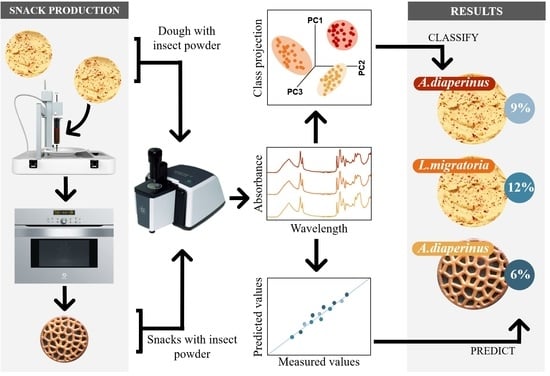
ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dough Preparation
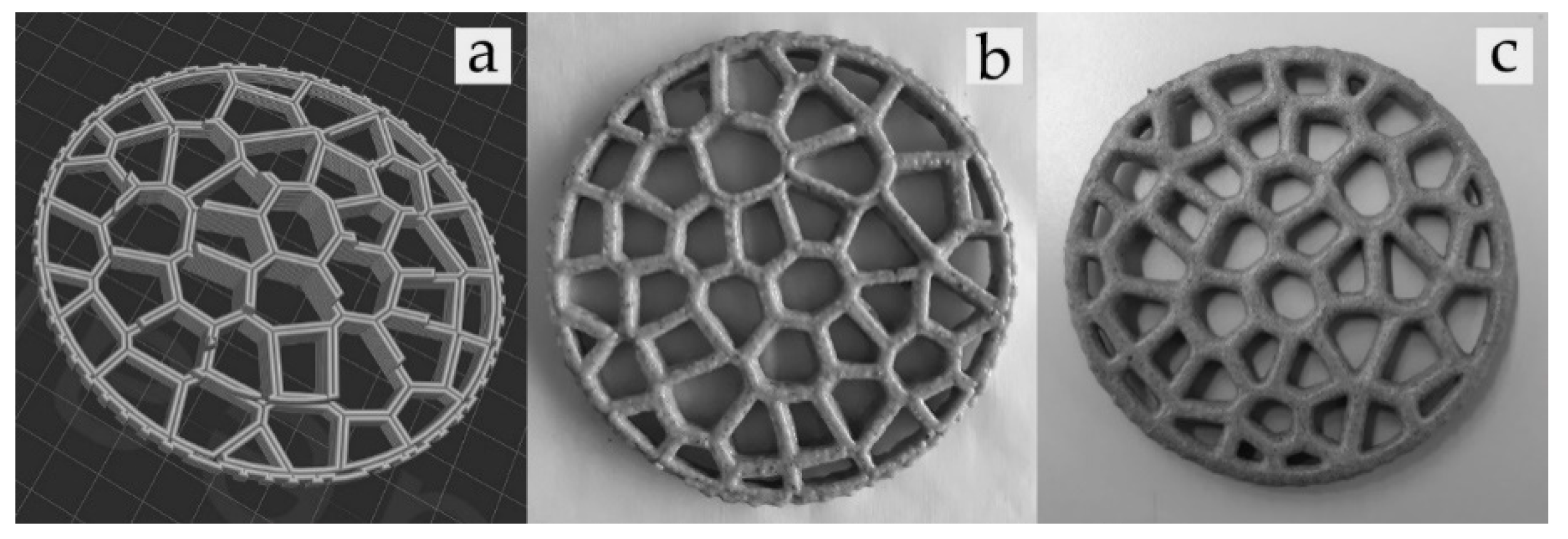
2.3. 3D Printing and Post-Processing Analysis
2.4. Spectral Data Acquisition by FTIR
2.5. Multivariate Analysis
3. Results and Discussion
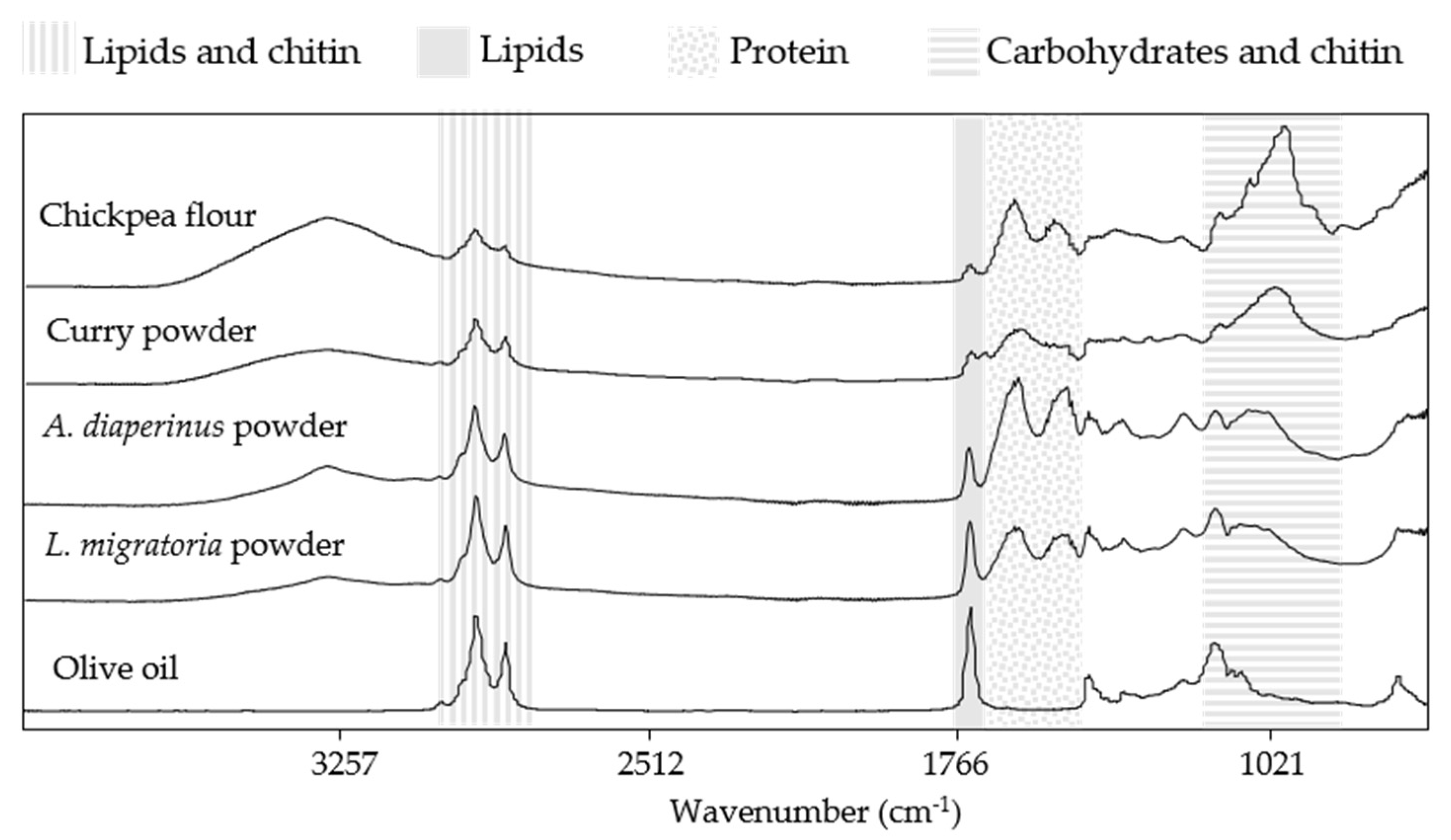
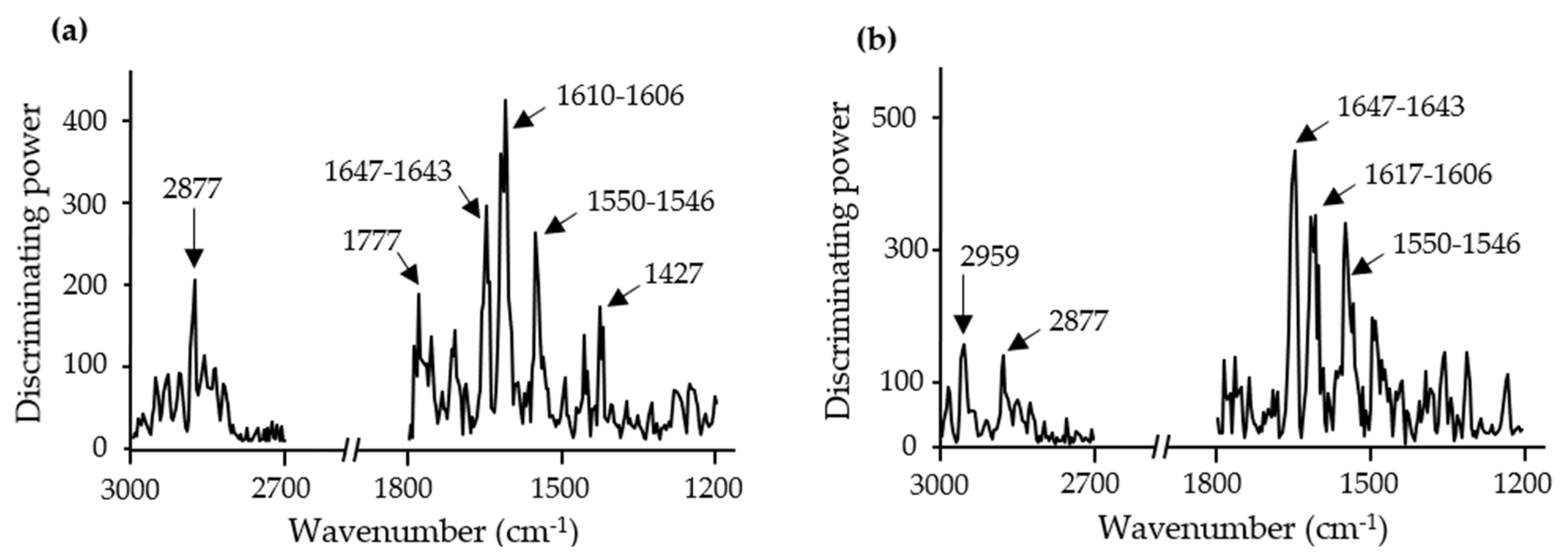
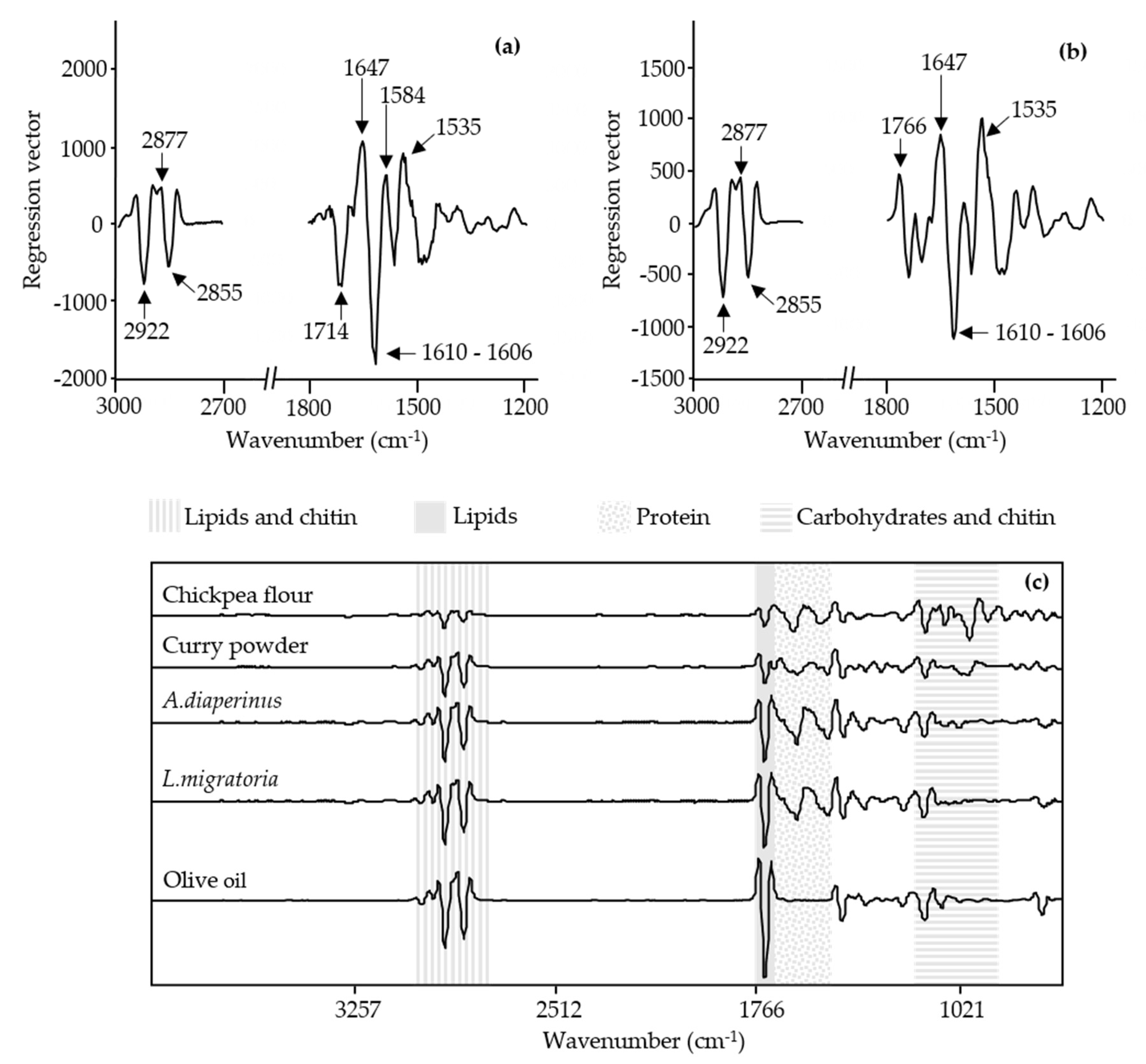
3.1. Spectral Information of Ingredients and Doughs
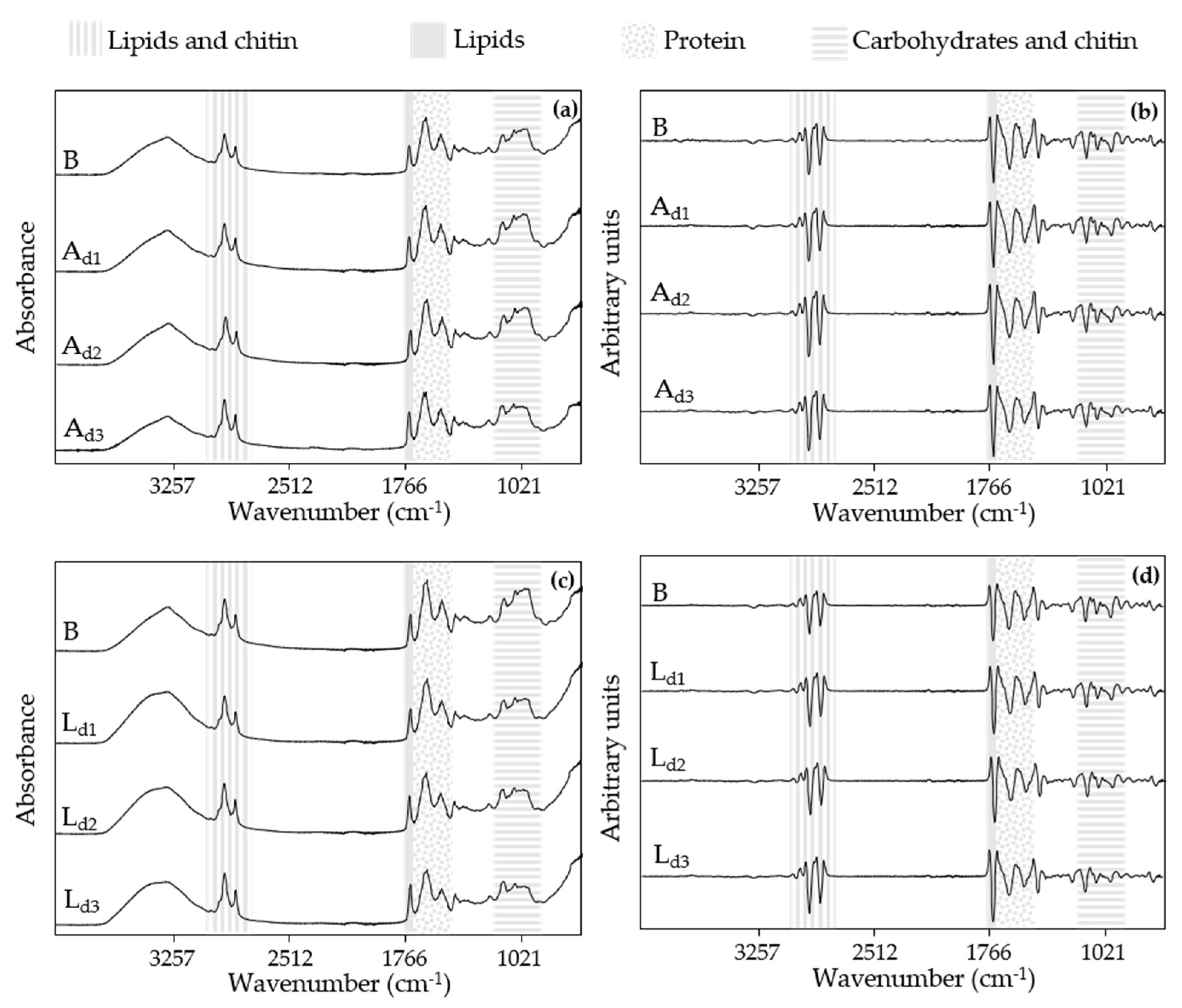
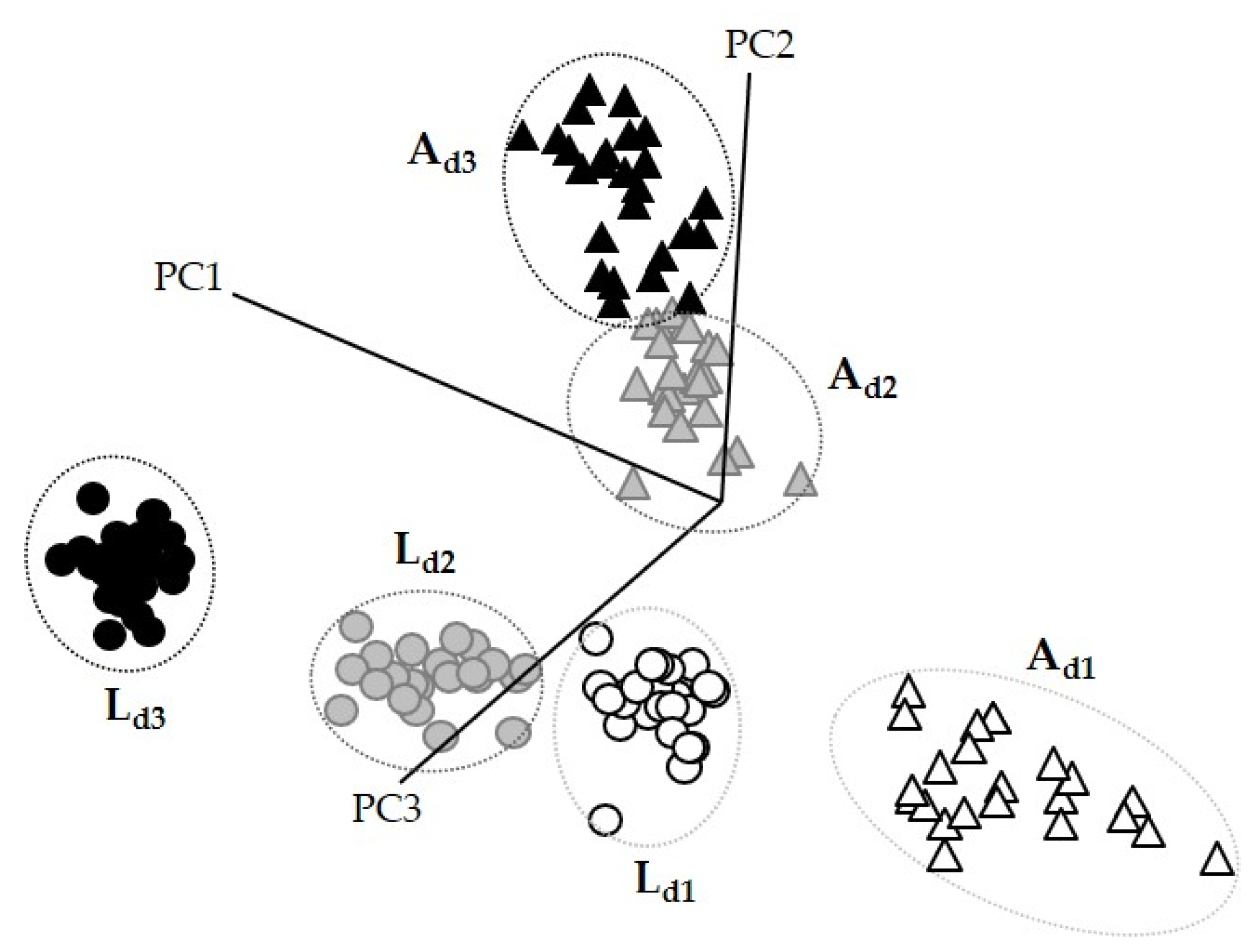
3.2. Discrimination and Classification of Doughs by ATR-FT-MIR Combined with SIMCA
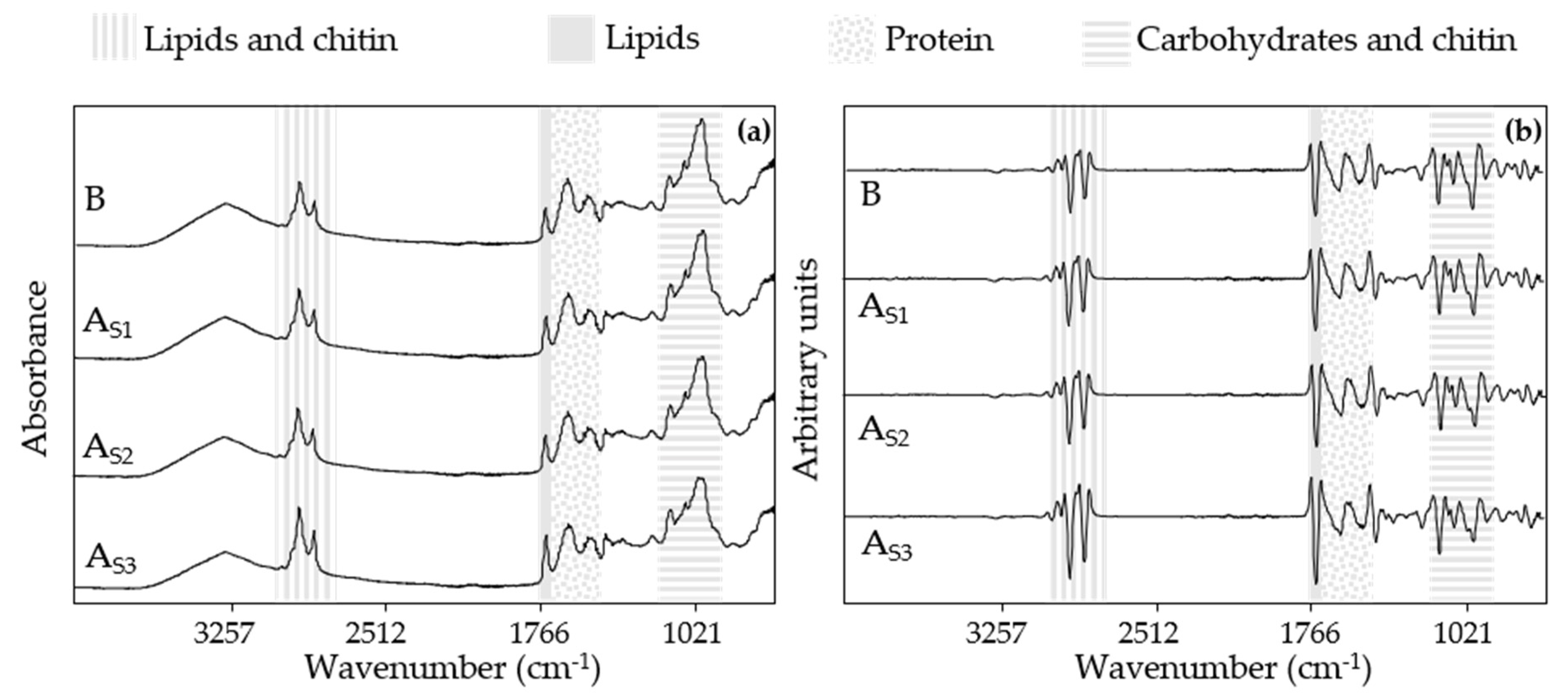
3.3. Spectral Information of 3D-Printed Snacks
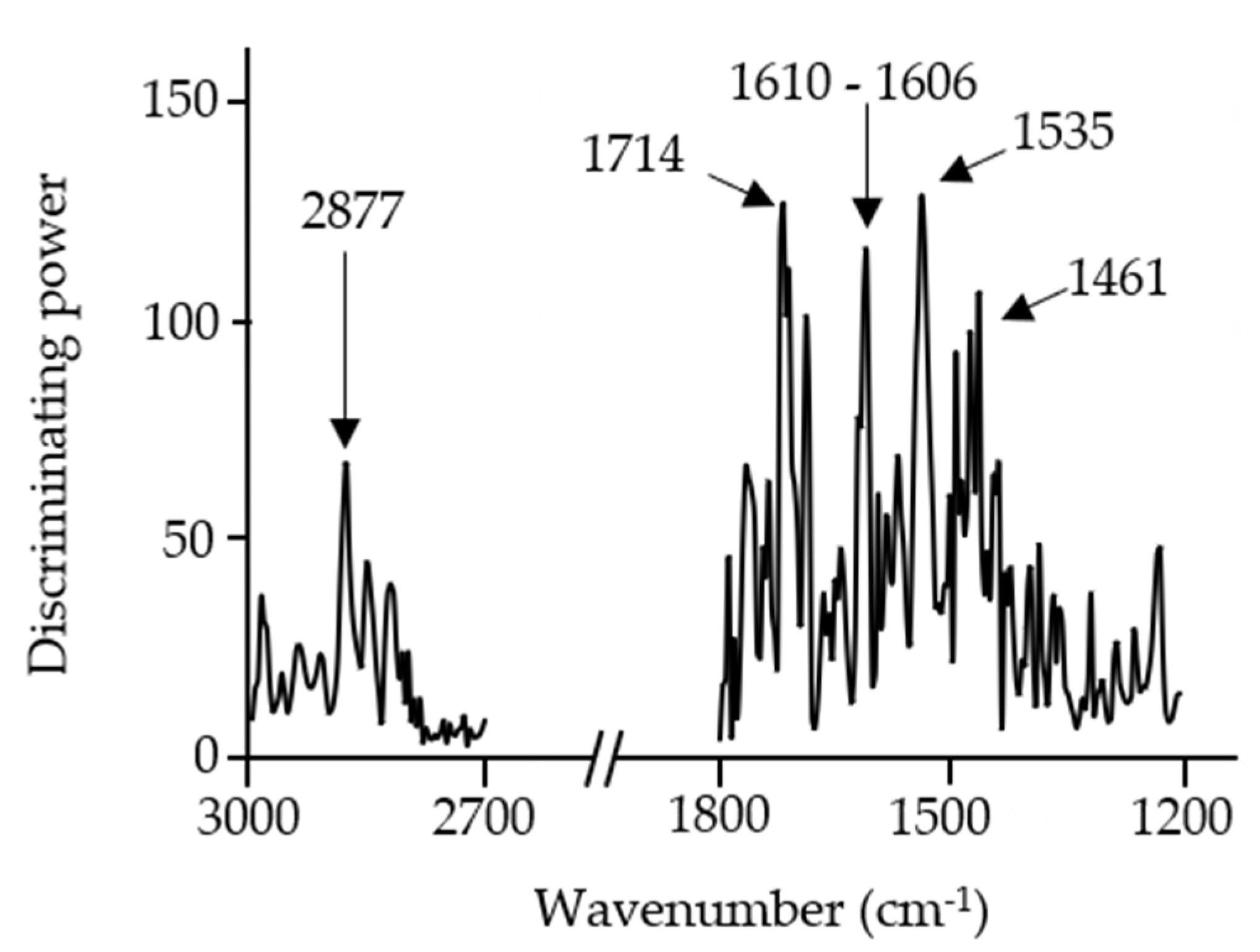
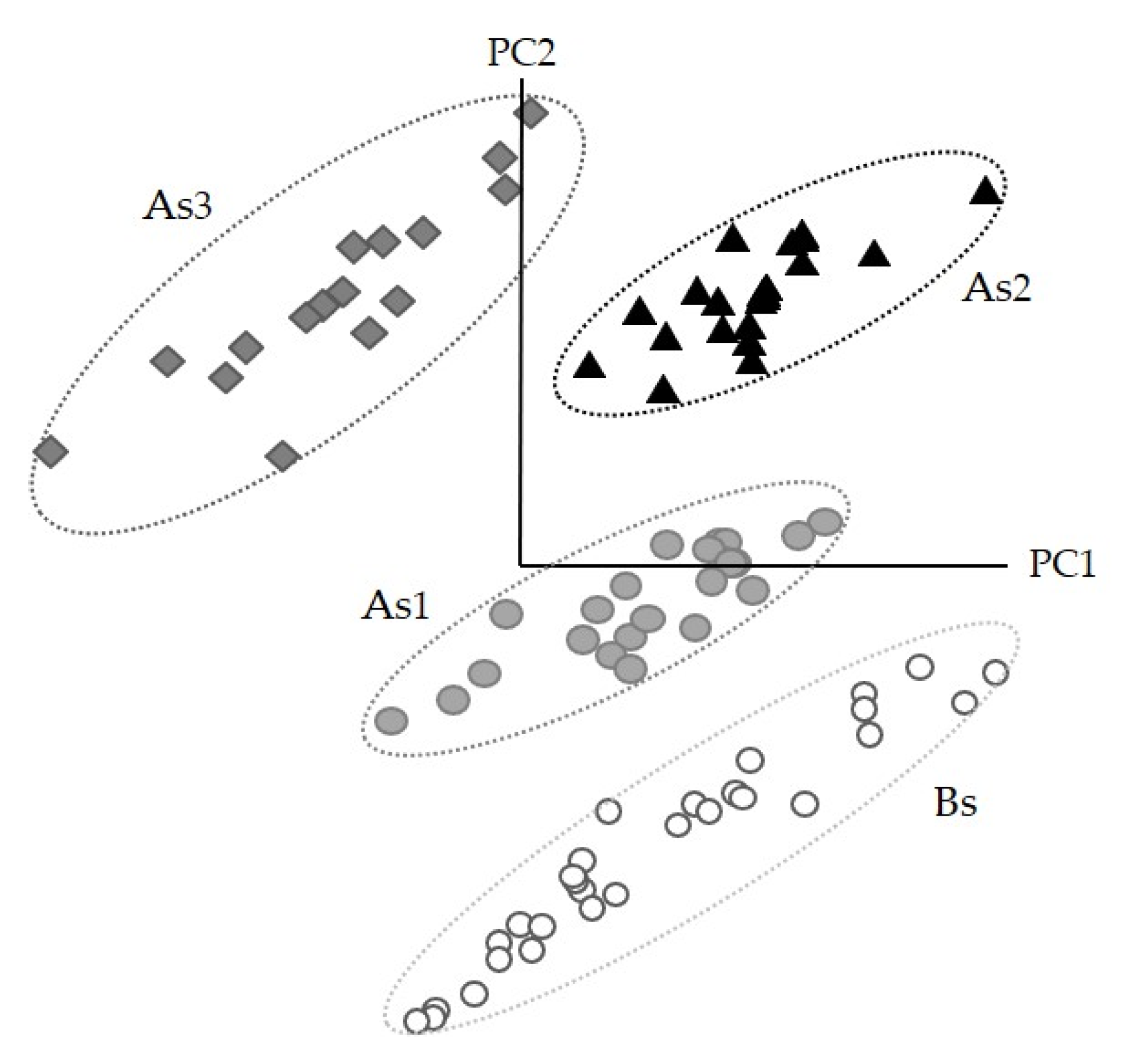
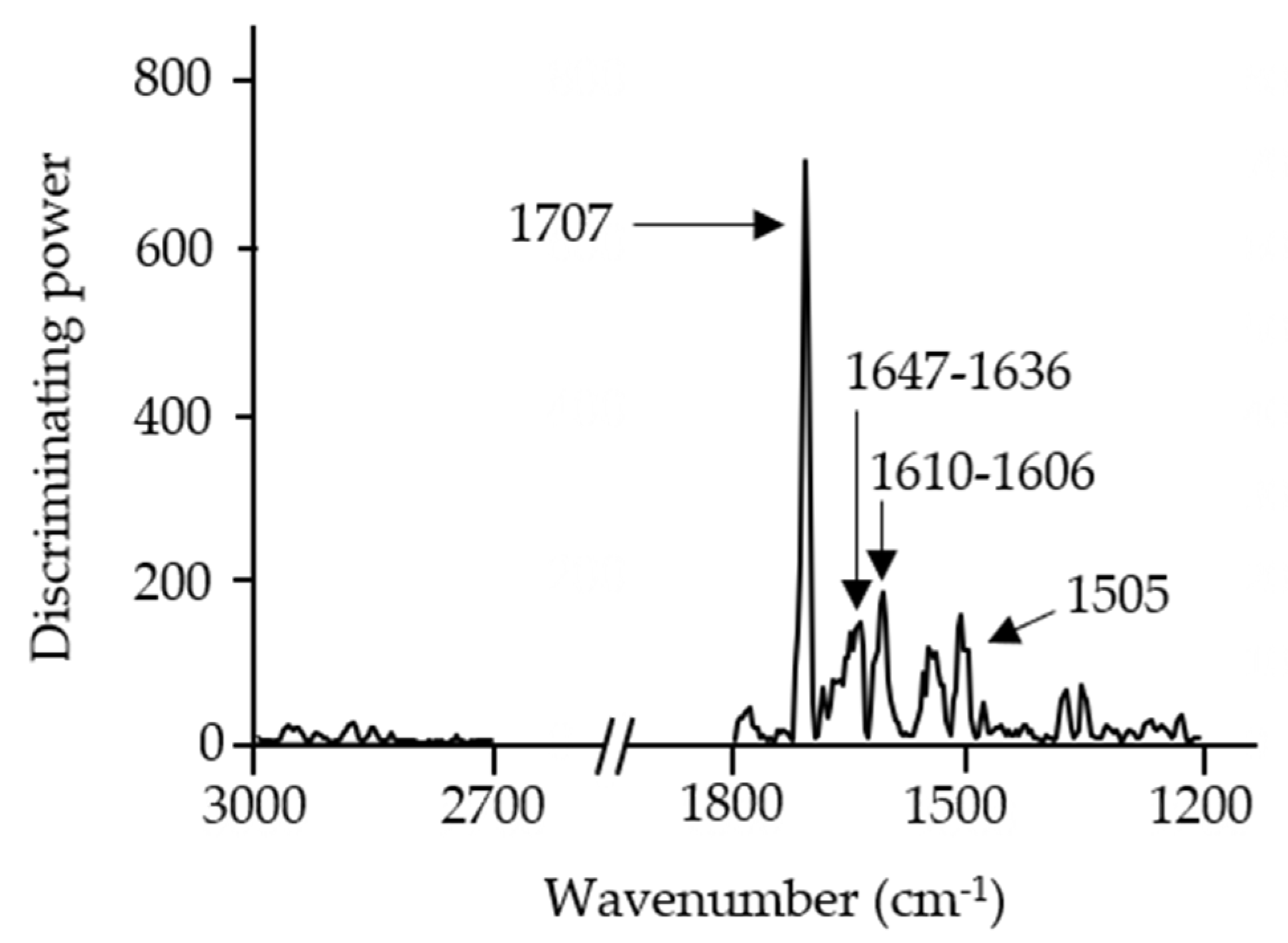
3.4. Discrimination and Classification of Snacks by ATR-FT-MIR Combined with SIMCA
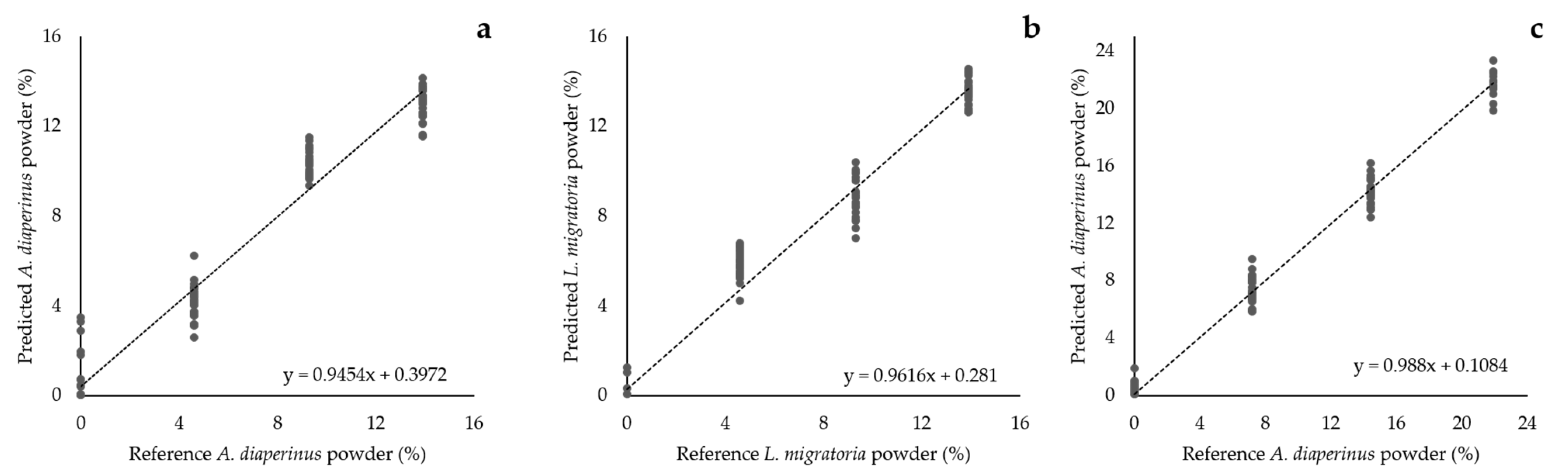
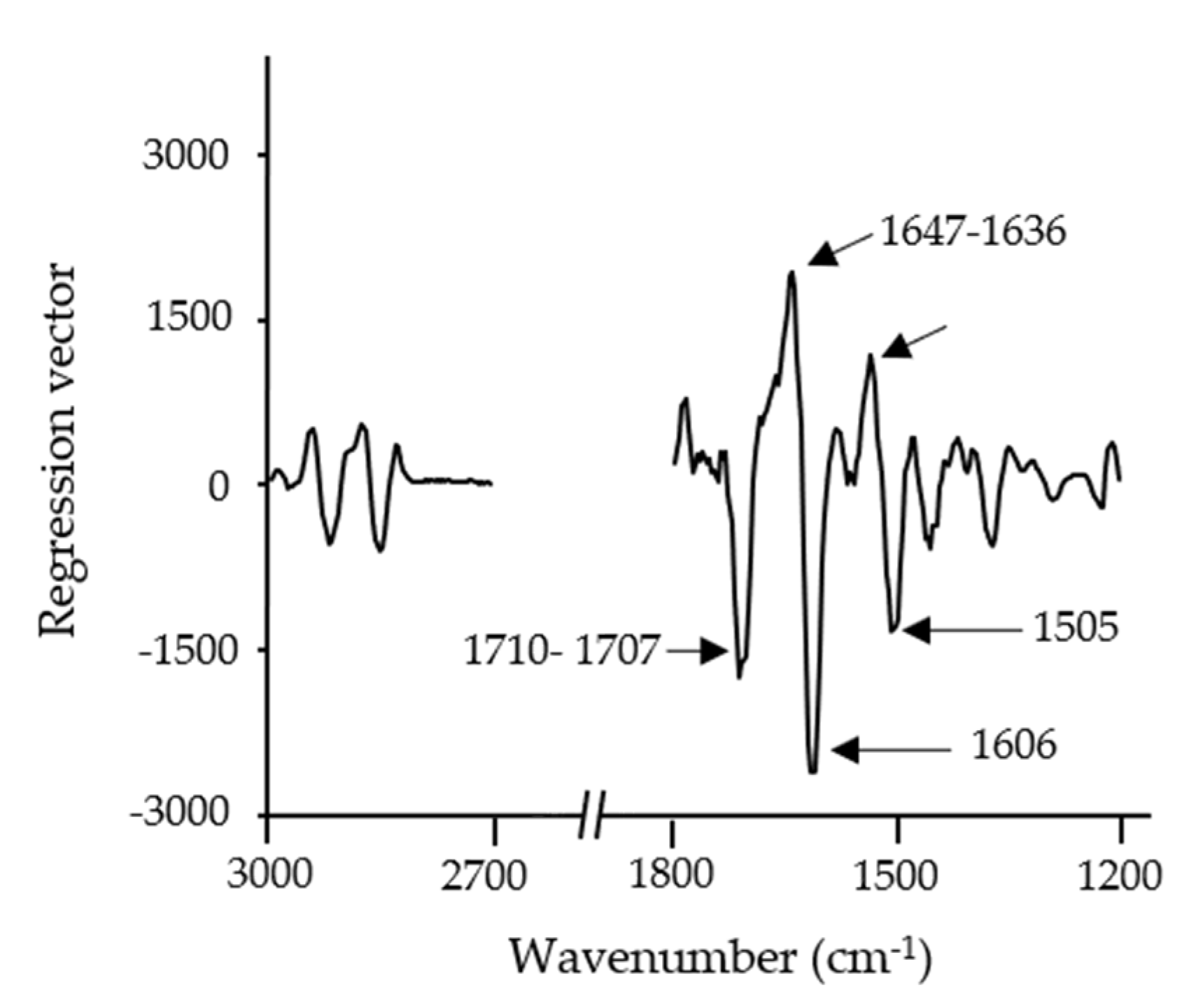
3.5. PLSR of Insect Powder Concentration in Doughs and Snacks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- United Nations: Department of Economic and Social Affairs: Population Division. World Population Prospects 2019: Highlights; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Production, F.; Cervantes-Godoy, D.; Dewbre, J.; PIN; Amegnaglo, C.J.; Soglo, Y.Y.; Akpa, A.F.; Bickel, M.; Sanyang, S.; Ly, S.; et al. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; Volume 4, ISBN 1815-6797. [Google Scholar]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. A J. Biosci. 2017, 72, 337–349. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects. Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; Van Huis, A.; Loon, J.J.A. Van Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-efficient food production to reduce global warming and ecodegradation: The use of edible insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef] [PubMed]
- Garino, C.; Zagon, J.; Braeuning, A. Insects in food and feed—Allergenicity risk assessment and analytical detection. EFSA J. 2019, 17, e170907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- International Platform of Insects for Food and Feed (IPIFF). Edible Insects on the European Market. Available online: https://ipiff.org/wp-content/uploads/2020/06/10-06-2020-IPIFF-edible-insects-market-factsheet.pdf (accessed on 4 August 2021).
- European Commission. Approval of First Insect as Novel Food. Available online: https://ec.europa.eu/food/safety/novel_food/authorisations/approval-first-insect-novel-food_en (accessed on 27 May 2021).
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Trends in Analytical Chemistry Food authentication: Techniques, trends & emerging approaches. Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Reed, J.D.; Krueger, C.G.; Vestling, M.M. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry 2005, 66, 2248–2263. [Google Scholar] [CrossRef]
- Ulrich, S.; Kühn, U.; Biermaier, B.; Piacenza, N.; Schwaiger, K.; Gottschalk, C.; Gareis, M. Direct identification of edible insects by MALDI-TOF mass spectrometry. Food Control 2017, 76, 96–101. [Google Scholar] [CrossRef]
- Rohman, A.; Sismindari; Erwanto, Y.; Che Man, Y.B. Analysis of pork adulteration in beef meatball using Fourier transform infrared (FTIR) spectroscopy. Meat Sci. 2011, 88, 91–95. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Allendorf, M.E. Use of FTIR for Rapid Authentication and Detection of Adulteration of Food. Annu. Rev. Food Sci. Technol. 2011, 2, 467–483. [Google Scholar] [CrossRef]
- Mellado-Carretero, J.; García-Gutiérrez, N.; Ferrando, M.; Güell, C.; García-Gonzalo, D.; De Lamo-Castellví, S. Rapid discrimination and classification of edible insect powders using ATR-FTIR spectroscopy combined with multivariate analysis. J. Insects Food Feed 2019, 6, 141–148. [Google Scholar] [CrossRef]
- Suhandy, D.; Yulia, M. Peaberry coffee discrimination using UV-visible spectroscopy combined with SIMCA and PLS-DA. Int. J. Food Prop. 2017, 20, S331–S339. [Google Scholar] [CrossRef]
- Mertens, B.; Thompson, M.; Fearn, T. Principal component outlier detection and SIMCA: A synthesis. Analyst 1994, 119, 2777–2784. [Google Scholar] [CrossRef]
- Afseth, N.K.; Segtnan, V.H.; Marquardt, B.J.; Wold, J.P. Raman and near-infrared spectroscopy for quantification of fat composition in a complex food model system. Appl. Spectrosc. 2005, 59, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G.; Rohman, A. The Use of FTIR Spectroscopy Combined with Multivariate Analysis in Food Composition Analysis. In Spectroscopic Techniques & Artificial Intelligence for Food and Beverage Analysis; Springer: Singapore, 2020; pp. 25–51. ISBN 978-981-15-6495-6. [Google Scholar]
- Kumar, Y.; Sharanagat, V.S.; Singh, L.; Mani, S. Effect of germination and roasting on the proximate composition, total phenolics, and functional properties of black chickpea (Cicer arietinum). Legum. Sci. 2020, 2, e20. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res. Int. 2010, 43, 886–892. [Google Scholar] [CrossRef]
- Majtán, J.; Bíliková, K.; Markovič, O.; Gróf, J.; Kogan, G.; Šimúth, J. Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int. J. Biol. Macromol. 2007, 40, 237–241. [Google Scholar] [CrossRef]
- Sun, B.; Rahman, M.M.; Tar’an, B.; Yu, P. Determine effect of pressure heating on carbohydrate related molecular structures in association with carbohydrate metabolic profiles of cool-climate chickpeas using Globar spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 8–18. [Google Scholar] [CrossRef]
- Meza-Márquez, O.G.; Gallardo-Velázquez, T.; Osorio-Revilla, G. Application of mid-infrared spectroscopy with multivariate analysis and soft independent modeling of class analogies (SIMCA) for the detection of adulterants in minced beef. MESC 2010, 86, 511–519. [Google Scholar] [CrossRef]
- Tsugawa, H.; Tsujimoto, Y.; Arita, M.; Bamba, T.; Fukusaki, E. GC/MS based metabolomics: Development of a data mining system for metabolite identification by using soft independent modeling of class analogy (SIMCA). BMC Bioinform. 2011, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Mantilla, S.M.O.; Alagappan, S.; Sultanbawa, Y.; Smyth, H.E.; Cozzolino, D. A Mid Infrared (MIR) Spectroscopy Study of the Composition of Edible Australian Green Ants (Oecophylla smaragdina)—A Qualitative Study. Food Anal. Methods 2020, 13, 1627–1633. [Google Scholar] [CrossRef]
- Peren, D.; Menevseoglu, A. A rapid method to detect green pea and peanut adulteration in pistachio by using portable FT-MIR and FT-NIR spectroscopy combined with chemometrics. Food Control 2021, 121, 107670. [Google Scholar] [CrossRef]
- Gómez-de-anda, F.; Dorantes-álvarez, L.; Gallardo-velázquez, T.; Osorio-revilla, G. Determination of Trichinella spiralis in pig muscles using Mid-Fourier Transform Infrared Spectroscopy (MID-FTIR) with Attenuated Total Re fl ectance (ATR) and Soft Independent Modeling of Class Analogy (SIMCA). MESC 2012, 91, 240–246. [Google Scholar] [CrossRef]
- De Lamo-Castellví, S.; Männing, A.; Rodríguez-Saona, L.E. Fourier-transform infrared spectroscopy combined with immunomagnetic separation as a tool to discriminate Salmonella serovars. Analyst 2010, 135, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, T.K.; Espina, L.; Pagán, R.; García-Gonzalo, D.; De Lamo-Castellví, S. Prediction of Injured and Dead Inactivated Escherichia coli O157:H7 Cells after Heat and Pulsed Electric Field Treatment with Attenuated Total Reflectance Infrared Microspectroscopy Combined with Multivariate Analysis Technique. Food Bioprocess Technol. 2014, 7, 2084–2092. [Google Scholar] [CrossRef]
- Kwok, K.C.; Liang, H.H.; Niranjan, K. Optimizing conditions for thermal processes of soy milk. J. Agric. Food Chem. 2002, 50, 4834–4838. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byler, D.M.; Purcell, J.M. FTIR Examination Of Thermal Denaturation And Gel-Formation In Whey Proteins. SPIE Digit. Libr. 1989, 1145, 415. [Google Scholar] [CrossRef]
- Sablani, S.S.; Marcotte, M.; Baik, O.D.; Castaigne, F. Modeling of simultaneous heat and water transport in the baking process. LWT Food Sci. Technol. 1998, 31, 201–209. [Google Scholar] [CrossRef]
- Frankel, E.N. Free radical oxidation. Lipid Oxid. 2012, 15–24. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Heat-Induced Changes in Milk. In Dairy Chemistry and Biochemistry; Springer International Publishing: Cham, Switzerland, 2015; pp. 345–375. [Google Scholar]
- Markoska, T.; Huppertz, T.; Grewal, M.K.; Vasiljevic, T. Structural changes of milk proteins during heating of concentrated skim milk determined using FTIR. Int. Dairy J. 2019, 89, 21–30. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.F.; Benning, R.; Haase, H. Effect of different drying methods on nutrient quality of the yellow mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Carl Hoseney, R. Chemical changes in carbohydrates produced by thermal processing. J. Chem. Educ. 1984, 61, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Mevik, B.H.; Cederkvist, H.R. Mean squared error of prediction (MSEP) estimates for principal component regression (PCR) and partial least squares regression (PLSR). J. Chemom. 2004, 18, 422–429. [Google Scholar] [CrossRef]
- Infometrix Inc. Pirouette Multivariate Data Analysis Software; Infometrix Inc.: Washington, DC, USA, 2011. [Google Scholar]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Candoğan, K.; Altuntas, E.G.; İğci, N. Authentication and Quality Assessment of Meat Products by Fourier-Transform Infrared (FTIR) Spectroscopy. Food Eng. Rev. 2021, 13, 66–91. [Google Scholar] [CrossRef]
- Cebi, N.; Taylan, O.; Abusurrah, M.; Sagdic, O. Detection of orange essential oil, isopropyl myristate, and benzyl alcohol in lemon essential oil by ftir spectroscopy combined with chemometrics. Foods 2021, 10, 27. [Google Scholar] [CrossRef] [PubMed]

| Ingredients (g) | Dough Formulation a | ||||||
|---|---|---|---|---|---|---|---|
| B | Ad1 | Ad2 | Ad3 | Ld1 | Ld2 | Ld3 | |
| Chickpea flour | 100.03 ± 0.01 | 90.05 ± 0.02 | 80.02 ± 0.01 | 70.03 ± 0.01 | 90.03 ± 0.05 | 80.03 ± 0.02 | 70.04 ± 0.05 |
| A. diaperinus powder | - | 10.02 ± 0.05 | 20.03 ± 0.04 | 30.02 ± 0.06 | - | - | - |
| L. migratoria powder | - | - | - | - | 10.07 ± 0.08 | 20.08 ± 0.09 | 30.06 ± 0.08 |
| Water | 85.01 ± 0.01 | 85.01 ± 0.01 | 85.00 ± 0.01 | 85.00 ± 0.01 | 85.01 ± 0.01 | 85.01 ± 0.01 | 85.01 ± 0.01 |
| Olive oil | 25.00 ± 0.01 | 25.01 ± 0.01 | 25.00 ± 0.01 | 25.01 ± 0.01 | 25.01 ± 0.01 | 25.00 ± 0.01 | 25.00 ± 0.01 |
| Curry powder | 4.00 ± 0.01 | 4.01 ± 0.01 | 4.00 ± 0.01 | 4.00 ± 0.01 | 4.01 ± 0.01 | 4.00 ± 0.01 | 4.00 ± 0.01 |
| Salt | 2.05 ± 0.03 | 2.04 ± 0.03 | 2.06 ± 0.02 | 2.02 ± 0.05 | 2.05 ± 0.04 | 2.07 ± 0.02 | 2.03 ± 0.03 |
| Insect powder (%) | 0.00 ± 0.00 | 4.64 ± 0.02 | 9.27 ± 0.02 | 13.89 ± 0.03 | 4.65 ± 0.02 | 9.28 ± 0.02 | 13.90 ± 0.02 |
| Snack Formulation | B | As1 | As2 | As3 |
|---|---|---|---|---|
| Water loss (%) a | 36.2 ± 0.2 | 36.0 ± 0.5 | 35.5 ± 1.5 | 36.5 ± 1.2 |
| A. diaperinus powder (wt%) a | 0.0 ± 0.0 | 7.2 ± 0.1 | 14.4 ± 0.4 | 21.9 ± 0.4 |
| Model | Class | Factor 1 (%) | Factor 2 (%) | Factor 3 (%) | Outliers |
|---|---|---|---|---|---|
| 4-class SIMCA A. diaperinus dough model | B | 82.2 | 92.7 | 95.0 | 0 |
| Ad1 | 81.2 | 92.5 | 96.3 | 5 | |
| Ad2 | 92.0 | 95.8 | 98.0 | 3 | |
| Ad3 | 91.7 | 97.0 | 98.5 | 5 | |
| 4-class SIMCA L. migratoria dough model | B | 82.2 | 95.7 | 95.0 | 0 |
| Ld1 | 86.0 | 91.7 | 95.7 | 4 | |
| Ld2 | 96.9 | 98.2 | 99.1 | 6 | |
| Ld3 | 75.0 | 85.7 | 91.9 | 5 |
| A. diaperinus | B | Ad1 | Ad2 | Ad3 |
| B | 0.0 | |||
| Ad1 | 3.1 | 0.0 | ||
| Ad2 | 8.7 | 4.5 | 0.0 | |
| Ad3 | 12.2 | 7.2 | 3.1 | 0.0 |
| L. migratoria | B | Ld1 | Ld2 | Ld3 |
| B | 0.0 | |||
| Ld1 | 5.8 | 0.0 | ||
| Ld2 | 8.4 | 3.0 | 0.0 | |
| Ld3 | 13.6 | 7.4 | 3.9 | 0.0 |
| Model | Dough | No. Spectra | Best Class | Next Best | Not Classified |
|---|---|---|---|---|---|
| 4-class SIMCA A. diaperinus | Ld1 | 26 | 0% | 0% | 100% |
| Ld2 | 24 | 0% | 0% | 100% | |
| Ld3 | 25 | 0% | 0% | 100% | |
| 4-class SIMCA L. migratoria | Ad1 | 25 | 0% | 0% | 100% |
| Ad2 | 27 | 0% | 0% | 100% | |
| Ad3 | 25 | 0% | 0% | 100% |
| Model | Class | Factor 1 (%) | Factor 2 (%) | Factor 3 (%) | Outliers |
|---|---|---|---|---|---|
| 6-class SIMCA A. diaperinus vs. L. migratoria dough model | Ld1 | 86.0 | 91.7 | 95.7 | 4 |
| Ad1 | 83.9 | 94.7 | 96.6 | 6 | |
| Ld2 | 96.7 | 98.2 | 99.1 | 7 | |
| Ad2 | 92.0 | 95.8 | 98.0 | 6 | |
| Ld3 | 75.9 | 85.7 | 91.9 | 5 | |
| Ad3 | 91.7 | 96.9 | 98.5 | 5 |
| Dough | Ld1 | Ad1 | Ld2 | Ad2 | Ld3 | Ad3 |
|---|---|---|---|---|---|---|
| Ld1 | 0.0 | |||||
| Ad1 | 3.0 | 0.0 | ||||
| Ld2 | 3.0 | 4.7 | 0.0 | |||
| Ad2 | 3.3 | 4.3 | 3.8 | 0.0 | ||
| Ld3 | 7.4 | 9.3 | 3.9 | 6.7 | 0.0 | |
| Ad3 | 6.4 | 7.3 | 5.1 | 3.1 | 5.0 | 0.0 |
| Model | Class | Factor 1 (%) | Factor 2 (%) | Outliers |
|---|---|---|---|---|
| 4-class SIMCA A. diaperinus snack model | Bs | 97.2 | 98.7 | 1 |
| As1 | 97.0 | 97.9 | 6 | |
| As2 | 89.5 | 93.5 | 4 | |
| As3 | 93.8 | 96.9 | 0 |
| Snack | Bs | AS1 | AS2 | AS3 |
|---|---|---|---|---|
| B | 0.0 | |||
| AS1 | 3.3 | 0.0 | ||
| AS2 | 5.9 | 3.0 | 0.0 | |
| AS3 | 8.2 | 5.1 | 3.3 | 0.0 |
| Model | Factors | n | Cumulative Variance (%) | SEP | R2val | SEC | R2cal |
|---|---|---|---|---|---|---|---|
| Ad PLSR | 2 | 93 | 96.2 | 1.24 | 0.970 | 1.21 | 0.972 |
| Ld PLSR | 2 | 91 | 95.6 | 1.08 | 0.978 | 1.06 | 0.979 |
| As PLSR | 2 | 89 | 97.8 | 0.90 | 0.994 | 0.88 | 0.994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gutiérrez, N.; Mellado-Carretero, J.; Bengoa, C.; Salvador, A.; Sanz, T.; Wang, J.; Ferrando, M.; Güell, C.; Lamo-Castellví, S.d. ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder. Foods 2021, 10, 1806. https://doi.org/10.3390/foods10081806
García-Gutiérrez N, Mellado-Carretero J, Bengoa C, Salvador A, Sanz T, Wang J, Ferrando M, Güell C, Lamo-Castellví Sd. ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder. Foods. 2021; 10(8):1806. https://doi.org/10.3390/foods10081806
Chicago/Turabian StyleGarcía-Gutiérrez, Nerea, Jorge Mellado-Carretero, Christophe Bengoa, Ana Salvador, Teresa Sanz, Junjing Wang, Montse Ferrando, Carme Güell, and Sílvia de Lamo-Castellví. 2021. "ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder" Foods 10, no. 8: 1806. https://doi.org/10.3390/foods10081806
APA StyleGarcía-Gutiérrez, N., Mellado-Carretero, J., Bengoa, C., Salvador, A., Sanz, T., Wang, J., Ferrando, M., Güell, C., & Lamo-Castellví, S. d. (2021). ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder. Foods, 10(8), 1806. https://doi.org/10.3390/foods10081806