Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Bacterial Strains, and Culture Conditions
2.2. Preparation of the Probiotic and Synbiotic Films
2.3. Microbiological Analysis
2.4. Physicochemical Properties
2.4.1. Thickness and Moisture Content (MC)
2.4.2. Water Solubility (WS) and Swelling Ratio (SR)
2.4.3. Color and Opacity Properties
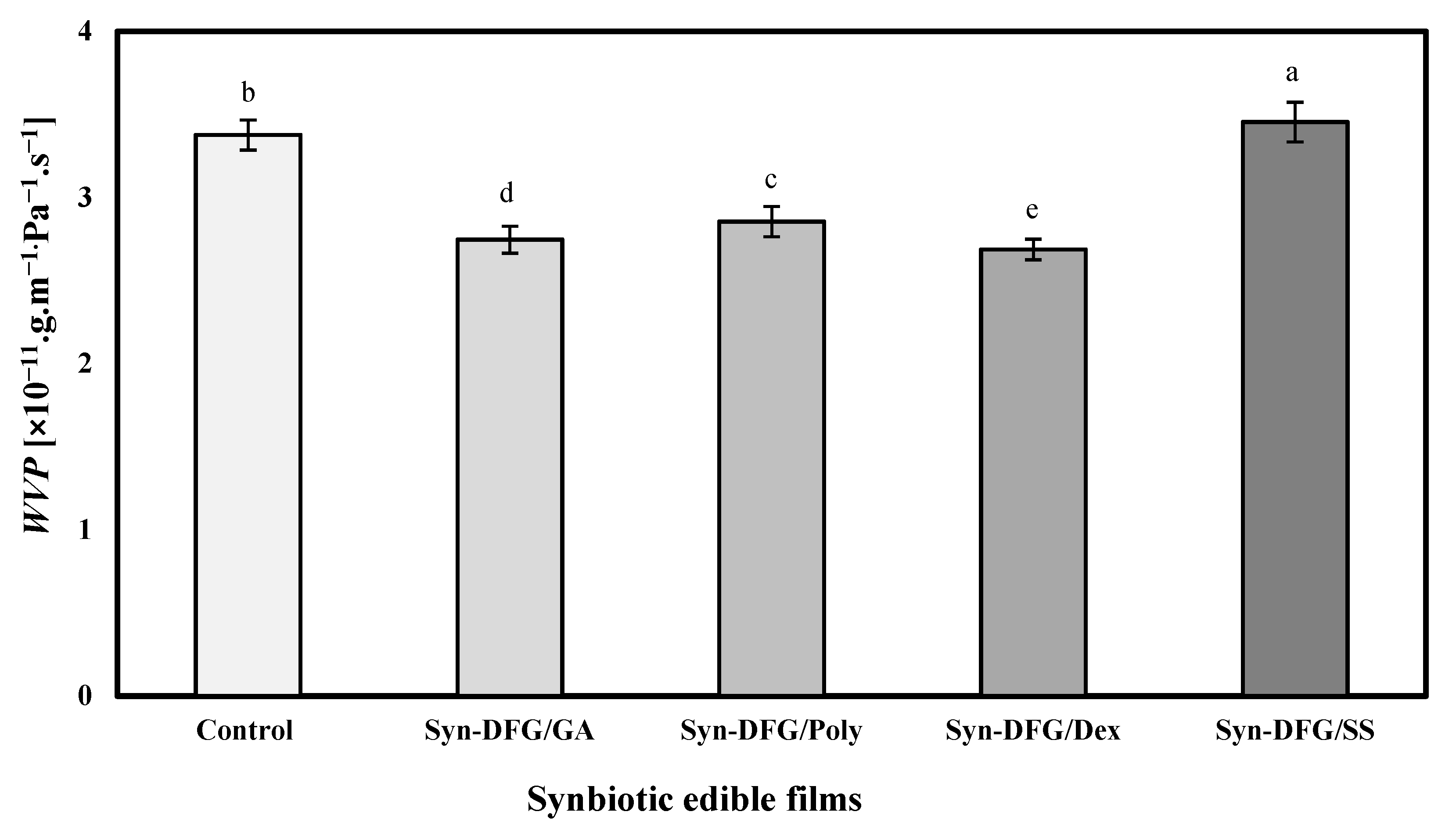
2.4.4. Water-Vapor Permeability (WVP)
2.4.5. Prevention against Oxidation (PAO)
2.5. Mechanical Properties of SEFs
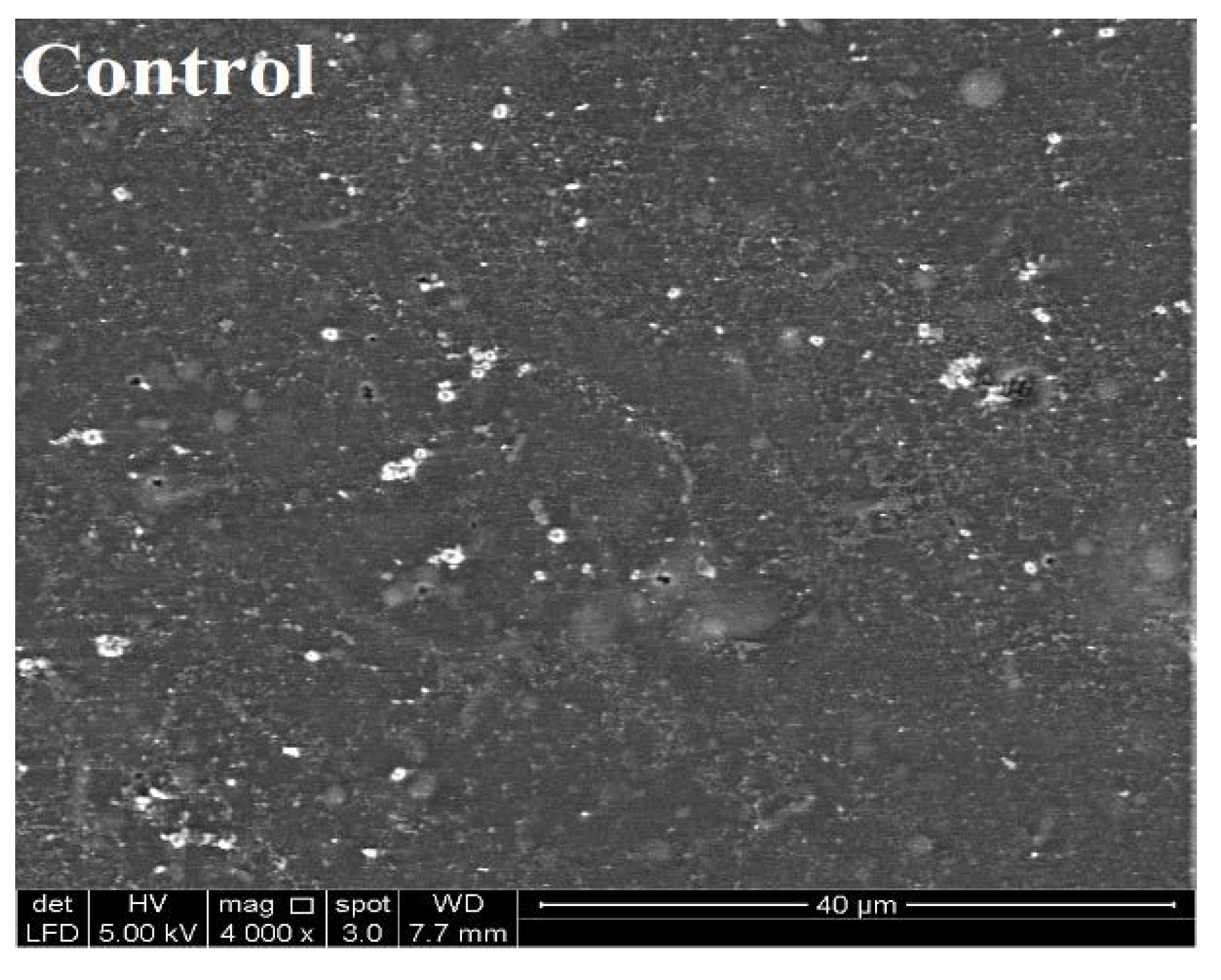
2.6. Scanning Electron Microscopy (SEM) of SEFs
2.7. Statistical Analysis
3. Results and Discussions
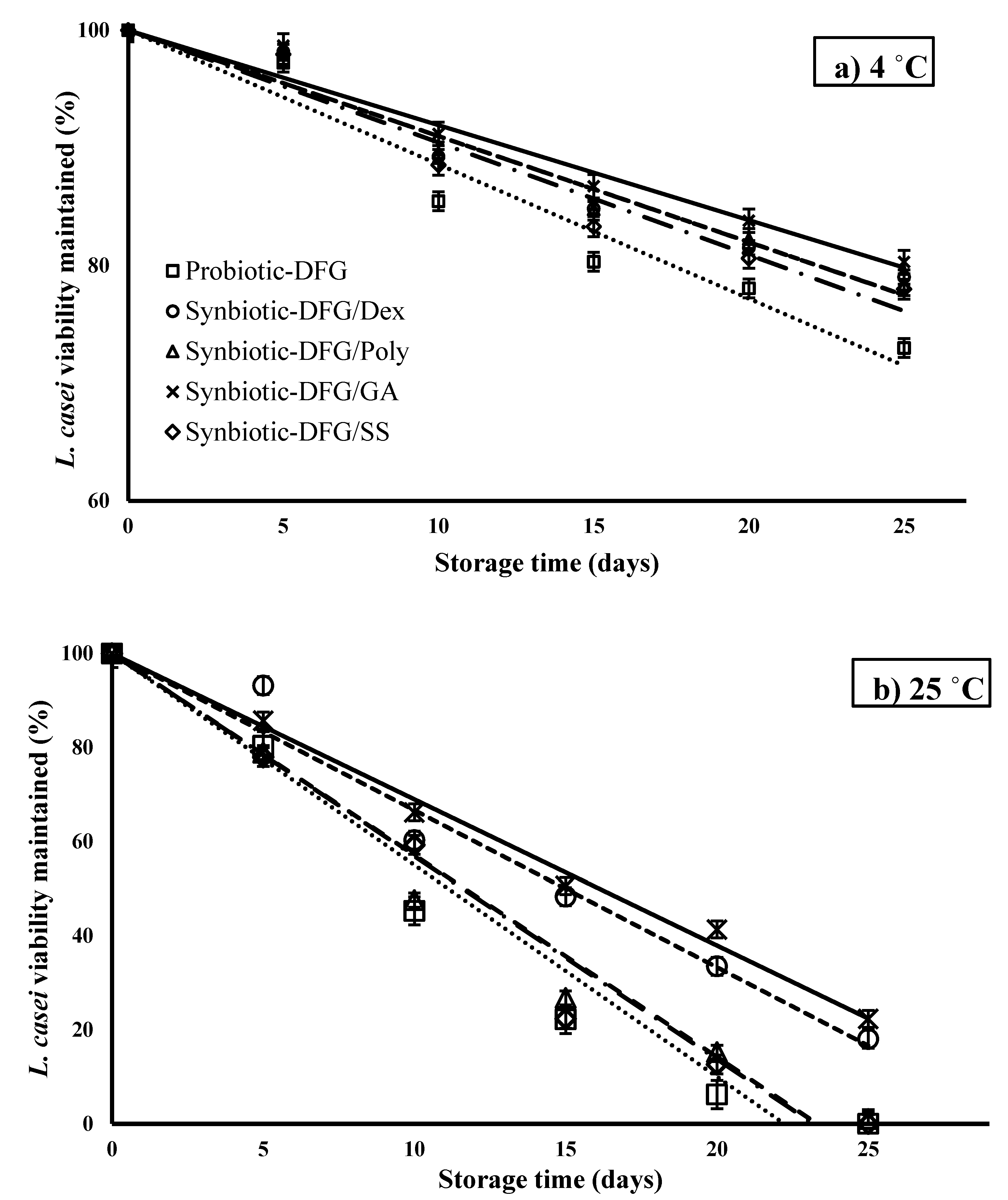
3.1. Effect of Gelatin Origin on the Storage Stability of L. casei
3.2. Color Properties and Morphology
3.3. Effect of Prebiotics on the Storage Stability of L. casei
3.4. Effect of Prebiotics on Physicochemical Properties of SEFs
3.5. Effect of Prebiotics on WVP and PAO of SEFs
3.6. Mechanical Properties of SEFs
4. Conclusions
- Several probiotic bacteria can be simultaneously used under the conditions of this study to evaluate the films’ ability to keep bacteria alive.
- The application of bioactive Syn-DFG/GA film to ready-to-eat or processed-meat packaging needs to be investigated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espitia, P.J.P.; Batista, R.A.; Azeredo, H.M.C.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.d.C.; Regal, P.; Miranda, J.M. Probiotics as a Possible Strategy for the Prevention and Treatment of Allergies. A Narrative Review. Foods 2021, 10, 701. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.L.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Scalfaro, C.; Iacobino, A.; Nardis, C.; Franciosa, G. Galleria mellonella as an in vivo model for assessing the protective activity of probiotics against gastrointestinal bacterial pathogens. FEMS Microbiol. Lett. 2017, 364, fnx064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, A.; Robertson, K.; Yung, A.; Que, M.; Randall, H.; Wellalagodage, D.; Cox, T.; Robertson, D.; Chi, C.; Sun, J. Efficacy of Probiotics in Patients of Cardiovascular Disease Risk: A Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 2020, 22, 1–27. [Google Scholar] [CrossRef]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo [a] pyrene: A review. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Farmer, S.; Cash, H.A.; Keller, D. Probiotic Bacillus coagulans GBI-30, 6086 improves protein absorption and utilization. Probiotics Antimicrob. Proteins 2018, 10, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, M.F.T.; Jalali, H.; Nafchi, A.M.; Nouri, L. Evaluating the effects of lactic acid bacteria and olive leaf extract on the quality of gluten-free bread. Gene Rep. 2020, 21, 100771. [Google Scholar] [CrossRef]
- Dhillon, P.; Singh, K. Therapeutic Applications of Probiotics in Ulcerative Colitis: An updated review. PharmaNutrition 2020, 13, 100194. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre-and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Delvarianzadeh, M.; Nouri, L.; Nafchi, A.M.; Ebrahimi, H. Physicochemical, rheological, and sensory evaluation of voluminous breads enriched by purslane (Portulaca oleracea L.). Ital. J. Food Sci. 2020, 32, 815–830. [Google Scholar]
- Chugh, B.; Kamal-Eldin, A. Bioactive compounds produced by probiotics in food products. Curr. Opin. Food Sci. 2020, 32, 76–82. [Google Scholar] [CrossRef]
- Asadzadeh, A.; Jalali, H.; Azizi, M.H.; Mohammadi Nafchi, A. Production of oat bran functional probiotic beverage using Bifidobacterium lactis. J. Food Meas. Charact. 2020, 15, 1301–1309. [Google Scholar] [CrossRef]
- Pandhi, S.; Kumar, A.; Alam, T. Probiotic Edible Films and Coatings: Concerns, Applications and Future Prospects. J. Packag. Technol. Res. 2019, 3, 261–268. [Google Scholar] [CrossRef]
- Piermaria, J.; Diosma, G.; Aquino, C.; Garrote, G.; Abraham, A. Edible kefiran films as vehicle for probiotic microorganisms. Innov. Food Sci. Emerg. Technol. 2015, 32, 193–199. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT Food Sci. Technol. 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Bekhit, M.; Arab-Tehrany, E.; Kahn, C.J.; Cleymand, F.; Fleutot, S.; Desobry, S.; Sánchez-González, L. Bioactive films containing alginate-pectin composite microbeads with Lactococcus lactis subsp. lactis: Physicochemical characterization and antilisterial activity. Int. J. Mol. Sci. 2018, 19, 574. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Moslehi, Z.; Nafchi, A.M.; Moslehi, M.; Jafarzadeh, S. Aflatoxin, microbial contamination, sensory attributes, and morphological analysis of pistachio nut coated with methylcellulose. Food Sci. Nutr. 2021, 9, 2576–2584. [Google Scholar] [CrossRef] [PubMed]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT Food Sci. Technol. 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; Li, S.; Liu, Y. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef]
- Jahdkaran, E.; Hosseini, S.E.; Nafchi, A.M.; Nouri, L. The effects of methylcellulose coating containing carvacrol or menthol on the physicochemical, mechanical, and antimicrobial activity of polyethylene films. Food Sci. Nutr. 2021, 9, 2768–2778. [Google Scholar] [CrossRef]
- Zabihollahi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S.; Hamishekar, H. Development and characterization of carboxymethyl cellulose based probiotic nanocomposite film containing cellulose nanofiber and inulin for chicken fillet shelf life extension. Int. J. Biol. Macromol. 2020, 160, 409–417. [Google Scholar] [CrossRef]
- Albadran, H.A.; Monteagudo-Mera, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Development of chitosan-coated agar-gelatin particles for probiotic delivery and targeted release in the gastrointestinal tract. Appl. Microbiol. Biotechnol. 2020, 5749–5757. [Google Scholar] [CrossRef] [PubMed]
- Pruksarojanakul, P.; Prakitchaiwattana, C.; Settachaimongkon, S.; Borompichaichartkul, C. Synbiotic edible film from konjac glucomannan composed of Lactobacillus casei-01® and Orafti® GR, and its application as coating on bread buns. J. Sci. Food Agric. 2020, 100, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Abedinia, A.; Nafchi, A.M.; Sharifi, M.; Ghalambor, P.; Oladzadabbasabadi, N.; Ariffin, F.; Huda, N. Poultry gelatin: Characteristics, developments, challenges, and future outlooks as a sustainable alternative for mammalian gelatin. Trends Food Sci. Technol. 2020, 104, 14–26. [Google Scholar] [CrossRef]
- Rama, G.R.; Dullius, D.; Agnol, W.D.; Esquerdo, V.M.; Lehn, D.N.; de Souza, C.F.V. Ricotta whey supplemented with gelatin and collagen for the encapsulation of probiotic lactic acid bacteria. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Abedinia, A.; Ariffin, F.; Huda, N.; Nafchi, A.M. Extraction and characterization of gelatin from the feet of Pekin duck (Anas platyrhynchos domestica) as affected by acid, alkaline, and enzyme pretreatment. Int. J. Biol. Macromol. 2017, 98, 586–594. [Google Scholar] [CrossRef]
- Nazmi, N.N.; Isa, M.I.N.; Sarbon, N.M. Preparation and characterization of chicken skin gelatin/CMC composite film as compared to bovine gelatin film. Food Biosci. 2017, 19, 149–155. [Google Scholar] [CrossRef]
- Qi, X.-M.; Liu, S.-Y.; Chu, F.-B.; Pang, S.; Liang, Y.-R.; Guan, Y.; Peng, F.; Sun, R.-C. Preparation and characterization of blended films from quaternized hemicelluloses and carboxymethyl cellulose. Materials 2016, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedinia, A.; Ariffin, F.; Huda, N.; Nafchi, A.M. Preparation and characterization of a novel biocomposite based on duck feet gelatin as alternative to bovine gelatin. Int. J. Biol. Macromol. 2018, 109, 855–862. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Kurek, M.; Bornaz, S.; Debeaufort, F. Barrier, structural and mechanical properties of bovine gelatin–chitosan blend films related to biopolymer interactions. J. Sci. Food Agric. 2014, 94, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.C.C.; Gardim, R.B.; Almeida, P.F.; Borini, G.B.; Quispe, A.P.B.; Llanos, S.A.V.; Heredia, J.A.; Zamuner, S.; Gamarra, F.M.C.; Farias, T.M.B.; et al. Valorization of Chicken Feet By-Product of the Poultry Industry: High Qualities of Gelatin and Biofilm from Extraction of Collagen. Polymers 2020, 12, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.-C.; Karim, A.A.; Uthumporn, U.; Ghazali, F.C. Effect of Thermal Treatment on the Physicochemical Properties of Emulsion Stabilized by Gelatin from Black Tilapia (Oreochromis mossambicus) Skin. Food Biophys. 2020, 15, 423–432. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on the evolution of flavor compounds in probiotic dry-fermented sausages during ripening. Meat Sci. 2015, 100, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.X.; Nafchi, A.M.; Ghasemipour, F.; Easa, A.M.; Jafarzadeh, S.; Al-Hassan, A.A. Characterization of pH sensitive sago starch films enriched with anthocyanin-rich torch ginger extract. Int. J. Biol. Macromol. 2020, 164, 4603–4612. [Google Scholar] [CrossRef]
- Soukoulis, C.; Yonekura, L.; Gan, H.-H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef]
- Akman, P.K.; Bozkurt, F.; Dogan, K.; Tornuk, F.; Tamturk, F. Fabrication and characterization of probiotic Lactobacillus plantarum loaded sodium alginate edible films. J. Food Meas. Charact. 2020, 15, 84–92. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists' Society. Method Cd 8b-90: Peroxide value. 2003. [Google Scholar]
- Dong, Q.Y.; Chen, M.Y.; Xin, Y.; Qin, X.Y.; Cheng, Z.; Shi, L.E.; Tang, Z.X. Alginate-based and protein-based materials for probiotics encapsulation: A review. Int. J. Food Sci. Technol. 2013, 48, 1339–1351. [Google Scholar] [CrossRef]
- Soukoulis, C.; Singh, P.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocoll. 2016, 52, 876–887. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Cailliez-Grimal, C.; Jeandel, C.; Scher, J. Encapsulation of Lactobacillus rhamnosus GG in microparticles: Influence of casein to whey protein ratio on bacterial survival during digestion. Innov. Food Sci. Emerg. Technol. 2013, 19, 233–242. [Google Scholar] [CrossRef]
- Burgain, J.; Scher, J.; Lebeer, S.; Vanderleyden, J.; Cailliez-Grimal, C.; Corgneau, M.; Francius, G.; Gaiani, C. Significance of bacterial surface molecules interactions with milk proteins to enhance microencapsulation of Lactobacillus rhamnosus GG. Food Hydrocoll. 2014, 41, 60–70. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Shakila, R.J.; Jeevithan, E.; Varatharajakumar, A.; Jeyasekaran, G.; Sukumar, D. Comparison of the properties of multi-composite fish gelatin films with that of mammalian gelatin films. Food Chem. 2012, 135, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Calame, W.; Weseler, A.R.; Viebke, C.; Flynn, C.; Siemensma, A.D. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 2008, 100, 1269–1275. [Google Scholar] [CrossRef]
- Fu, N.; Chen, X.D. Towards a maximal cell survival in convective thermal drying processes. Food Res. Int. 2011, 44, 1127–1149. [Google Scholar] [CrossRef]
- Braber, N.V.; Vergara, L.D.; Rossi, Y.; Aminahuel, C.; Mauri, A.; Cavaglieri, L.; Montenegro, M. Effect of microencapsulation in whey protein and water-soluble chitosan derivative on the viability of the probiotic Kluyveromyces marxianus VM004 during storage and in simulated gastrointestinal conditions. LWT 2020, 118, 108844. [Google Scholar] [CrossRef]
- Ying, D.; Sun, J.; Sanguansri, L.; Weerakkody, R.; Augustin, M.A. Enhanced survival of spray-dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose. J. Food Eng. 2012, 109, 597–602. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, A.; Khosravi-Darani, K.; Mohammadi, R. Application of edible films containing probiotics in food products. J. Consum. Prot. Food Saf. 2020, 15, 307–320. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A.; Voilley, A.; Debeaufort, F. Effect of oxidized potato starch on the physicochemical properties of soy protein isolate-based edible films. Food Technol. Biotechnol. 2013, 51, 403–409. [Google Scholar]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Lee, K.Y.; Shim, J.; Lee, H.G. Mechanical properties of gellan and gelatin composite films. Carbohydr. Polym. 2004, 56, 251–254. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic viability in yoghurt: A review of influential factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]




| Probiotic Film | k 25 °C (%/day−1) | Estimated Shelf-Life at 25 °C (day) | R2 |
|---|---|---|---|
| Probiotic-DFG | 4.47 ± 0.12 a | 20.11 | 0.957 |
| Probiotic-BG | 4.66 ± 0.12 a | 19.27 | 0.908 |
| Probiotic-FG | 4.72 ± 0.12 a | 19.03 | 0.887 |
| Synbiotic Film | L* | a* | b* | ΔE* | Opacity Values |
|---|---|---|---|---|---|
| Control | 95.35 ± 0.07 a | −0.39 ± 0.01 d | 3.42 ± 0.01 e | - | 0.82 ± 0.04 c |
| Syn-DFG/Dex | 94.17 ± 0.08 c | −0.05 ± 0.003 a | 4.41 ± 0.01 c | 3.9 ± 0.02 c | 1.79 ± 0.15 b |
| Syn-DFG/GA | 94.59 ± 0.08 b | −0.33 ± 0.02 c | 4.78 ± 0.05 b | 4.33 ± 0.06 b | 3.94 ± 0.11 a |
| Syn-DFG/Poly | 94.51 ± 0.1 b | −0.74 ± 0.03 e | 6.24 ± 0.08 a | 5.74 ± 0.1 a | 3.24 ± 0.08 a |
| Syn-DFG/SS | 94.47 ± 0.12 b | −0.25 ± 0.02 b | 3.80 ± 0.04 d | 3.32 ± 0.07 d | 1.92 ± 0.12 b |
| Synbiotic Film | k 4 °C (%/day−1) | Estimated Shelf- Life * at 4 °C | R2 | k 25 °C (%/day−1) | Estimated Shelf- Life at 25 °C | R2 |
|---|---|---|---|---|---|---|
| Control | 1.14 ± 0.09 c | 78 | 0.95 | 4.49 ± 0.12 b | 20 | 0.95 |
| Syn-DFG/Dex | 0.9 ± 0.05 b | 99 | 0.96 | 3.33 ± 0.08 a | 27 | 0.97 |
| Syn-DFG/Poly | 0.9 ± 0.03 b | 99 | 0.97 | 4.28 ± 0.05 b | 21 | 0.97 |
| Syn-DFG/GA | 0.8 ± 0.04 a | 111 | 0.97 | 3.1 ± 0.07 a | 29 | 0.99 |
| Syn-DFG/SS | 0.95 ± 0.08 b | 94 | 0.95 | 4.31 ± 0.11 b | 21 | 0.97 |
| Synbiotic Film | Thickness (mm) | MC (%) | SR (%) | WS (%) |
|---|---|---|---|---|
| Control | 0.17 ± 0.01 c | 10.15 ± 0.1 b | 662.1 ± 13.2 a | 19.37 ± 1.12 c |
| Syn-DFG/Dex | 0.21 ± 0.05 a | 9.88 ± 0.21 b | 444.34 ± 10.1 c | 18.08 ± 0.95 c |
| Syn-DFG/Poly | 0.21 ± 0.02 a | 10.64 ± 0.15 b | 675.55 ± 19.8 a | 44.07 ± 1.4 a |
| Syn-DFG/GA | 0.19 ± 0.01 b | 10.19 ± 0.11 b | 602.34 ± 9.2 b | 29.49 ± 0.78 b |
| Syn-DFG/SS | 0.21 ± 0.01 a | 10.95 ± 0.05 a | 569.32 ± 12.11 b | 18.57 ± 1.1 c |
| Synbiotic Film | TS (MPa) | EB (%) | YM (MPa) |
|---|---|---|---|
| Control | 17.1 ± 2.2 ab | 31.3 ± 1.2 c | 516.81 ± 11.2 ab |
| Syn-DFG/Dex | 18.36 ± 0.18 a | 27.93 ± 3.2 c | 574.66 ± 12.2 a |
| Syn-DFG/Poly | 6.97 ± 0.5 c | 94.64 ± 6.4 a | 78.89 ± 8.7 c |
| Syn-DFG/GA | 18.2 ± 0.22 a | 49.53 ± 4.1 b | 407.2 ± 10.2 b |
| Syn-DFG/SS | 15.01 ± 0.9 b | 48.52 ± 2.5 b | 413.04 ± 19.41 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedinia, A.; Alimohammadi, F.; Teymori, F.; Razgardani, N.; Saeidi Asl, M.R.; Ariffin, F.; Mohammadi Nafchi, A.; Huda, N.; Roslan, J. Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics. Foods 2021, 10, 1761. https://doi.org/10.3390/foods10081761
Abedinia A, Alimohammadi F, Teymori F, Razgardani N, Saeidi Asl MR, Ariffin F, Mohammadi Nafchi A, Huda N, Roslan J. Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics. Foods. 2021; 10(8):1761. https://doi.org/10.3390/foods10081761
Chicago/Turabian StyleAbedinia, Ahmadreza, Faezeh Alimohammadi, Farangis Teymori, Najibeh Razgardani, Mohammad Reza Saeidi Asl, Fazilah Ariffin, Abdorreza Mohammadi Nafchi, Nurul Huda, and Jumardi Roslan. 2021. "Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics" Foods 10, no. 8: 1761. https://doi.org/10.3390/foods10081761
APA StyleAbedinia, A., Alimohammadi, F., Teymori, F., Razgardani, N., Saeidi Asl, M. R., Ariffin, F., Mohammadi Nafchi, A., Huda, N., & Roslan, J. (2021). Characterization and Cell Viability of Probiotic/Prebiotics Film Based on Duck Feet Gelatin: A Novel Poultry Gelatin as a Suitable Matrix for Probiotics. Foods, 10(8), 1761. https://doi.org/10.3390/foods10081761










