Red Beetroot’s NMR-Based Metabolomics: Phytochemical Profile Related to Development Time and Production Year
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Materials
2.2. Sample Preparation
2.3. Nitrate Determination
2.4. NMR Experiments
2.5. Statistical Analysis
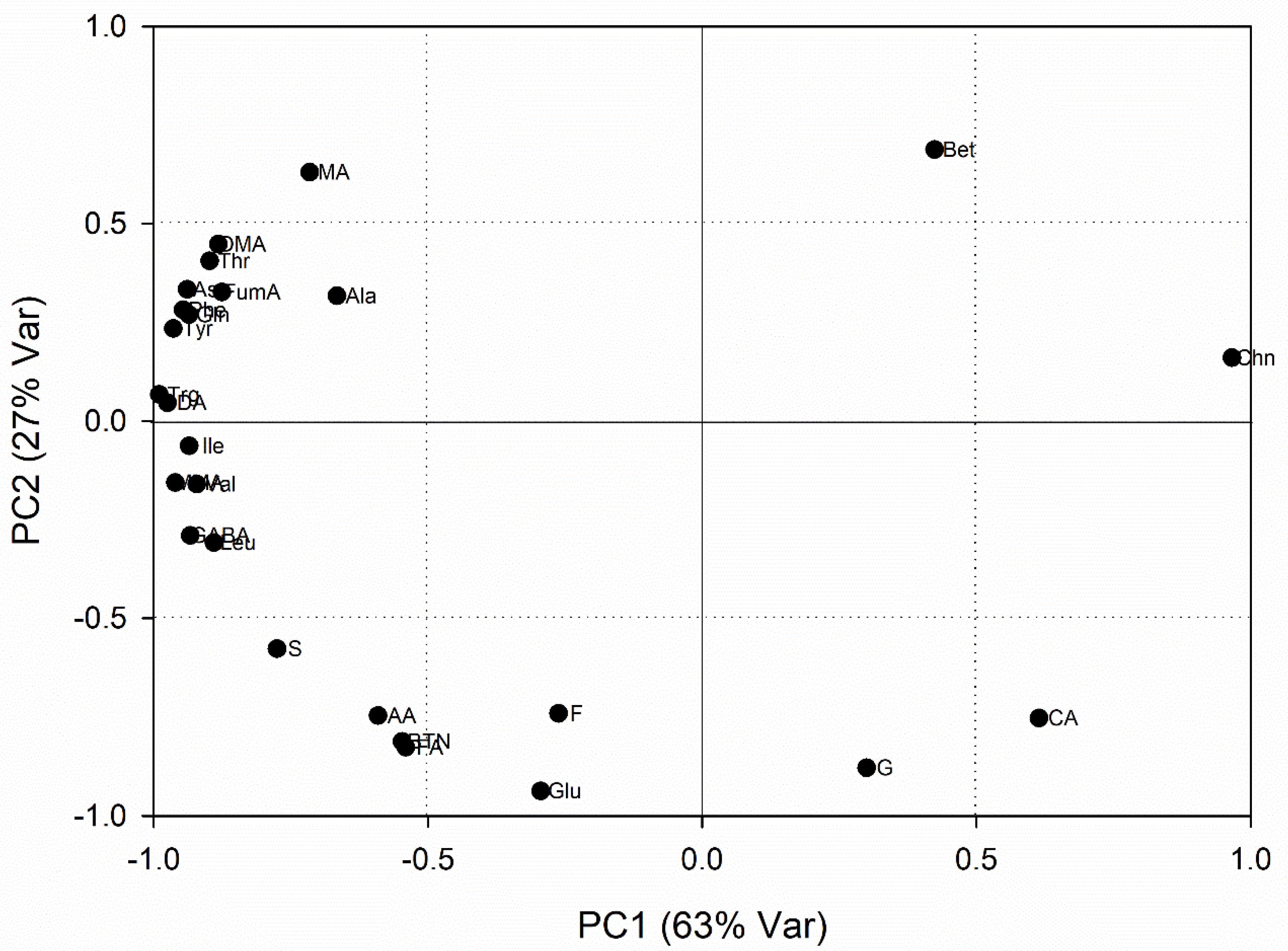
3. Results
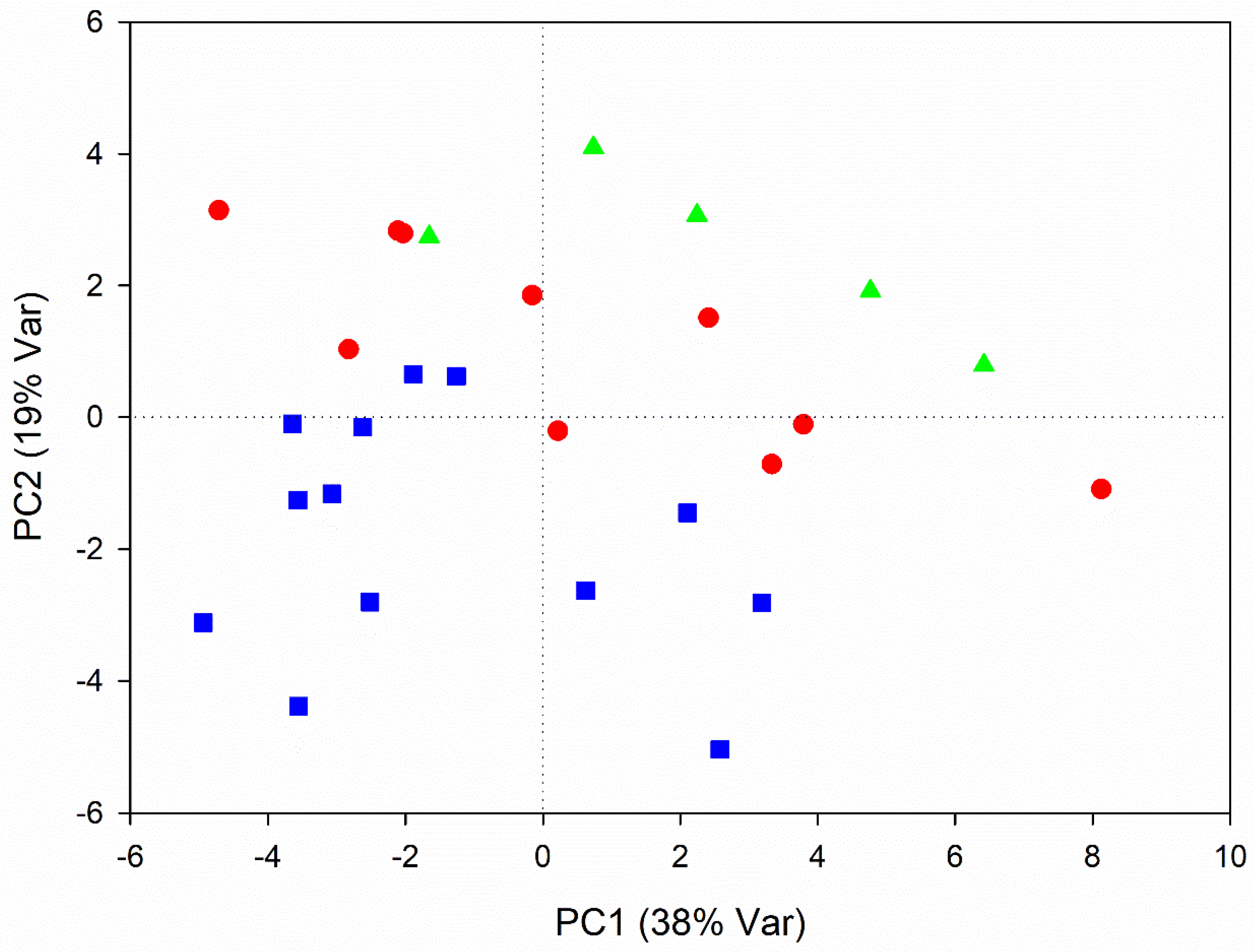
3.1. Metabolic Variations Related to Root Development
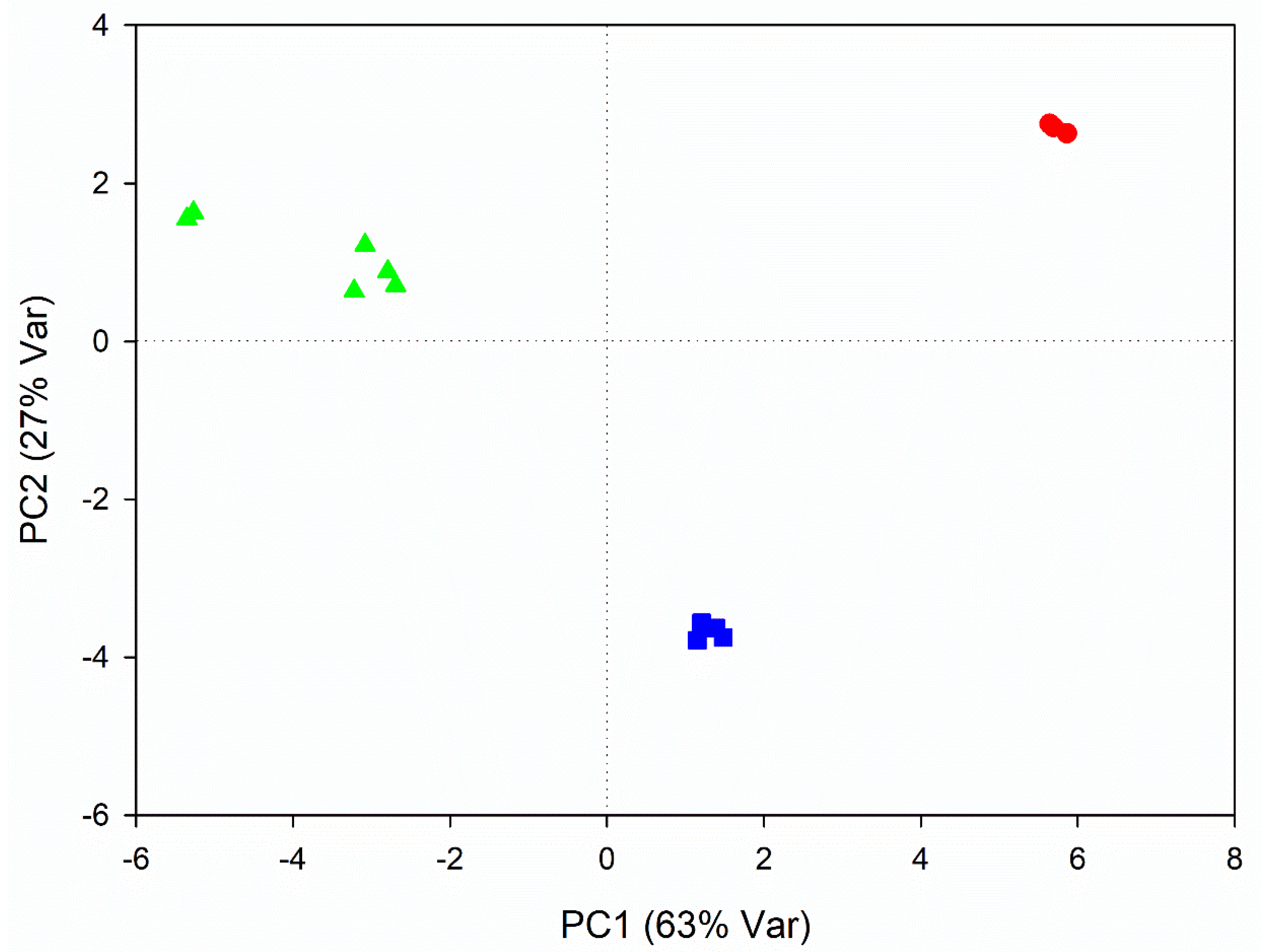
3.2. Metabolic Variations Related to Seasonal Changes
4. Discussion
4.1. Metabolic Variations Related to Root Development
4.2. Metabolic Variations Related to Seasonal Changes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 9 November 2020).
- Neha, P.; Jain, S.; Jain, N.; Jain, H.; Mittal, H. Chemical and Functional Properties of Beetroot (Beta vulgaris L.) for Product Development: A Review. Int. J. Chem. Stud. 2018, 6, 3190–3194. [Google Scholar]
- Babarykin, D.; Smirnova, G.; Pundinsh, I.; Vasiljeva, S.; Krumina, G.; Agejchenko, V. Red Beet (Beta vulgaris) Impact on Human Health. J. Biosci. Med. 2019, 7, 61–79. [Google Scholar] [CrossRef] [Green Version]
- Zamani, H.; de Joode, M.E.J.R.; Hossein, I.J.; Henckens, N.F.T.; Guggeis, M.A.; Berends, J.E.; de Kok, T.M.C.M.; van Breda, S.G.J. The benefits and risks of beetroot juice consumption: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 788–804. [Google Scholar] [CrossRef] [Green Version]
- Lidder, S.; Webb, A.J. Vascular Effects of Dietary Nitrate (as Found in Green Leafy Vegetables and Beetroot) via the Nitrate-Nitrite-Nitric Oxide Pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [Green Version]
- Arazi, H.; Eghbali, E. Possible Effects of Beetroot Supplementation on Physical Performance through Metabolic, Neuroendocrine, and Antioxidant Mechanisms: A Narrative Review of the Literature. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Baião, D.D.S.; da Silva, V.; Paschoalin, M. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Song, P.; Wu, L.; Guan, W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 94, 1–412. [Google Scholar]
- Vinson, J.A.; Hao, Y.; Su, A.X.; Zubik, L. Phenol Antioxidant Quantity and Quality in Foods: Vegetables. J. Agric. Food Chem. 1998, 46, 3630–3634. [Google Scholar] [CrossRef]
- Žitňanová, I.; Ranostajová, S.; Sobotová, H.; Demelová, D.; Pecháň, I.; Ďuračková, Z. Antioxidative activity of selected fruits and vegetables. Biologia 2006, 61, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Shah, S.; Butt, A.M.; Shah, S.M.; Jabeen, Z.; Nadeem, A. Biochemical Profiling and Elucidation of Biological Activities of Beta vulgaris L. Leaves and Roots Extracts. Saudi J. Biol. Sci. 2021, 28, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Arif, T.U.; Vachova, P.; Hejnak, V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.-M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant Activity and Phenolic Content of Betalain Extracts from Intact Plants and Hairy Root Cultures of the Red Beetroot Beta vulgaris cv. Detroit Dark Red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Sciubba, F.; Tomassini, A.; Giorgi, G.; Brasili, E.; Pasqua, G.; Capuani, G.; Aureli, W.; Miccheli, A. NMR-Based Metabolomic Study of Purple Carrot Optimal Harvest Time for Utilization as a Source of Bioactive Compounds. Appl. Sci. 2020, 10, 8493. [Google Scholar] [CrossRef]
- Sciubba, F.; Avanzato, D.; Vaccaro, A.; Capuani, G.; Spagnoli, M.; Di Cocco, M.E.; Tzareva, I.N.; Delfini, M. Monitoring of pistachio (Pistacia Vera) ripening by high field nuclear magnetic resonance spectroscopy. Nat. Prod. Res. 2016, 31, 765–772. [Google Scholar] [CrossRef]
- Tomassini, A.; Sciubba, F.; Di Cocco, M.E.; Capuani, G.; Delfini, M.; Aureli, W.; Miccheli, A. 1H NMR-Based Metabolomics Reveals a Pedoclimatic Metabolic Imprinting in Ready-to-Drink Carrot Juices. J. Agric. Food Chem. 2016, 64, 5284–5291. [Google Scholar] [CrossRef]
- Capitani, D.; Mannina, L.; Proietti, N.; Sobolev, A.P.; Tomassini, A.; Miccheli, A.; Di Cocco, M.E.; Capuani, G.; De Salvador, F.R.; Delfini, M. Metabolic Profiling and Outer Pericarp Water State in Zespri, CI.GI, and Hayward Kiwifruits. J. Agric. Food Chem. 2012, 61, 1727–1740. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris ) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Webb, K.M.; Delgrosso, S.J.; West, M.S.; Freeman, C.; Brenner, T. Influence of environment, crop age and cultivar on the development and severity of Fusarium yellows in field-grown sugar beet. Can. J. Plant Pathol. 2017, 39, 37–47. [Google Scholar] [CrossRef]
- Wolfgang, A.; Zachow, C.; Müller, H.; Grand, A.; Temme, N.; Tilcher, R.; Berg, G. Understanding the Impact of Cultivar, Seed Origin, and Substrate on Bacterial Diversity of the Sugar Beet Rhizosphere and Suppression of Soil-Borne Pathogens. Front. Plant Sci. 2020, 11, 560869. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.S.; Zhang, H.W.; Zhan, Z.X.; Liu, B.J.; Chen, Z.T.; Liang, Y. Transcriptome Analysis of Sucrose Metabolism during Bulb Swelling and Development in Onion (Allium cepa L.). Front. Plant Sci. 2016, 7, 1425. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-H.; Offler, C.E.; Ruan, Y.-L. Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Front. Plant Sci. 2013, 4, 282. [Google Scholar] [CrossRef] [Green Version]
- Sturm, A. Invertases. Primary Structures, Functions, and Roles in Plant Development and Sucrose Partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Godt, D.; Roitsch, T. The developmental and organ specific expression of sucrose cleaving enzymes in sugar beet suggests a transition between apoplasmic and symplasmic phloem unloading in the tap roots. Plant Physiol. Biochem. 2006, 44, 656–665. [Google Scholar] [CrossRef]
- Gillaspy, G.E. The cellular language of myo-inositol signaling. New Phytol. 2011, 192, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P.N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, Sources, and Applications of Betalain Pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Montes-Lora, S.; Rodríguez-Pulido, F.J.; Cejudo-Bastante, M.J.; Heredia, F.J. Implications of the Red Beet Ripening on the Colour and Betalain Composition Relationships. Plant Foods Hum. Nutr. 2018, 73, 216–221. [Google Scholar] [CrossRef]
- Archivio Report Climatici. Available online: https://www.regione.abruzzo.it/archivio-report-climatici (accessed on 9 December 2020).
- Laine, P.; Bigot, J.; Ourry, A.; Boucaud, J. Effects of low temperature on nitrate uptake, and xylem and phloem flows of nitrogen, in Secale cereale L. and Brassica napus L. New Phytol. 1994, 127, 675–683. [Google Scholar] [CrossRef]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2007, 132, 220–235. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the Temperature-Stress Metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef] [Green Version]
- Mayer, R.R.; Cherry, J.H.; Rhodes, D. Effects of Heat Shock on Amino Acid Metabolism of Cowpea Cells. Plant Physiol. 1990, 94, 796–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuramalingam, P.; Krishnan, S.R.; Pandian, S.; Mareeswaran, N.; Aruni, W.; Pandian, S.K.; Ramesh, M. Global analysis of threonine metabolism genes unravel key players in rice to improve the abiotic stress tolerance. Sci. Rep. 2018, 8, 9270. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, A.; Rut, G.; Krupa, J. Effect of abiotic stress factors on fluctuations in contents of malate and citrate and on malic enzyme activity in moss gametophores. Photosynthetica 2009, 47, 141–145. [Google Scholar] [CrossRef]
- Volino-Souza, M.; De Oliveira, G.V.; Conte-Junior, C.A.; Alvares, T.S. Covid-19 Quarantine: Impact of Lifestyle Behaviors Changes on Endothelial Function and Possible Protective Effect of Beetroot Juice. Front. Nutr. 2020, 7, 582210. [Google Scholar] [CrossRef] [PubMed]
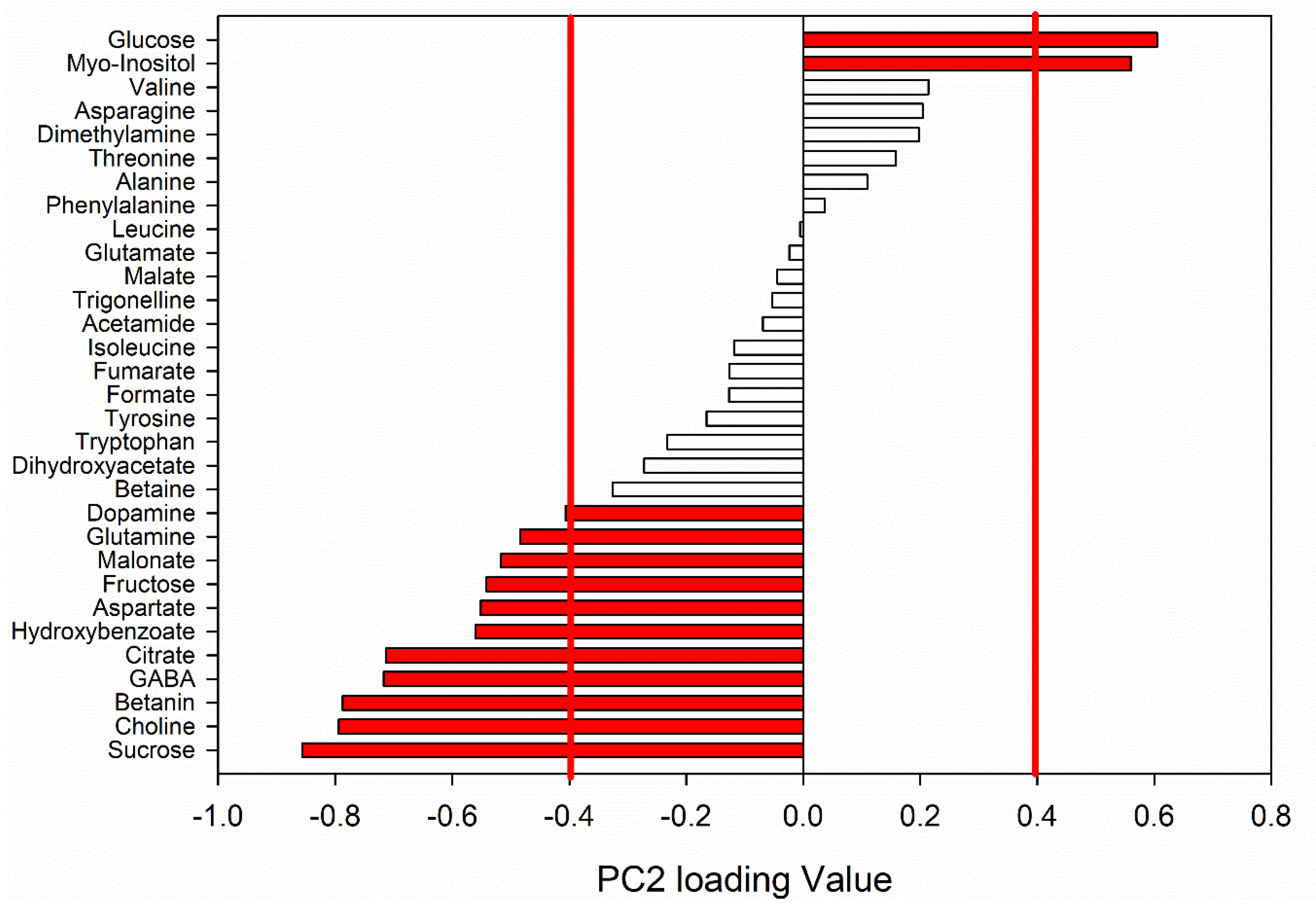
| Metabolite | (mg/100 g) | ||
|---|---|---|---|
| July | August | September | |
| Glucose | 20.0 ± 5.0 | 42.1 ± 8.6 § ** | 118 ± 50 † * |
| Myo-Inositol | 0.25 ± 0.27 | 0.50 ± 0.43 § * | 9.72 ± 1.98 † ** |
| Dopamine | 7.67 ± 0.18 | 5.60 ± 0.86 § * | 5.81 ± 2.45 |
| Glutamine | 245 ± 27 | 260 ± 34 | 250 ± 48 |
| Malonate | 3.05 ± 0.31 | 3.19 ± 0.37 | 3.46 ± 0.53 |
| Fructose | 32.2 ± 5.4 | 23.4 ± 6.5 §* | 9.38 ± 0.45 † * |
| Aspartate | 21.8 ± 3.1 | 14.5 ± 2.8 §* | 8.3 ± 1.9 † * |
| Hydroxybenzoate | 1.20 ± 0.15 | 0.97 ± 0.22 | 0.48 ± 0.15 † * |
| Citrate | 57.8 ± 6.7 | 52.2 ± 3.1 | 40.9 ± 6.7 |
| GABA | 10.06 ± 0.81 | 9.80 ± 0.92 | 9.4 ± 1.3 |
| Betanin | 56.7 ± 6.6 | 37.9 ± 4.5 § * | 23.0 ± 3.1 † * |
| Choline | 3.14 ± 0.22 | 2.29 ± 0.17 § ** | 1.63 ± 0.22 † * |
| Sucrose | 3196 ± 267 | 2514 ± 228 § * | 2049 ± 144 † * |
| Metabolite | (mg/100 mL) | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Leucine | 31.7 ± 4.1 a | 9.35 ± 0.79 b | 31.2 ± 3.4 a |
| Isoleucine | 19.4 ± 2.1 a | 6.48 ± 0.54 b | 22.5 ± 2.1 a |
| Valine | 16.6 ± 1.8 a | 6.70 ± 0.55 b | 16.3 ± 1.4 a |
| Threonine | 6.38 ± 0.63 a | 4.09 ± 0.30 a | 24.5 ± 2.9 b |
| Alanine | 47.5 ± 4.6 a | 31.5 ± 2.5 a | 44.8 ± 3.7 a |
| Glutamate | 177 ± 20 a | 24.3 ± 1.9 b | 82.8 ± 6.6 c |
| Glutamine | 233 ± 14 a | 134 ± 10 b | 291 ± 13 c |
| Asparagine | 29.8 ± 2.8 a | 16.4 ± 1.5 a | 57.2 ± 4.4 b |
| GABA | 39.8 ± 3.5 a | 12.5 ± 1.0 b | 40.0 ± 3.5 a |
| Tyrosine | 6.53 ± 0.56 a | 3.48 ± 0.24 b | 8.94 ± 0.74 c |
| Phenylalanine | 1.83 ± 0.17 a | 0.90 ± 0.07 b | 3.47 ± 0.30 c |
| Fructose | 497 ± 109 a | 111.1 ± 6.5 b | 266 ± 28 c |
| Glucose | 1185 ± 113 a | 560 ± 42 b | 647 ± 46 b |
| Sucrose | 14482 ± 1453 a | 6725 ± 538 b | 12005 ± 1077 a |
| Citrate | 147 ± 13 a | 54.0 ± 3.5 b | 17.9 ± 2.7 c |
| Malonate | 5.54 ± 0.48 a | 2.09 ± 0.31 b | 5.82 ± 0.45 c |
| Malate | 137 ± 12 a | 114.0 ± 8.8 b | 189 ± 14 c |
| Fumarate | 0.44 ± 0.15 a | 0.25 ± 0.04 a | 0.91 ± 0.07 b |
| Formate | 4.45 ± 0.43 a | 0.80 ± 0.08 b | 2.77 ± 0.23 c |
| Acetamide | 5.78 ± 0.94 a | 0.12 ± 0.06 b | 3.60 ± 0.47 a |
| Dimethylamine | 5.45 ± 0.77 a | 10.22 ± 0.63 b | 130.5 ± 9.3 c |
| Choline | 23.3 ± 2.6 a | 30.4 ± 2.4 a | 6.15 ± 0.48 b |
| Betaine | 297 ± 28 a | 248 ± 20 a | 260 ± 22 a |
| Dopamine | 19.4 ± 1.5 a | 4.16 ± 0.24 b | 31.5 ± 2.9 c |
| Betanin | 193 ± 30 a | 17.8 ± 1.4 b | 115.0 ± 8.8 c |
| Trigonelline | 1.42 ± 0.14 a | 0.51 ± 0.14 b | 1.97 ± 0.16 a |
| Year | Climatic Parameters | Month | |||
|---|---|---|---|---|---|
| June | July | August | September | ||
| 2016 | Average Min. Temperature (°C) | 12.8 | 15.7 | 14.2 | 12.0 |
| N° of days with Temperature < 11 °C | 2 | 1 | 1 | 3 | |
| Average Max. Temperature (°C) | 29.0 | 32.8 | 35.4 | 25.5 | |
| Total mm of precipitations | 39.2 | 44.4 | 86.4 | 75.8 | |
| 2017 | Average Min. Temperature (°C) | 14.5 | 15.2 | 15.7 | 11.5 |
| N° of days with Temperature < 11 °C | 1 | 1 | 1 | 4 | |
| Average Max. Temperature (°C) | 32.5 | 34.4 | 35.8 | 26.1 | |
| Total mm of precipitations | 5.2 | 15.8 | 4.6 | 69.9 | |
| 2018 | Average Min. Temperature (°C) | 12.9 | 15.5 | 14.1 | 12.0 |
| N° of days with Temperature < 11 °C | 6 | 2 | 5 | 8 | |
| Average Max. Temperature (°C) | 29.2 | 33.5 | 32.3 | 28.3 | |
| Total mm of precipitations | 41.4 | 15.8 | 133.6 | 19.2 | |
| Year | Nitrate Content (ppm) |
|---|---|
| 2016 | 3263 ± 262 |
| 2017 | 2883 ± 47 |
| 2018 | 1709 ± 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giampaoli, O.; Sciubba, F.; Conta, G.; Capuani, G.; Tomassini, A.; Giorgi, G.; Brasili, E.; Aureli, W.; Miccheli, A. Red Beetroot’s NMR-Based Metabolomics: Phytochemical Profile Related to Development Time and Production Year. Foods 2021, 10, 1887. https://doi.org/10.3390/foods10081887
Giampaoli O, Sciubba F, Conta G, Capuani G, Tomassini A, Giorgi G, Brasili E, Aureli W, Miccheli A. Red Beetroot’s NMR-Based Metabolomics: Phytochemical Profile Related to Development Time and Production Year. Foods. 2021; 10(8):1887. https://doi.org/10.3390/foods10081887
Chicago/Turabian StyleGiampaoli, Ottavia, Fabio Sciubba, Giorgia Conta, Giorgio Capuani, Alberta Tomassini, Giorgio Giorgi, Elisa Brasili, Walter Aureli, and Alfredo Miccheli. 2021. "Red Beetroot’s NMR-Based Metabolomics: Phytochemical Profile Related to Development Time and Production Year" Foods 10, no. 8: 1887. https://doi.org/10.3390/foods10081887
APA StyleGiampaoli, O., Sciubba, F., Conta, G., Capuani, G., Tomassini, A., Giorgi, G., Brasili, E., Aureli, W., & Miccheli, A. (2021). Red Beetroot’s NMR-Based Metabolomics: Phytochemical Profile Related to Development Time and Production Year. Foods, 10(8), 1887. https://doi.org/10.3390/foods10081887