the 7th International Workshop Gluten 2000, Bristol, UK, 2–6 April 2000; pp. 125–131. [Google Scholar]
- Greenwood, C.; Ewart, J. Hypothesis for the structure of glutenin in relation to rheological properties of gluten and dough [Wheat]. Cereal Chem. 1975, 52, 146–153. [Google Scholar]
- Ewart, J. A hypothesis for the structure and rheology of glutenin. J. Sci. Food Agric. 1968, 19, 617–623. [Google Scholar] [CrossRef]
- Lindsay, M.P.; Skerritt, J.H. The glutenin macropolymer of wheat flour doughs: Structure–function perspectives. Trends Food Sci. Technol. 1999, 10, 247–253. [Google Scholar] [CrossRef]
- Ewart, J.A. Calculated molecular weight distribution for glutenin. J. Sci. Food Agric. 1987, 38, 277–289. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. https://doi.org/10.3390/foods10092124
de Sousa T, Ribeiro M, Sabença C, Igrejas G. The 10,000-Year Success Story of Wheat! Foods. 2021; 10(9):2124. https://doi.org/10.3390/foods10092124
Chicago/Turabian Stylede Sousa, Telma, Miguel Ribeiro, Carolina Sabença, and Gilberto Igrejas. 2021. "The 10,000-Year Success Story of Wheat!" Foods 10, no. 9: 2124. https://doi.org/10.3390/foods10092124
APA Stylede Sousa, T., Ribeiro, M., Sabença, C., & Igrejas, G. (2021). The 10,000-Year Success Story of Wheat! Foods, 10(9), 2124. https://doi.org/10.3390/foods10092124