Influence of Selenium Biofortification on the Growth and Bioactive Metabolites of Ganoderma lucidum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Strain and Culture Conditions
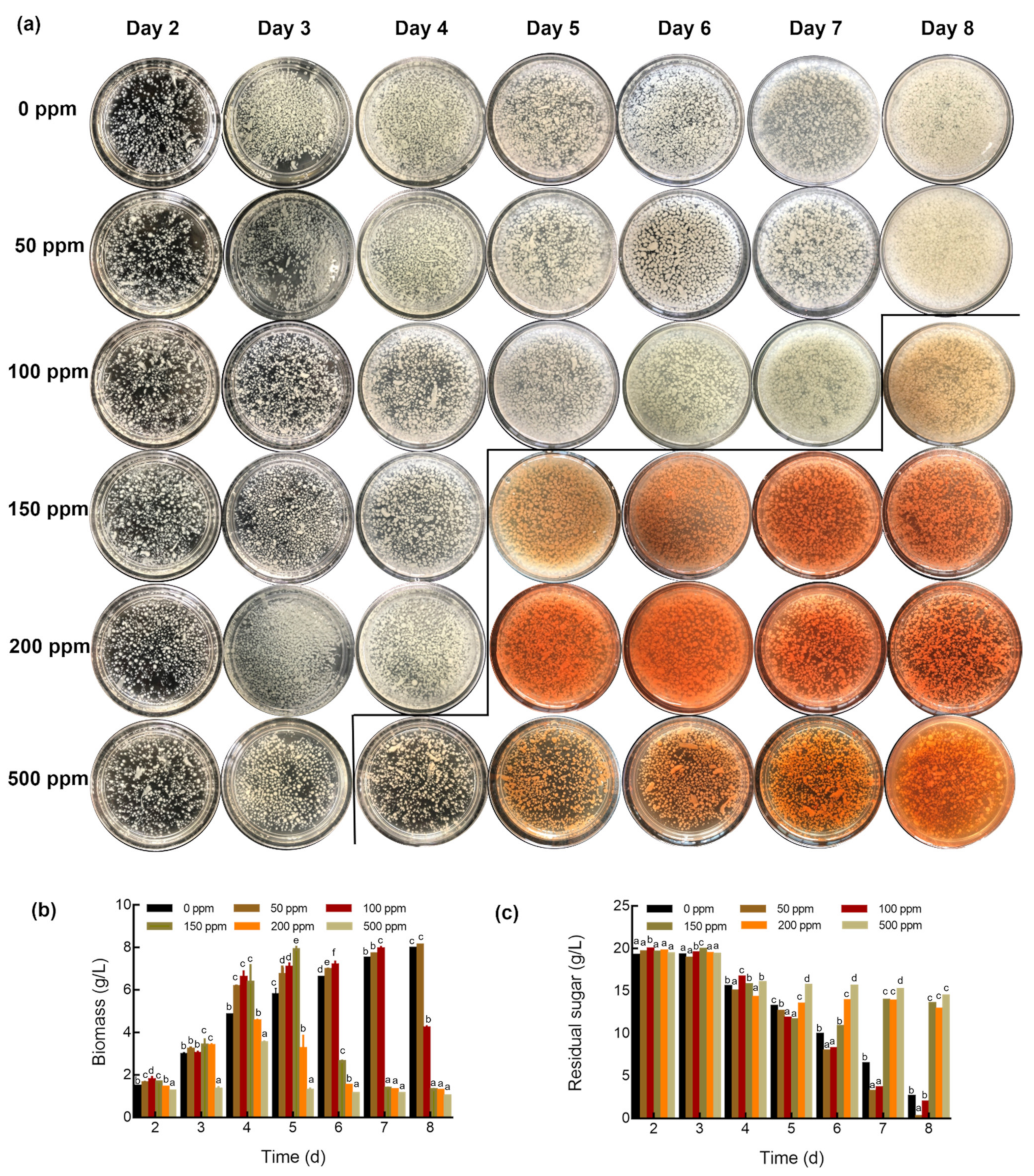
2.3. Determination of Biomass, Residual Sugar, Polysaccharide Content
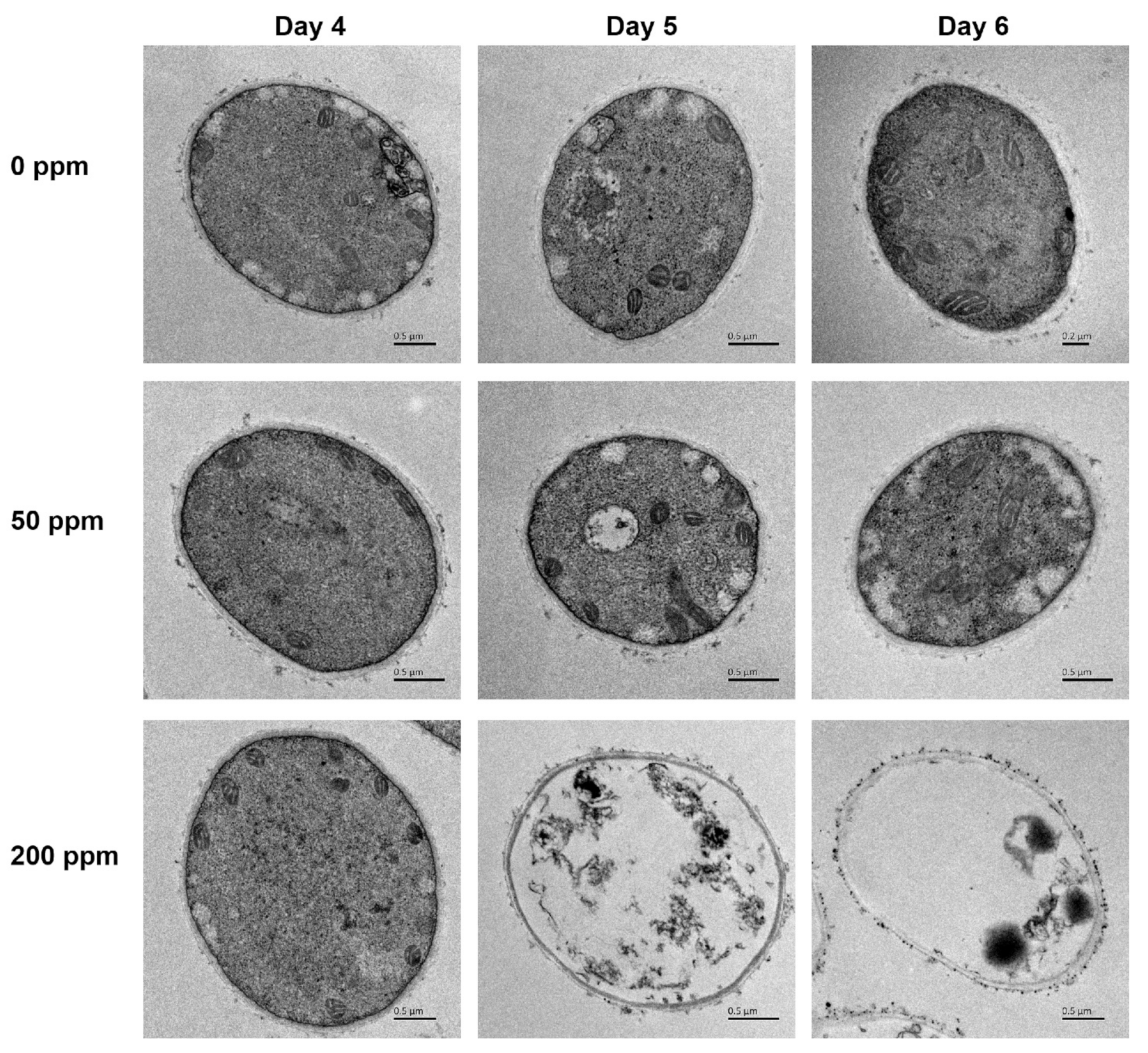
2.4. Ultra-Structural Analysis
2.5. Quantification of Nucleic Acid, Protein, Chitin, β-1,3-Glucan, and Ergosterol
2.6. Determination of Total Se Content and Selenoamino Acid Analysis
2.7. Volatile Se Compound Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth and Morphology of G. lucidum Cultured with Different Concentrations of Na2SeO3
3.2. Ultra-Structure Alterations
3.3. Changes in Mycelial Cell Chemical Composition
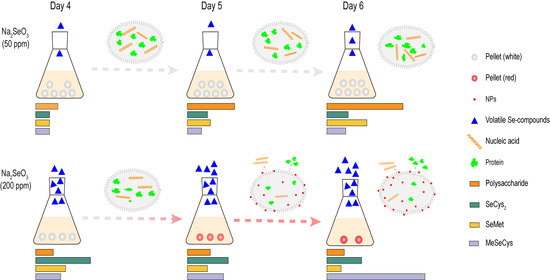
3.4. Volatile Se Compounds in G. lucidum
3.5. Bioactive Metabolites
3.5.1. Polysaccharide
3.5.2. Se Enrichment Capacity
3.5.3. Selenoamino Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kieczykowska, M.; Kocot, J.; Padzior, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.; Mo, H.Y.; Lau, A.T.Y.; Xu, Y.M. Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 75. [Google Scholar] [CrossRef] [Green Version]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current knowledge on Selenium biofortification to improve nutraceutical profile of food: A comprehensive review. J. Agric. Food Chem. 2020, 68, 4057–4097. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.H.; Ding, Z.Y. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Li, L.; Hui, G.F.; Zhang, J.J.; Li, H.F.; Wu, W.L.; Wei, X.H.; Guo, Y.B. Selenium biofortification and its effect on multi-element change in Auricularia auricular. Food Chem. 2019, 295, 206–213. [Google Scholar] [CrossRef]
- Perfileva, A.; Tsivileva, O. Selenium Compounds Biotransformed by Mushrooms: Not Only Dietary Sources, But Also Toxicity Mediators. Curr. Nutr. Food Sci. 2017, 13, 82–96. [Google Scholar]
- Miletić, D.; Turło, J.; Podsadni, P.; Pantić, M.; Nedović, V.; Levic, S.; Nikšic, M. Selenium enriched Coriolus versicolor mushroom biomass: Potential novel food supplement with improved selenium bioavailability. J. Sci. Food Agric. 2019, 99, 5122–5130. [Google Scholar] [CrossRef]
- Wang, B.J.; Zhao, N.; Li, J.; Xu, R.Y.; Wang, T.R.; Guo, L.; Ma, M.; Fan, M.T.; Wei, X.Y. Selenium-enriched Lactobacillus plantarum improves the antioxidant activity and flavor properties of fermented Pleurotus eryngii. Food Chem. 2021, 345, 128770. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, G.G.; Wang, W.; Zhang, W.J.; Ding, Y.Y.; Zhao, T.; Li, F.; Mao, G.H.; Feng, W.W.; Wang, Q.; et al. A novel Se-polysaccharide from Se-enriched G. frondosa protects against immunosuppression and low Se status in Se-deficient mice. Int. J. Biol. Macromol. 2018, 117, 878–889. [Google Scholar] [CrossRef]
- Hu, T.; Hui, G.F.; Li, H.F.; Guo, Y.B. Selenium biofortification in Hericium erinaceus (Lion’s Mane mushroom) and its in vitro bioaccessibility. Food Chem. 2020, 331, 127287. [Google Scholar] [CrossRef]
- Wang, J.P.; Wang, B.; Zhang, D.; Wu, Y.H. Selenium uptake, tolerance and reduction in Flammulina velutipes supplied with selenite. Peerj 2016, 4, e1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elisashvili, V. Submerged cultivation of medicinal mushrooms: Bioprocesses and products. Int. J. Med. Mushrooms 2012, 14, 211–239. [Google Scholar] [CrossRef]
- Leskosek-Cukalovic, I.; Despotovic, S.; Lakic, N.; Niksic, M.; Nedovic, V.; Tesevic, V. Ganoderma lucidum—Medical mushroom as a raw material for beer with enhanced functional properties. Food Res. Int. 2010, 43, 2262–2269. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.S.; Batra, P.; Beniwal, V.; Gupta, G.K.; Sharma, A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17, 100268. [Google Scholar] [CrossRef]
- Ma, Z.M.; Xu, M.M.; Wang, Q.; Wang, F.; Zheng, H.H.; Gu, Z.H.; Li, Y.R.; Shi, G.Y.; Ding, Z.Y. Development of an Efficient Strategy to Improve Extracellular Polysaccharide Production of Ganoderma lucidum Using L-Phenylalanine as an Enhancer. Front. Microbiol. 2019, 10, 2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazawa, K.; Yoshimi, A.; Kasahara, S.; Sugahara, A.; Koizumi, A.; Yano, S.; Kimura, S.; Iwata, T.; Sano, M.; Abe, K. Molecular Mass and Localization of α-1,3-Glucan in Cell Wall Control the Degree of Hyphal Aggregation in Liquid Culture of Aspergillus nidulans. Front. Microbiol. 2018, 9, 2623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, M.M.; Zhao, L.T.; Wang, F.; Li, Y.R.; Shi, G.Y.; Ding, Z.Y. Transcriptome dynamics and metabolite analysis revealed the candidate genes and regulatory mechanism of ganoderic acid biosynthesis during liquid superficial-static culture of Ganoderma lucidum. Microb. Biotechnol. 2021, 14, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Wang, J.H.; Liu, X.; Kuang, H.C.; Huang, X.N. Determination of ergosterol in ganoderma spore lipid from the germinating spores of Ganoderma lucidum by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 6172–6176. [Google Scholar] [CrossRef]
- Yang, M.Y.; Tarun, B.; Hair, P.D.; Li, L.; Luo, Z.S. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar]
- Niedzielski, P.; Mleczek, M.; Siwulski, M.; Rzymski, P.; Kozak, L. Supplementation of cultivated mushroom species with selenium: Bioaccumulation and speciation study. Eur. Food Res. Technol. 2015, 241, 419–426. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet—Relationship between morphology and productivity. Appl. Microbiol. Biot. 2018, 102, 2997–3006. [Google Scholar] [CrossRef] [Green Version]
- Slusarczyk, J.; Kuraś, M.; Malinowska, E.; Skalicka-Woźniak, K.; Głowniak, K. Ultrastructural changes in the mycelium of Hericium erinaceum (Bull.; Fr.) Pers. under selenium-induced oxidative stress. J. Sci. Food Agric. 2014, 94, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Yang, M.Y.; Ma, B.; Zhao, Y.; Zhuang, H.N.; Zhang, J.S.; Chen, D. Volatile profiles of two genotype Agaricus bisporus species at different growth stages. Food Res. Int. 2021, 140, 109761. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Martin, G.; Sanz-Landaluze, J.; León-Gonzalez, M.E.; Madrid, Y. In-vivo solid phase microextraction for quantitative analysis of volatile organoselenium compounds in plants. Anal. Chim. Acta. 2019, 1081, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Guadayol, M.; Cortina, M.; Guadayol, J.M.; Caixach, J. Determination of dimethyl selenide and dimethyl sulphide compounds causing off-flavours in bottled mineral waters. Water Res. 2016, 92, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Radhika, B.; Gonzalo, A.P.; Rebecca, A.M.; Claudio, C.V.; Thomas, G.C. Production of dimethyl triselenide and dimethyl diselenenyl sulfide in the headspace of metalloid-resistant Bacillus species grown in the presence of selenium oxyanions. Anal. Biochem. 2010, 396, 217–222. [Google Scholar]
- Turło, J.; Gutkowska, B.; Herold, F.; Dawidowski, M.; Słowinski, T.; Zobe, A. Relationship between selenium accumulation and mycelial cell composition in Lentinula edodes (Berk.) cultures. J. Toxicol. Environ. Health Part A 2010, 73, 1211–1219. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Sun, P.P.; Li, H.R.; Zhu, Z.Y. Effects of Na2SeO3 on growth, metabolism, antioxidase and enzymes involved in polysaccharide synthesis of Cordyceps militaris. Process Biochem. 2020, 97, 64–71. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of selenium, selenomethionine and selenocysteine from foods and influence of heat processing on the same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef]
- Maseko, T.; Callahan, D.L.; Dunshea, F.R.; Doronila, A.; Kolev, S.D.; Ng, K. Chemical characterisation and speciation of organic selenium in cultivated selenium-enriched Agaricus bisporus. Food Chem. 2013, 141, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Dinh, T.Q.; Yang, W.X.; Wang, M.K.; Xue, M.Y.; Bañuelos, G.S.; Liang, D.L. Assessment of speciation and in vitro bioaccessibility of selenium in Se-enriched Pleurotus ostreatus and potential health risks. Ecotoxicol. Environ. Saf. 2019, 185, 109675. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, W.X.; Wang, M.K.; Miao, Y.X.; Cui, Z.W.; Li, Z.; Liang, D.L. Effects of selenium application on Se content and speciation in Lentinula edodes. Food Chem. 2018, 265, 182–188. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, M.J.; Hunter, D.A.; Pathirana, R.; Watson, L.M.; Joyce, N.I.; Matich, A.J.; Rowan, D.D.; Brummell, D.A. Accumulation of an organic anticancer selenium compound in a transgenic Solanaceous species shows wider applicability of the selenocysteine methyltransferase transgene from selenium hyperaccumulators. Transgen. Res. 2009, 18, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Vetchinkina, E.; Loshchinina, E.; Kursky, V.; Nikitina, V. Reduction of organic and inorganic selenium compounds by the edible medicinal basidiomycete Lentinula edodes and the accumulation of elemental selenium nanoparticles in its mycelium. J. Microbiol. 2013, 51, 829–835. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Faizy, E.D.; Abdalla, N.; Taha, H.; Brevik, E.C. Selenium and Nano-Selenium Biofortification for Human Health: Opportunities and Challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
- Hu, S.Q.; Hu, W.C.; Li, Y.R.; Li, S.J.; Tian, H.F.; Lu, A.; Wang, J.G. Construction and structure-activity mechanism of polysaccharide nano-selenium carrier. Carbohydr. Polym. 2020, 236, 116052. [Google Scholar] [CrossRef]
- Ali, S.M.; Raman, J.; Lakshmanan, H.; Ling, T.C.; Phan, C.W.; Tan, Y.S.; Sabaratnam, V. Surface Decoration of Selenium Nanoparticles by Proteins from the Culinary-Medicinal Shiitake Mushroom, Lentinus edodes (Agaricomycetes), for Enhanced Fibrinolytic Activity. Int. J. Med. Mushrooms 2018, 20, 1021–1030. [Google Scholar] [CrossRef] [PubMed]

| Se Compound | 50 ppm | ||
|---|---|---|---|
| Day 4 | Day 5 | Day 6 | |
| Dimethyl selenide | 1.000 b | 1.748 ± 0.027 b | 1.634 ± 0.046 b |
| Carbo selenide | ND | ND | 0.088 ± 0.006 a |
| Dimethyl selenone | 0.053 ± 0.001 a | 0.115 ± 0.004 a | 0.170 ± 0.009 a |
| Se Compound | 200 ppm | ||
| Day 4 | Day 5 | Day 6 | |
| Dimethyl selenide | 0.689 ± 0.097 a | 0.596 ± 0.007 a | 0.134 ± 0.017 a |
| Carbo selenide | 0.765 ± 0.027 a | 0.681 ± 0.068 a | 0.245 ± 0.002 b |
| Carbon diselenide | 9.467 ± 0.012 | 0.669 ± 0.071 | 0.313 ± 0.082 |
| Methylselenoacetate | ND | 0.235 ± 0.005 | 0.052 ± 0.012 |
| Dimethyl selenone | 0.132 ± 0.009 b | 4.092 ± 0.015 b | 4.990 ± 0.601 b |
| Methyl diselenide | 0.538 ± 0.069 | 35.329 ± 0.032 | 44.242 ± 0.022 |
| 1-(methylselanyl)propan-2-one | 0.068 ± 0.008 | 0.108 ± 0.011 | 0.080 ± 0.015 |
| Dimethyl selenodisulfide | ND | ND | 0.058 ± 0.006 |
| Dimethyl diselenenyl_sulfide | 0.081 ± 0.002 | 6.925 ± 0.013 | 6.813 ± 0.068 |
| Triselenothane | ND | 21.495 ± 0.053 | 31.129 ± 0.021 |
| Bioactive Metabolites | Day 4 | Day 5 | Day 6 | |
|---|---|---|---|---|
| Polysaccharide (mg/L) | 0 ppm | 269.81 ± 16.81 a | 560.12 ± 14.67 b | 1253.42 ± 9.72 b |
| 50 ppm | 331.21 ± 3.89 b | 702.81 ± 25.68 c | 1365.97 ± 10.93 c | |
| 200 ppm | 262.23 ± 8.06 a | 340.93 ± 28.81 a | 259.11 ± 32.86 a | |
| Se(total) (mg/kg) | 0 ppm | 2.92 ± 0.65 a | 3.31 ± 0.89 a | 2.86 ± 0.23 a |
| 50 ppm | 275.11 ± 27.39 b | 316.87 ± 30.45 a | 421.69 ± 29.07 a | |
| 200 ppm | 1398.41 ± 126.96c | 22,472.65 ± 1271.83 b | 41,574.27 ± 1933.50 b | |
| SeCys2 (mg/kg) | 50 ppm | 55.37 ± 0.39 a | 82.85 ± 4.52 a | 92.01 ± 3.22 a |
| SeMet (mg/kg) | 146.55 ± 7.47 a | 243.96 ± 9.31 a | 445.83 ± 30.95 a | |
| MeSeCys (mg/kg) | 39.99 ± 0.42 a | 40.69 ± 0.57 a | 54.44 ± 1.25 a | |
| SeCys2 (mg/kg) | 200 ppm | 237.84 ± 13.22 b | 177.23 ± 12.21 b | 160.99 ± 14.51 b |
| SeMet (mg/kg) | 301.78. ± 10.41 b | 157.25 ± 8.66 b | 149.44 ± 29.16 b | |
| MeSeCys (mg/kg) | 72.23 ± 5.07 b | 104.00 ± 5.11 b | 304.04 ± 30.71 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Zhu, S.; Wang, L.; Wei, Z.; Zhao, L.; Shi, G.; Ding, Z. Influence of Selenium Biofortification on the Growth and Bioactive Metabolites of Ganoderma lucidum. Foods 2021, 10, 1860. https://doi.org/10.3390/foods10081860
Xu M, Zhu S, Wang L, Wei Z, Zhao L, Shi G, Ding Z. Influence of Selenium Biofortification on the Growth and Bioactive Metabolites of Ganoderma lucidum. Foods. 2021; 10(8):1860. https://doi.org/10.3390/foods10081860
Chicago/Turabian StyleXu, Mengmeng, Song Zhu, Lingling Wang, Zhiyi Wei, Liting Zhao, Guiyang Shi, and Zhongyang Ding. 2021. "Influence of Selenium Biofortification on the Growth and Bioactive Metabolites of Ganoderma lucidum" Foods 10, no. 8: 1860. https://doi.org/10.3390/foods10081860
APA StyleXu, M., Zhu, S., Wang, L., Wei, Z., Zhao, L., Shi, G., & Ding, Z. (2021). Influence of Selenium Biofortification on the Growth and Bioactive Metabolites of Ganoderma lucidum. Foods, 10(8), 1860. https://doi.org/10.3390/foods10081860