O/W Pickering Emulsions Stabilized with Cellulose Nanofibrils Produced through Different Mechanical Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mechanical Treatments of Cellulose and Tomato Pomace
2.3. Preparation of Pickering Emulsions
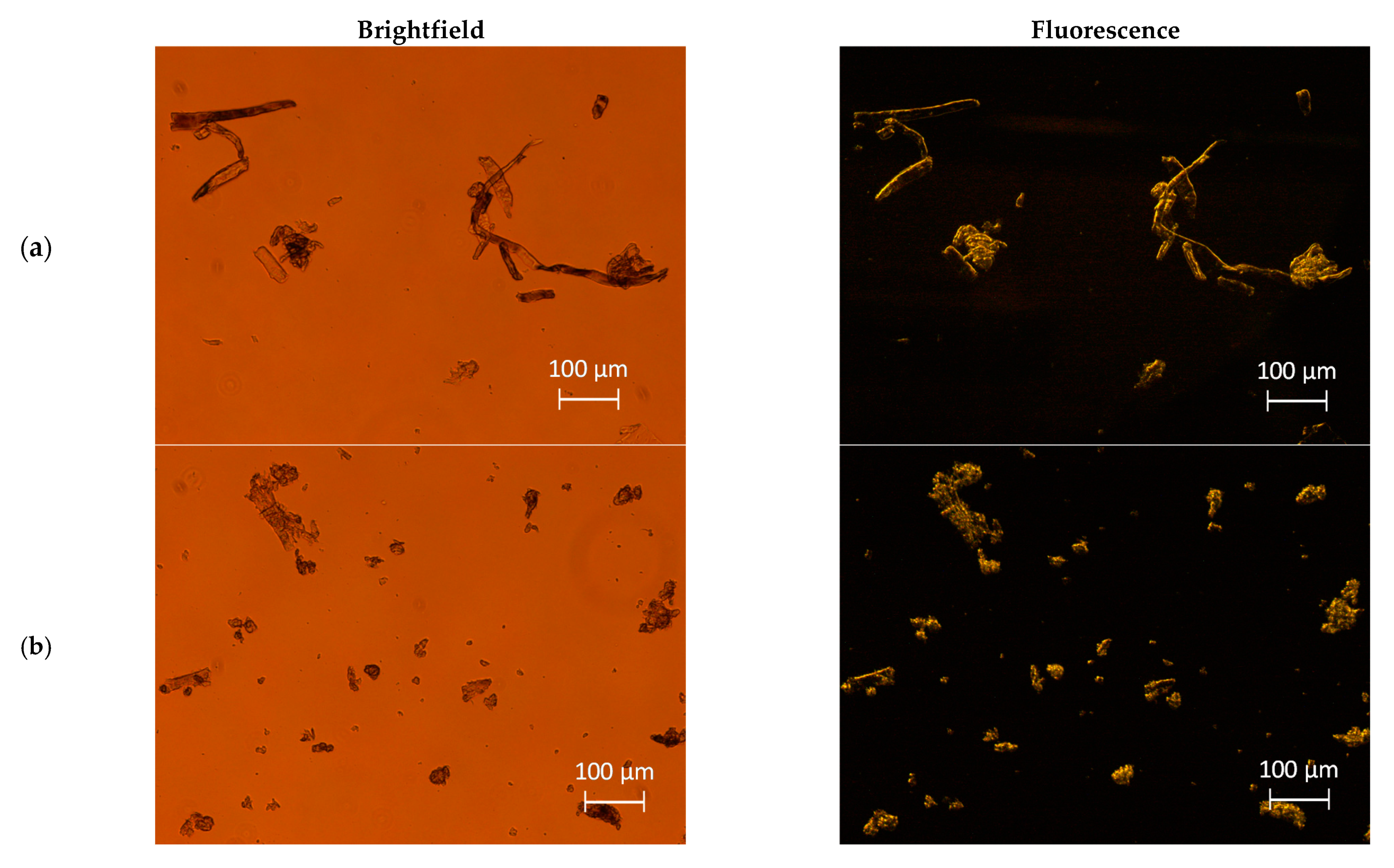
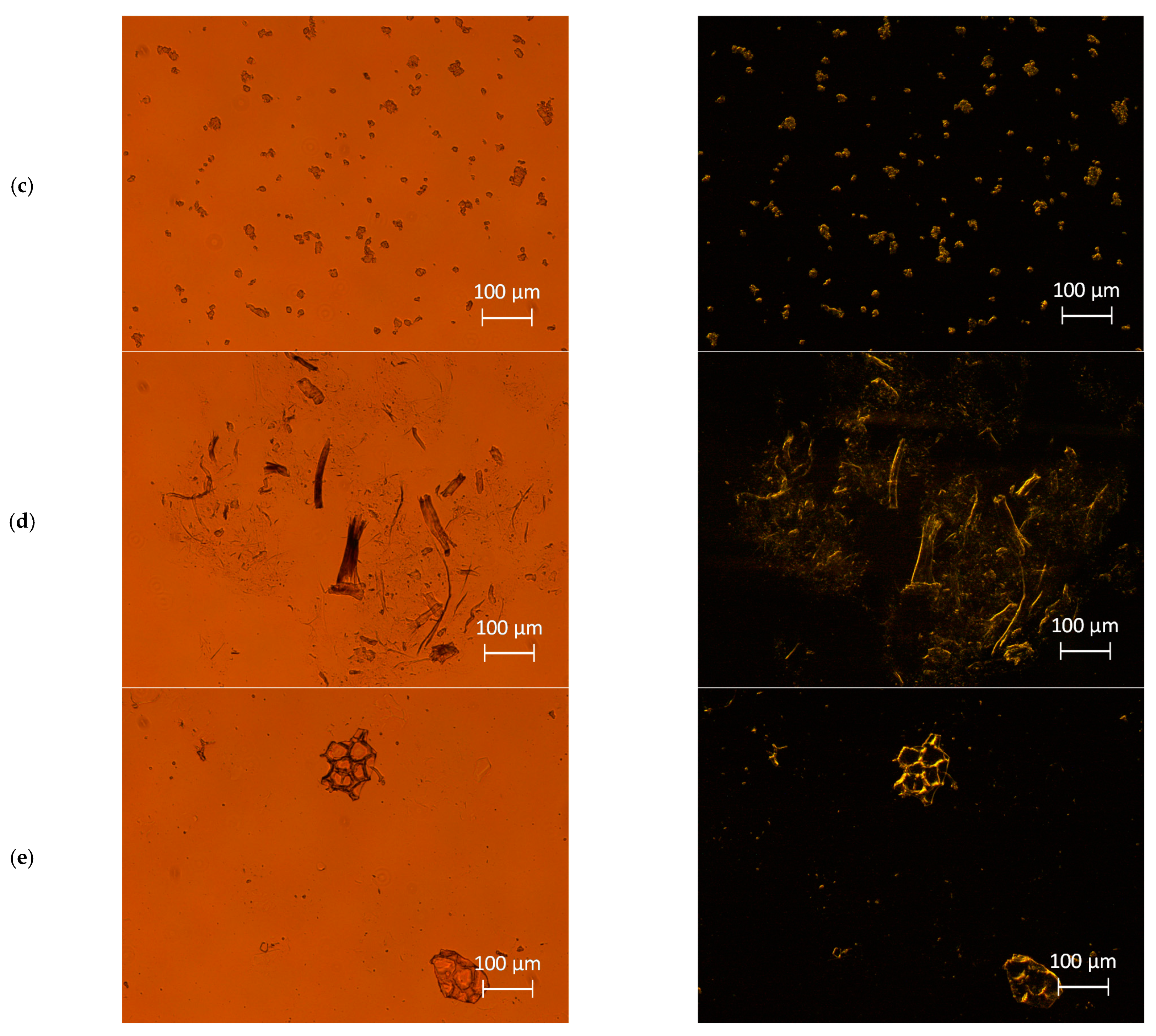
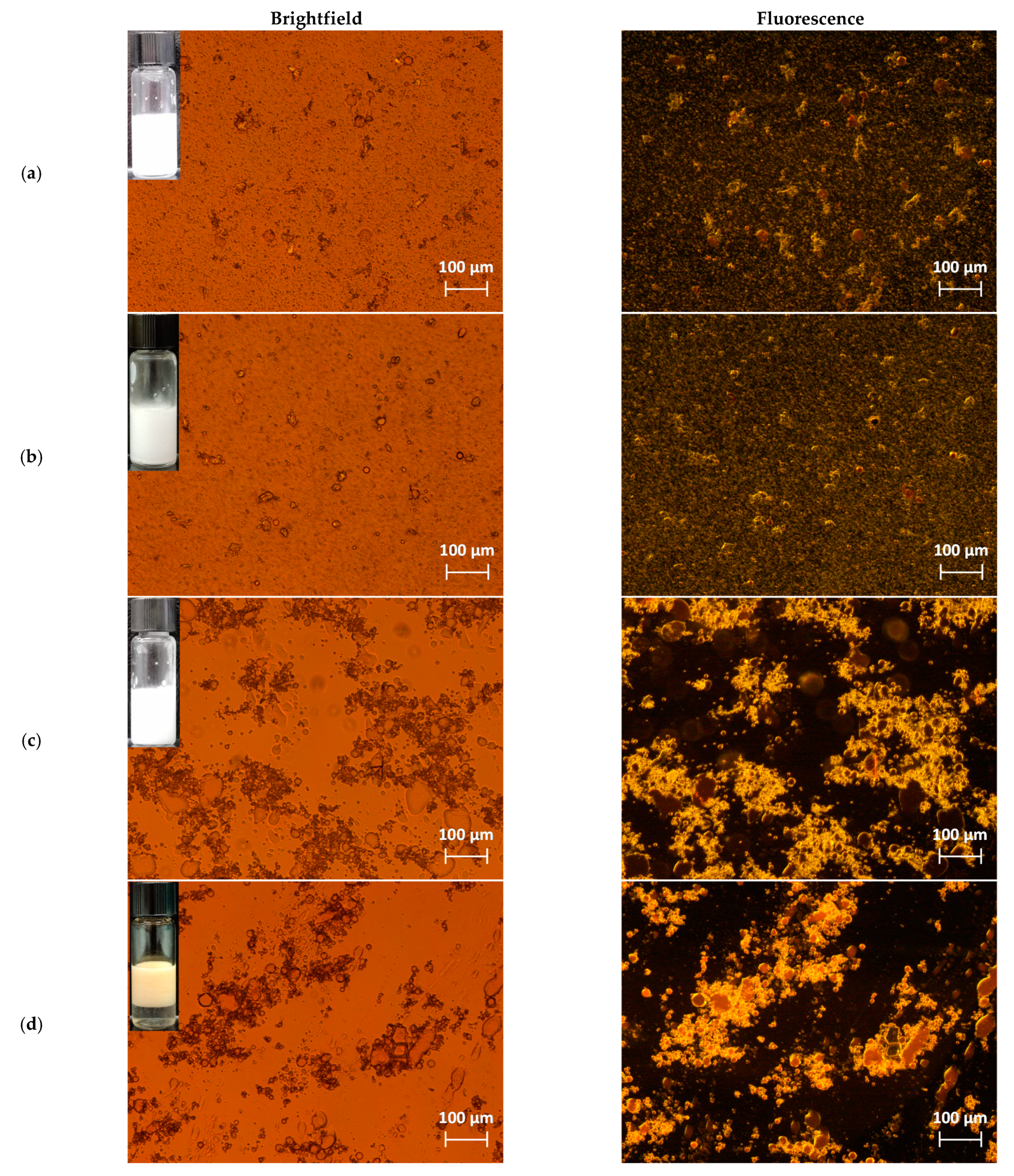
2.4. Light Microscopy Analysis
2.5. Scanning Electron Microscopy Analysis
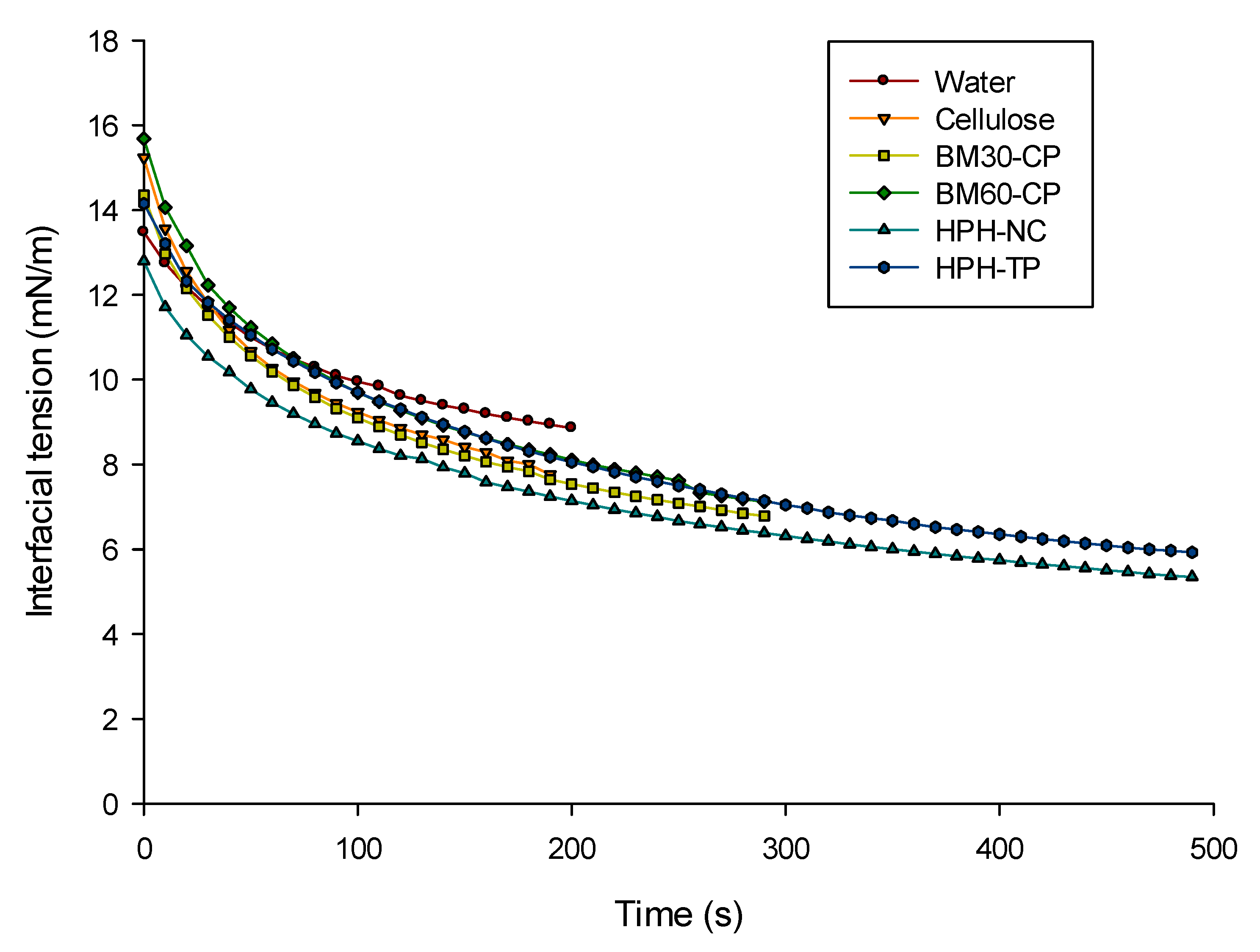
2.6. Interfacial Tension Measurements
2.7. Size Distribution of BM-CP, NC, HPH-TP, and Emulsions
2.8. Emulsion Rheological Analysis
2.9. Influence of Environmental Factors on Emulsions Stability
2.10. Statistical Analysis
3. Results and Discussion
3.1. Structural Analysis of CP, CNF, and HPH-TP
3.1.1. Morphology Analysis
3.1.2. Interfacial Tension
3.1.3. Effect of Mechanical Treatments on the Particle Size Distribution
3.2. Characterization of Nanocellulose-Stabilized Pickering Emulsions
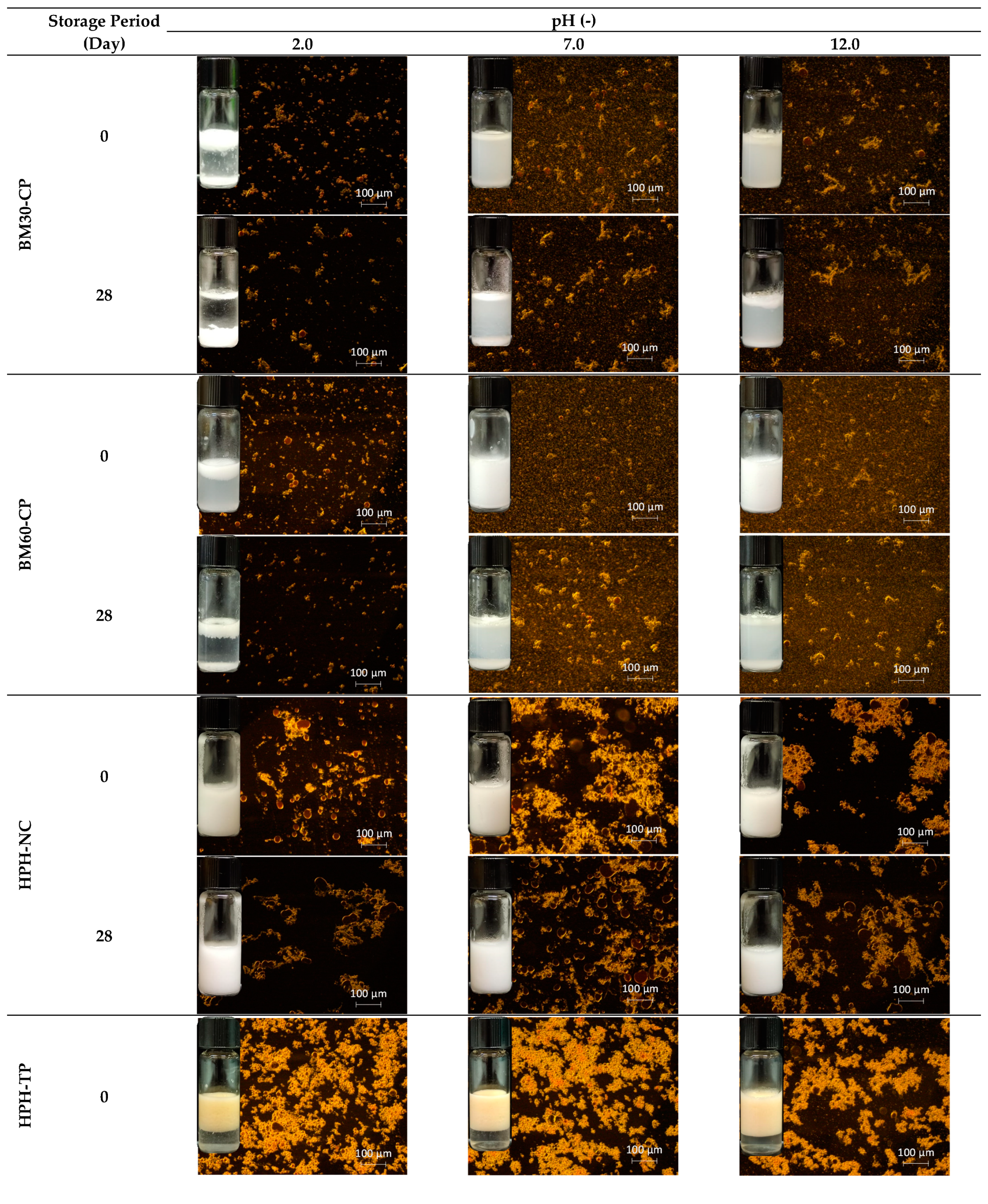
3.3. Emulsion Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-grade pickering emulsions: Preparation, stabilization and applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.G.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Donsì, F.; Velikov, K.P. Encapsulation of food ingredients by single O/W and W/O nanoemulsions. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–87. [Google Scholar]
- McClements, D.J.D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Dong, H.; Ding, Q.; Jiang, Y.; Li, X.; Han, W. Pickering emulsions stabilized by spherical cellulose nanocrystals. Carbohydr. Polym. 2021, 265, 118101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef]
- Mauriello, E.; Ferrari, G.; Donsì, F. Effect of formulation on properties, stability, carvacrol release and antimicrobial activity of carvacrol emulsions. Colloids Surf. B Biointerfaces 2021, 197, 111424. [Google Scholar] [CrossRef]
- Sarkar, A.; Ademuyiwa, V.; Stubley, S.; Esa, N.H.; Goycoolea, F.M.; Qin, X.; Gonzalez, F.; Olvera, C. Pickering emulsions co-stabilized by composite protein/polysaccharide particle-particle interfaces: Impact on in vitro gastric stability. Food Hydrocoll. 2018, 84, 282–291. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Wu, J.; Ma, G.H. Recent Studies of Pickering Emulsions: Particles Make the Difference. Small 2016, 12. [Google Scholar] [CrossRef]
- Baek, J.; Wahid-Pedro, F.; Kim, K.; Kim, K.; Tam, K.C. Phosphorylated-CNC/modified-chitosan nanocomplexes for the stabilization of Pickering emulsions. Carbohydr. Polym. 2019, 206, 520–527. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Chun, S.-J.; Kang, I.-A.; Park, J.-Y. Preparation of cellulose nanofibrils by high-pressure homogenizer and cellulose-based composite films. J. Ind. Eng. Chem. 2009, 15, 50–55. [Google Scholar] [CrossRef]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Cocca, M.; Avolio, R.; Gentile, G.; Di Pace, E.; Errico, M.E.; Avella, M. Amorphized cellulose as filler in biocomposites based on poly(ε-caprolactone). Carbohydr. Polym. 2015, 118, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Avolio, R.; Graziano, V.; Pereira, Y.D.F.; Cocca, M.; Gentile, G.; Errico, M.E.; Ambrogi, V.; Avella, M. Effect of cellulose structure and morphology on the properties of poly(butylene succinate-co-butylene adipate) biocomposites. Carbohydr. Polym. 2015, 133, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci., Appl. Polym. Symp. 1983, 37, 815–827. [Google Scholar]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Pickering Emulsions Containing Cellulose Microfibers Produced by Mechanical Treatments as Stabilizer in the Food Industry. Appl. Sci. 2019, 9, 359. [Google Scholar] [CrossRef]
- Restu, W.K.; Sampora, Y.; Meliana, Y.; Haryono, A. Effect of Accelerated Stability Test on Characteristics of Emulsion Systems with Chitosan as a Stabilizer. Procedia Chem. 2015, 16, 171–176. [Google Scholar] [CrossRef]
- Ni, Y.; Li, J.; Fan, L. Production of nanocellulose with different length from ginkgo seed shells and applications for oil in water Pickering emulsions. Int. J. Biol. Macromol. 2020, 149, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.G.; Harris, P.J.; Melton, L.D.; Newman, R.H. Crystalline cellulose in hydrated primary cell walls of three monocotyledons and one dicotyledon. Plant Cell Physiol. 1998, 39, 711–720. [Google Scholar] [CrossRef]
- Abe, M.; Ogura, T.; Imura, T.; Misono, T.; Sakai, K.; Tsuchiya, K.; Torigoe, K.; Endo, T. Measurement Techniques and Practices of Colloid and Interface Phenomena; Abe, M., Ed.; Springer Nature: Singapore, 2019. [Google Scholar]
- Bertsch, P.; Diener, M.; Adamcik, J.; Scheuble, N.; Geue, T.; Mezzenga, R.; Fischer, P. Adsorption and Interfacial Layer Structure of Unmodified Nanocrystalline Cellulose at Air/Water Interfaces. Langmuir 2018, 34, 15195–15202. [Google Scholar] [CrossRef]
- Bergfreund, J.; Sun, Q.; Fischer, P.; Bertsch, P. Adsorption of charged anisotropic nanoparticles at oil-water interfaces. Nanoscale Adv. 2019, 1, 4308–4312. [Google Scholar] [CrossRef]
- Danov, K.D.; Kralchevsky, P.A. Capillary forces between particles at a liquid interface: General theoretical approach and interactions between capillary multipoles. Adv. Colloid Interface Sci. 2010, 154, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Chen, X.; Liu, C.; Liao, Y.; Huang, Y.; Hao, L.; Yan, H.; Lin, Q. Extraction of cellulose nanocrystals from microcrystalline cellulose for the stabilization of cetyltrimethylammonium bromide-enhanced Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125442. [Google Scholar] [CrossRef]
- Seta, L.; Baldino, N.; Gabriele, D.; Lupi, F.R.; de Cindio, B. Rheology and adsorption behaviour of β-casein and β-lactoglobulin mixed layers at the sunflower oil/water interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 669–677. [Google Scholar] [CrossRef]
- Tenorio, A.T.; Gieteling, J.; Nikiforidis, C.V.; Boom, R.M.; van der Goot, A.J. Interfacial properties of green leaf cellulosic particles. Food Hydrocoll. 2017, 71, 8–16. [Google Scholar] [CrossRef]
- Wüstenberk, T. Cellulose and Cellulose Derivatives in the Food Industry. Fundamental and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; ISBN 9780874216561. [Google Scholar]
- Trujillo-Cayado, L.A.; Natera, A.; García, M.C.; Muñoz, J.; Alfaro, M.C. Rheological properties and physical stability of ecological emulsions stabilized by a surfactant derived from cocoa oil and high pressure homogenization. Grasas Aceites 2015. [Google Scholar] [CrossRef][Green Version]
- Yuan, T.; Zeng, J.; Wang, B.; Cheng, Z.; Chen, K. Pickering emulsion stabilized by cellulosic fibers: Morphological properties-interfacial stabilization-rheological behavior relationships. Carbohydr. Polym. 2021, 269, 118339. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Sun, C.; Wei, Y.; Mao, L.; Gao, Y. Characterization of Pickering emulsion gels stabilized by zein/gum arabic complex colloidal nanoparticles. Food Hydrocoll. 2018, 74, 239–248. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, B.; Xie, F.; Hu, M.; Sun, Y.; Han, L.; Li, L.; Zhang, S.; Li, Y. Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocoll. 2021, 113, 106391. [Google Scholar] [CrossRef]
- Qi, W.; Yu, J.; Zhang, Z.; Xu, H.N. Effect of pH on the aggregation behavior of cellulose nanocrystals in aqueous medium. Mater. Res. Express 2019, 6, 125078. [Google Scholar] [CrossRef]

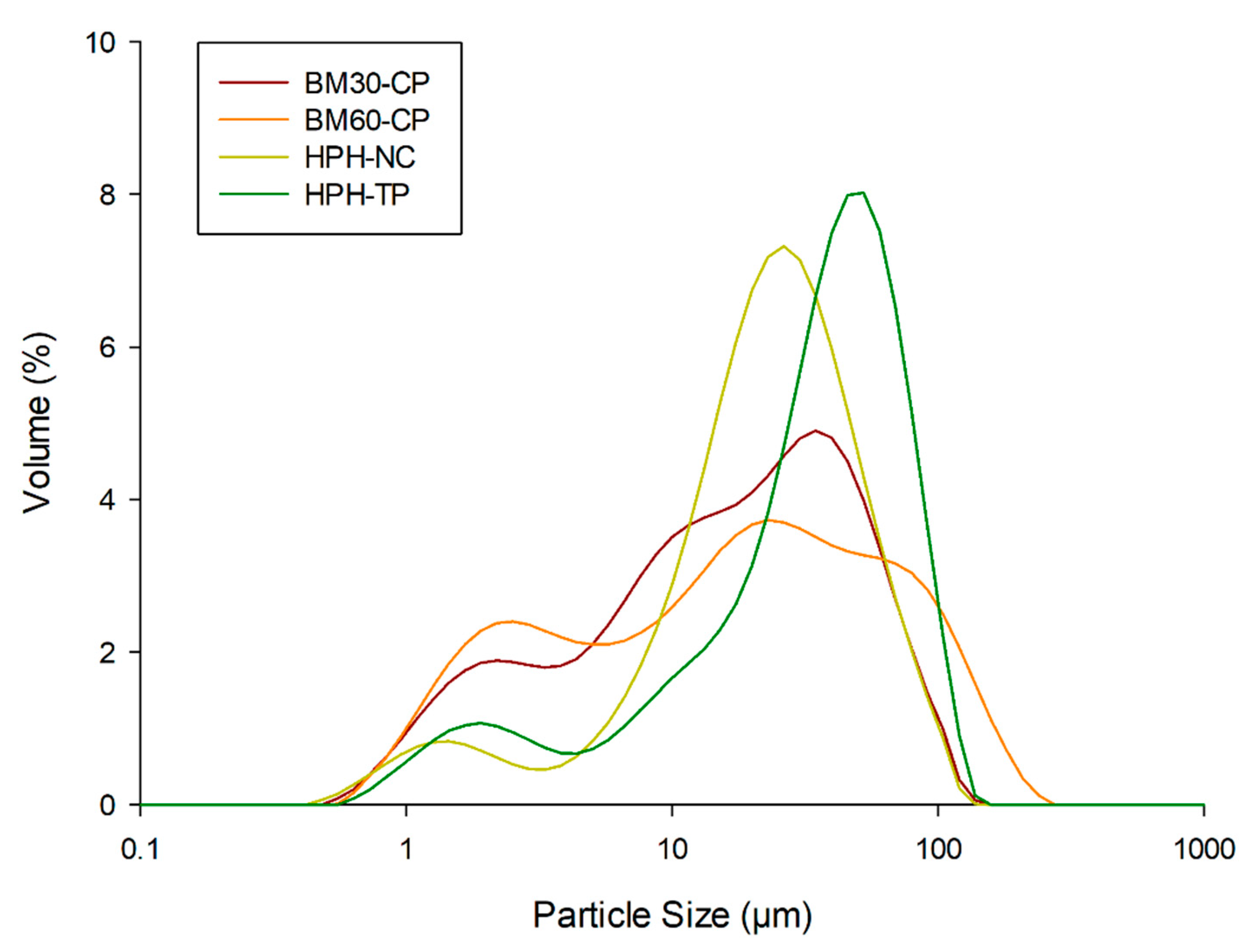


| Cellulose | BM30-CP | BM60-CP | HPH-NC | HPH-TP | |
|---|---|---|---|---|---|
| d(0.1) (µm) | 14.20 ± 017 a | 7.16 ± 0.14 c,d | 6.89 ± 0.12 d | 9.56 ± 0.11 b | 7,78 ± 0.14 c |
| d(0.5) (µm) | 53.23 ± 2.24 a | 31.69 ± 0.34 b | 30.66 ± 0.32 b,c | 32.04 ± 0.12 b | 29.97 ± 0.25 c |
| d(0.9) (µm) | 206.46 ± 13.96 a | 88.82 ± 0.69 c | 82.93 ± 0.59 d | 108.73 ± 1.16 b | 78.22 ± 0.50 e |
| Span (-) | 3.61 ± 0.15 a | 2.58 ± 0.02 c | 2.48 ± 0.02 dc | 3.10 ± 0.03 b | 2.35 ± 0.01 d |
| D[4,3] (µm) | 100.43 ± 5.32 a | 45.01 ± 0.65 c | 44.99 ± 0.49 c | 54.44 ± 0.43 b | 38.47 ± 1.06 d |
| D[3,2] (µm) | 23.25 ± 0.29 a | 12.79 ± 0.18 d | 11.68 ± 0.17 d | 16.85 ± 0.12 b | 14.47 ± 0.16 c |
| Pickering Stabilizer | ||||
|---|---|---|---|---|
| BM30-CP | BM60-CP | HPH-NC | HPH-TP | |
| d(0.1) (µm) | 1.92 | 1.79 | 5.02 | 3.82 |
| d(0.5) (µm) | 15.60 | 16.65 | 21.75 | 34.40 |
| d(0.9) (µm) | 54.44 | 84.25 | 53.85 | 72.73 |
| Span (-) | 3.37 | 4.95 | 2.245 | 2.00 |
| D[4,3] (µm) | 22.90 | 31.11 | 26.38 | 37.05 |
| D[3,2] (µm) | 5.70 | 5.44 | 8.45 | 9.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirozzi, A.; Capuano, R.; Avolio, R.; Gentile, G.; Ferrari, G.; Donsì, F. O/W Pickering Emulsions Stabilized with Cellulose Nanofibrils Produced through Different Mechanical Treatments. Foods 2021, 10, 1886. https://doi.org/10.3390/foods10081886
Pirozzi A, Capuano R, Avolio R, Gentile G, Ferrari G, Donsì F. O/W Pickering Emulsions Stabilized with Cellulose Nanofibrils Produced through Different Mechanical Treatments. Foods. 2021; 10(8):1886. https://doi.org/10.3390/foods10081886
Chicago/Turabian StylePirozzi, Annachiara, Roberta Capuano, Roberto Avolio, Gennaro Gentile, Giovanna Ferrari, and Francesco Donsì. 2021. "O/W Pickering Emulsions Stabilized with Cellulose Nanofibrils Produced through Different Mechanical Treatments" Foods 10, no. 8: 1886. https://doi.org/10.3390/foods10081886
APA StylePirozzi, A., Capuano, R., Avolio, R., Gentile, G., Ferrari, G., & Donsì, F. (2021). O/W Pickering Emulsions Stabilized with Cellulose Nanofibrils Produced through Different Mechanical Treatments. Foods, 10(8), 1886. https://doi.org/10.3390/foods10081886











