Extraction and Microencapsulation of Bioactive Compounds from Muicle (Justicia spicigera) and Their Use in the Formulation of Functional Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction of Bioactive Compounds from Muicle Leaves
2.4. Determination of the Best Conditions to Maximize Extraction of Bioactive Compounds from Muicle Leaves
2.5. Concentration of Bioactive Compounds Extract
2.6. Analytical Methods
2.6.1. Total Phenolic Compounds
2.6.2. Flavonoids Determination
2.6.3. Antioxidant Capacity
2.6.4. Condensed Tannins
2.6.5. Hydrolyzable Tannins
2.6.6. Monomeric Anthocyanins
2.7. Preparation of Wall Material-Concentrated Extract Solution for the Spray Drying Process
2.7.1. Soy Protein Isolate-Concentrated Extract Solution
2.7.2. Maltodextrin DE10-Concentrated Extract Solution
2.8. Microencapsulation of Muicle Leaves Extract Solution
2.9. Retention Efficiency
2.10. Moisture Content Determination
2.11. Characterization of Microcapsules Obtained under the Best Spray Drying Conditions
2.11.1. Particle Size Distribution
2.11.2. Microcapsules’ Surface Morphology
2.12. Preparation of Yogurt with Added Muicle Extract Microcapsules
Analysis of Yogurt with Added Maltodextrin or Isolate Soy Protein Microcapsules
2.13. Sensory Evaluation
2.14. Preparation of Jelly with Added Muicle Extract Microcapsules
Analysis of Jelly with Maltodextrin Muicle Extract Microcapsules
2.15. Bioactive Compounds and Antioxidant Capacity Stability in Manufactured Functional Foods during Storage
2.16. Statistics Analysis
3. Results and Discussion
3.1. Moisture Content and Remnant Phenolic Compounds in Dried Muicle Leaves
3.2. Best Extraction Conditions for Maximum Phenolic Compounds and Flavonoids Content
3.3. Bioactive Compounds in Dry Muicle Leaves Using Optimal Extracting Conditions
3.4. Determination of Bioactive Compounds Present in Optimized muicle Extract and Concentrated Extract
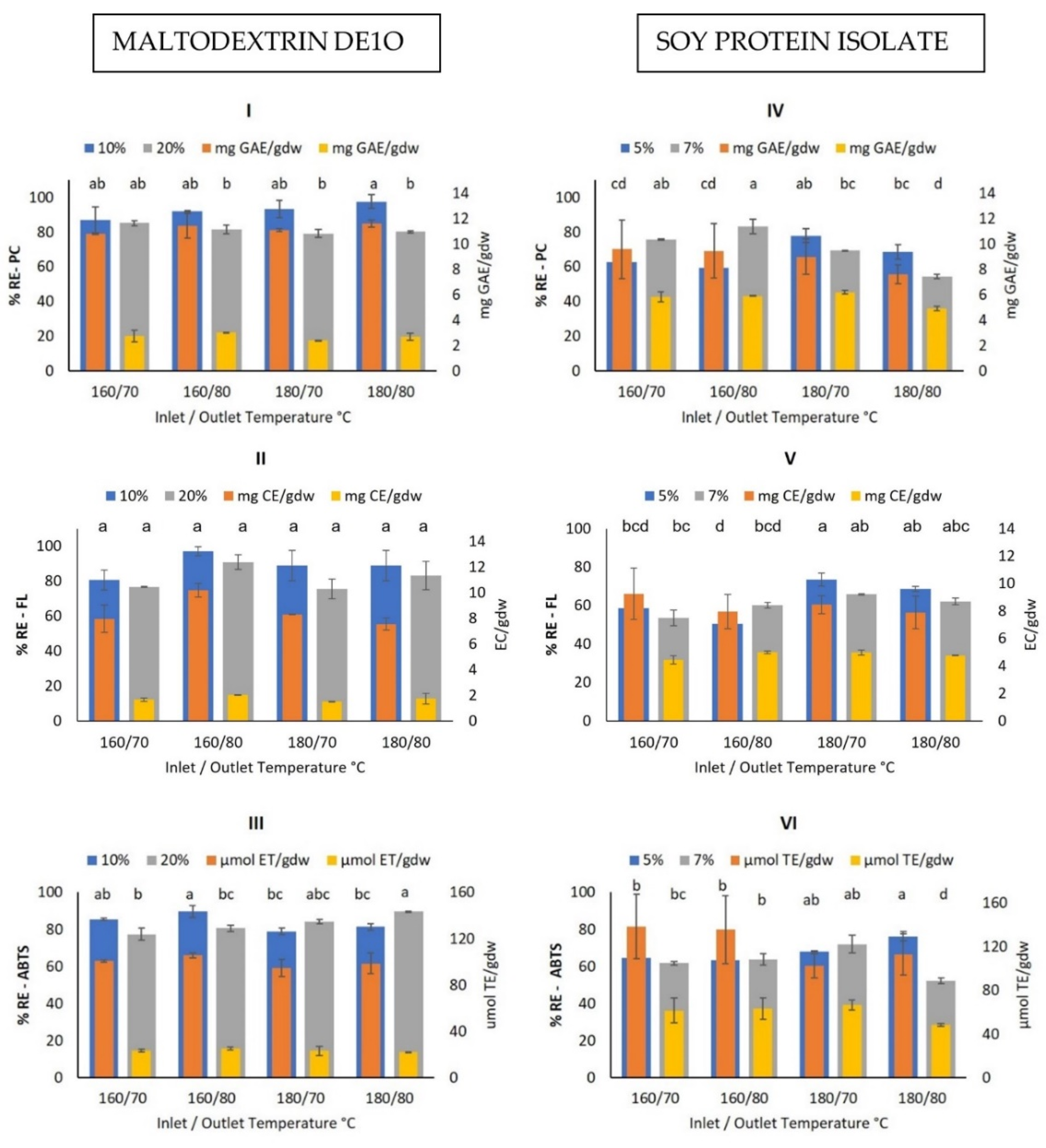
3.5. Muicle Microcapsules Characterization
Bioactive Compounds Retention Efficiency
3.6. Best Spray Drying Conditions for Maximum BC Retention
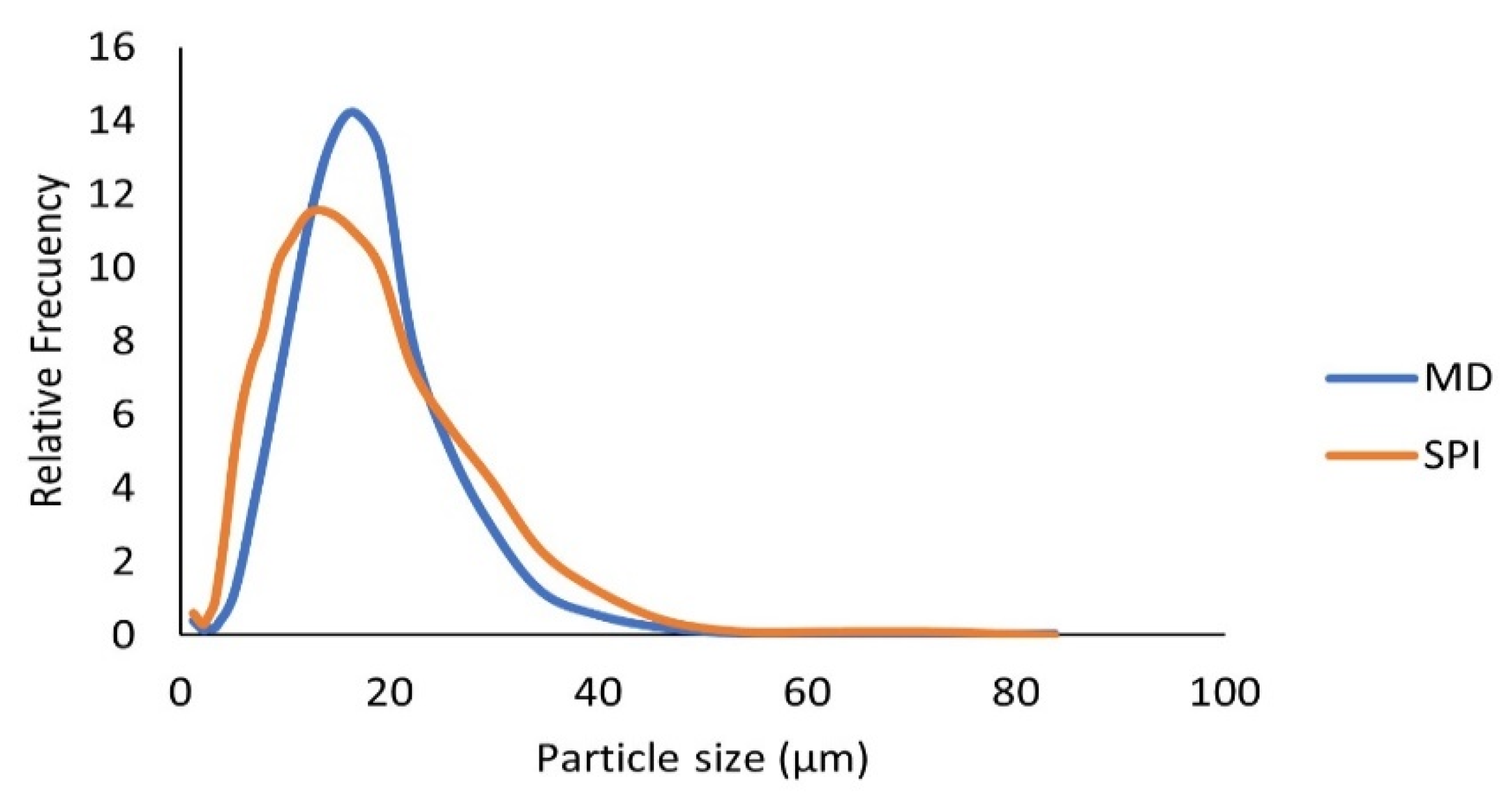
3.6.1. Particle Size Distribution for the Microcapsules Obtained in Optimal Conditions
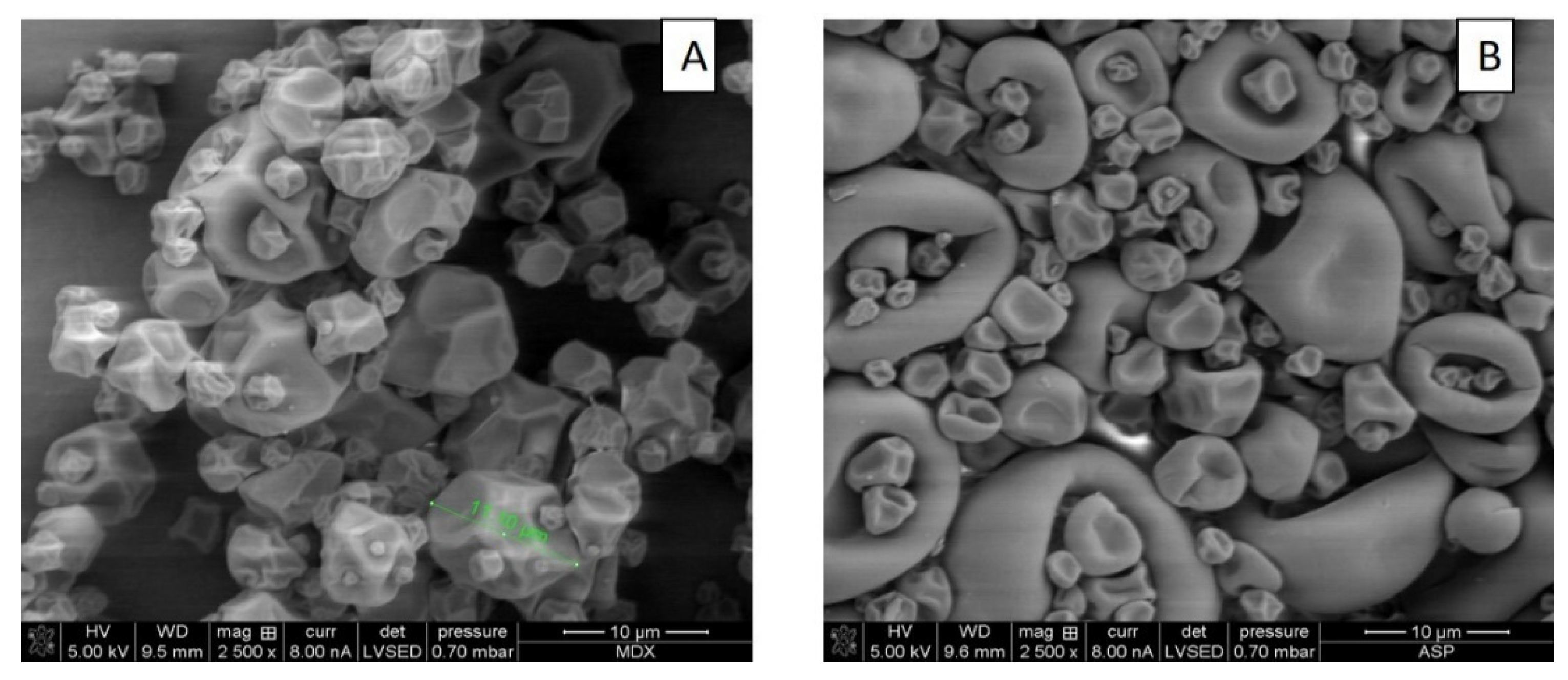
3.6.2. Surface Morphology of Microcapsules Obtained in Optimal Conditions
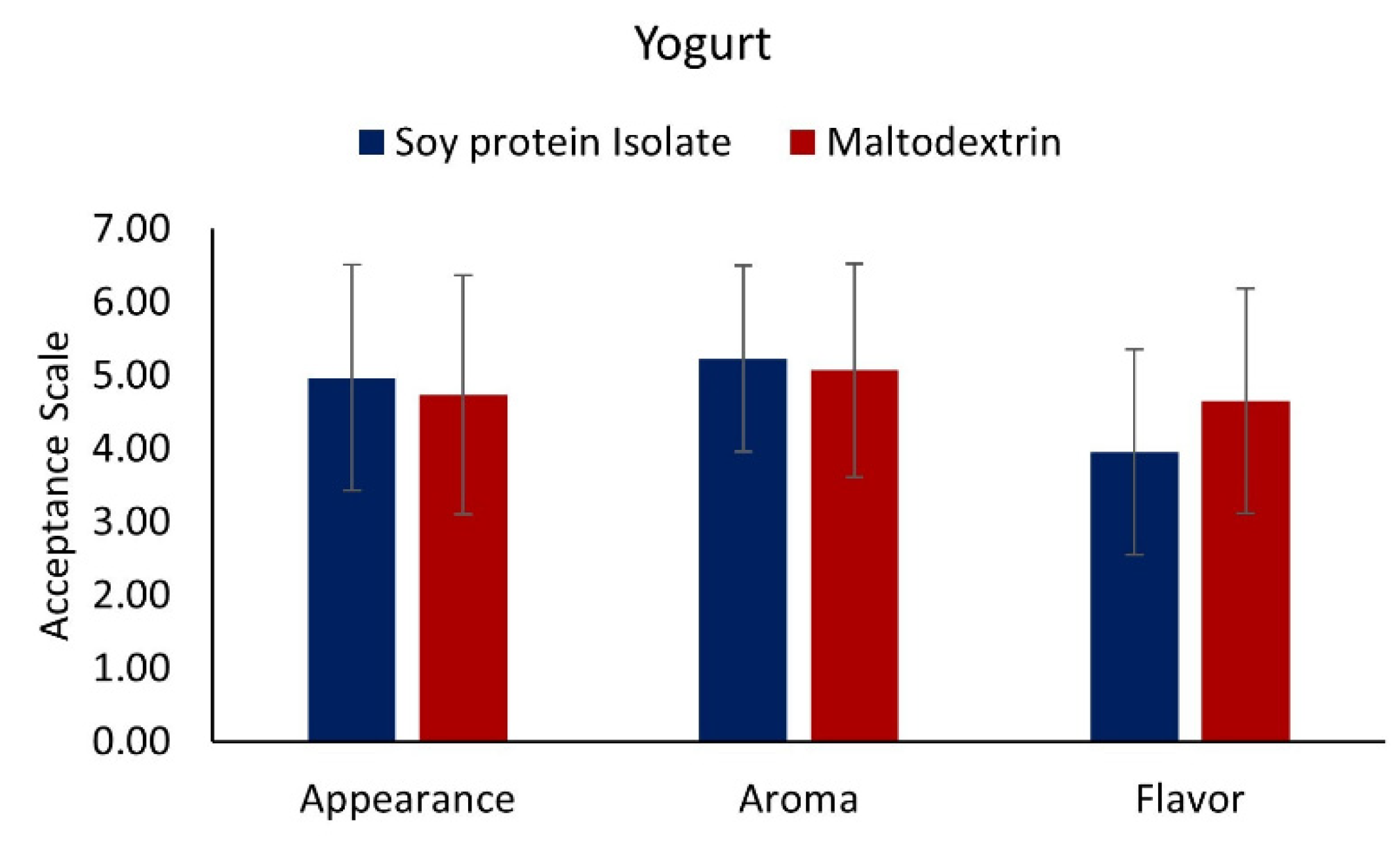
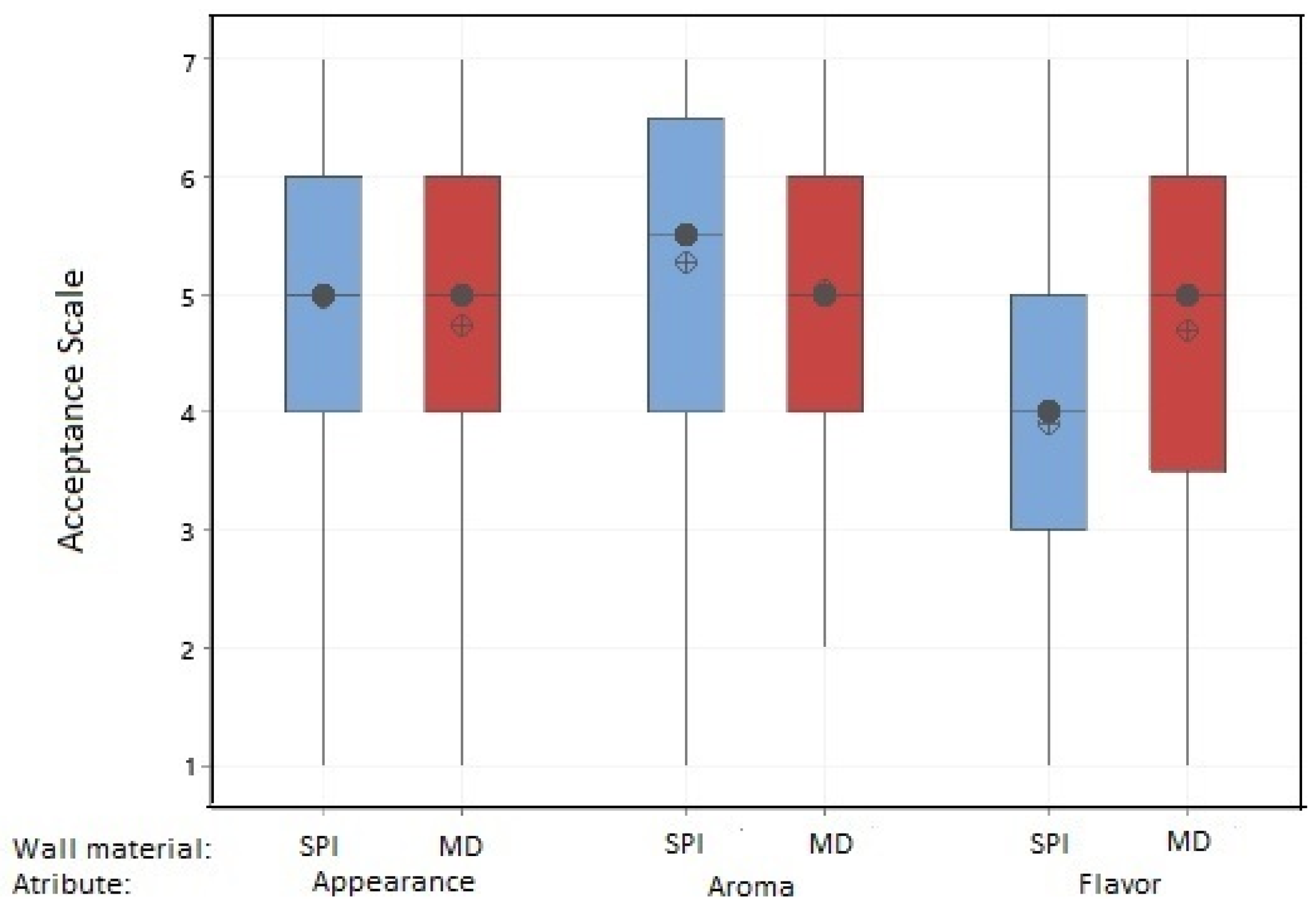
3.7. Sensory Assessment of Functional Food Products
3.7.1. Yogurt with Added Muicle Extract MD and SPI Microcapsules
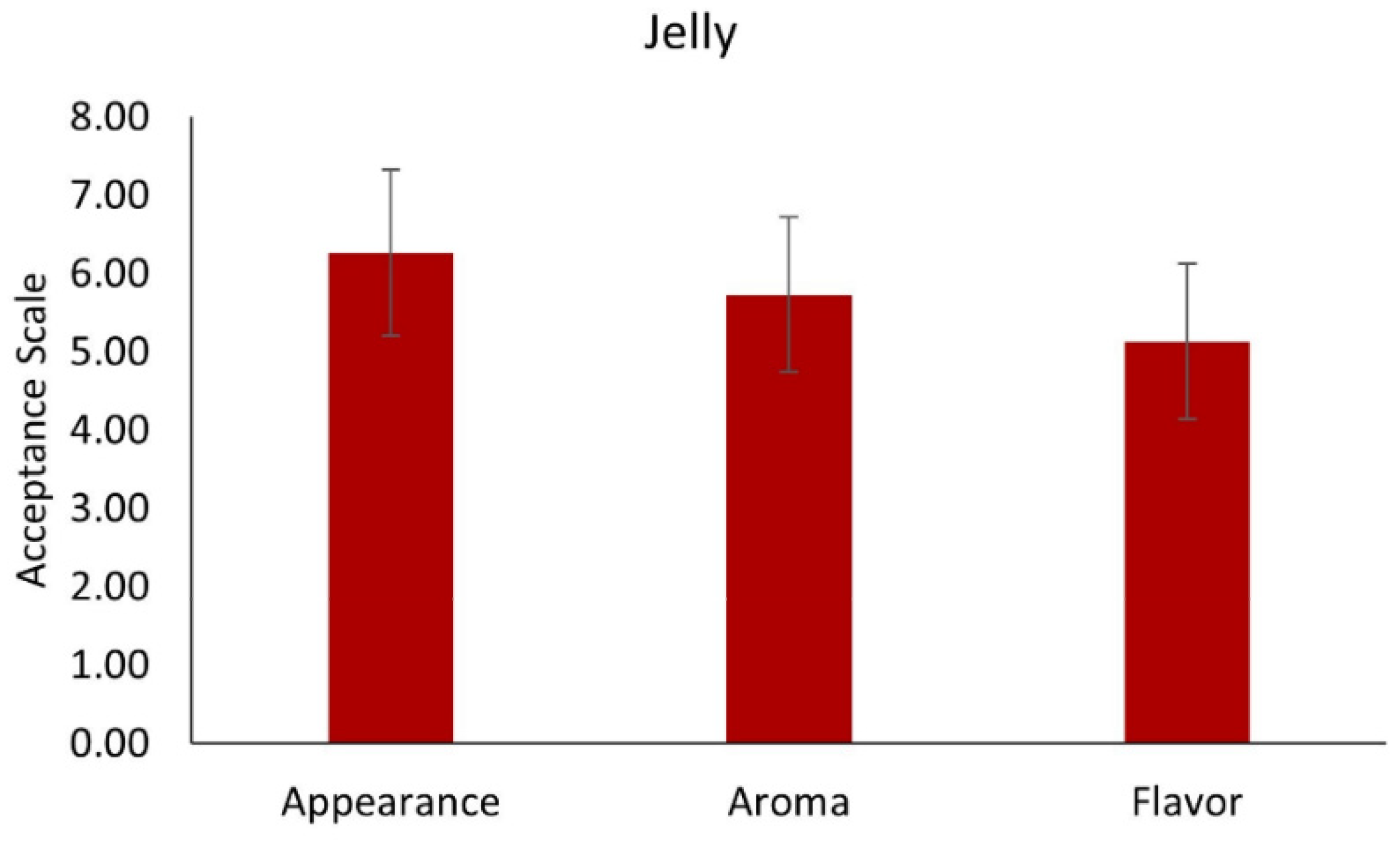
3.7.2. Jelly with MD Microcapsules
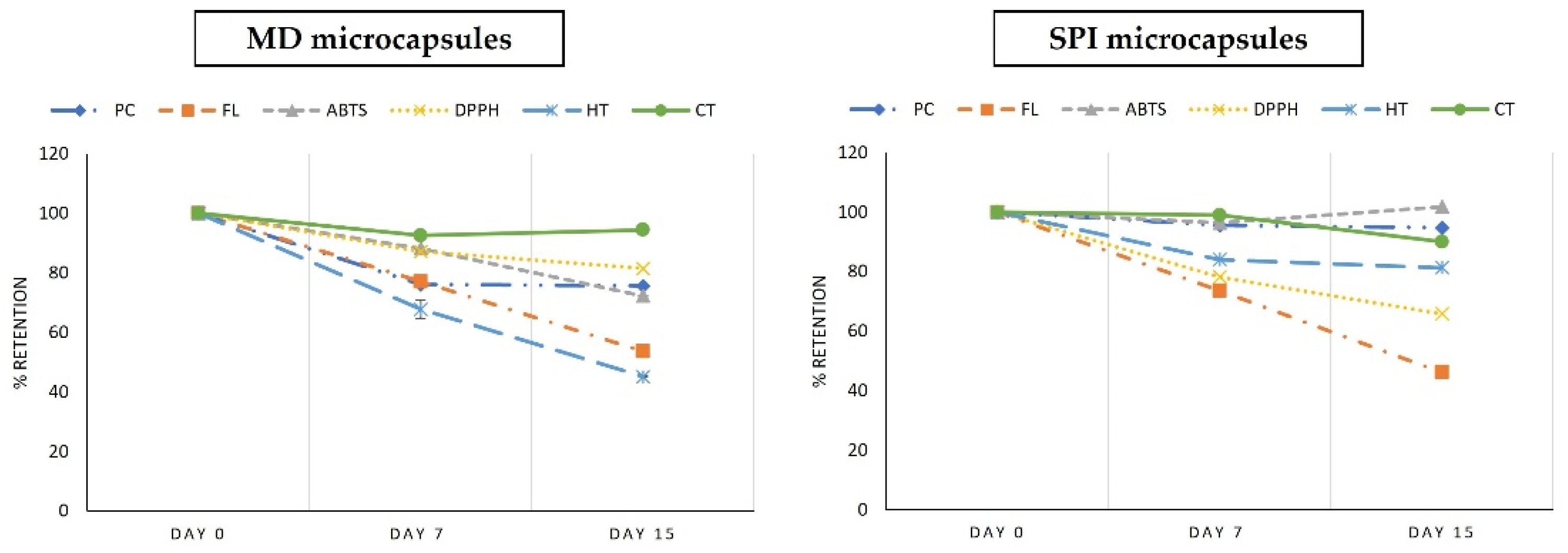
3.8. Preservation of Bioactive Compounds and Antioxidant Capacity during Storage in Manufactured Functional Foods Products
3.8.1. Preservation of Bioactive Compounds in Yogurt Added with Muicle Extract MD and SPI Microcapsules
3.8.2. Preservation of Bioactive Compounds in Jelly with MD Microcapsules
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of natural antioxidants for food application—The specific case of coffee antioxidants. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Miranda, J.S.; Costa, B.V.; de Oliveira, I.V.; de Lima, D.C.N.; Martins, E.M.F.; Júnior, B.R.D.C.L.; Benevenuto, W.C.A.D.N.; de Queiroz, I.C.; da Silva, R.R.; Martins, M. Probiotic jelly candies enriched with native Atlantic Forest fruits and Bacillus coagulans GBI-30 6086. LWT 2020, 126, 109275. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Díaz-Monroy, G.; Medina-Pérez, G.; Franco-Fernández, M.J.; Ludeña-Urquizo, F.E.; Vieyra-Alberto, R.; Campos-Montiel, R.G.; Cenobio-Galindo, A.D.J. Multiple Emulsions with Extracts of Cactus Pear Added in A Yogurt: Antioxidant Activity, In Vitro Simulated Digestion and Shelf Life. Foods 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, R.N.; Veggi, P.; Meireles, M.A. Supercritical fluid extraction with a modifier of antioxidant compounds from jabuticaba (Myrciaria cauliflora) by products: Economic viability. Procedia Food Sci. 2011, 1, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Sepúlveda-Jiménez, G.; Reyna-Aquino, C.; Chaires-Martínez, L.; Bermúdez-Torres, K.; Rodríguez-Monroy, M. Antioxidant activity and content of phenolic compounds and flavonoids from Justicia spicigera. J. Biol. Sci. 2009, 9, 629–632. [Google Scholar] [CrossRef] [Green Version]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J.Á. Physicochemical and antioxidant characterization of Justicia spicigera. Food Chem. 2017, 218, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Gao, C.; Xia, N.; Peng, H. Wuacanthus (Acanthaceae), a new Chinese endemic genus segregated from Justicia (Acanthaceae). Plant Diversity 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.; Calderas, F.; González-Laredo, R.F.; Guzmán, N.E.R.; Ochoa-Martínez, L.; Bernad-Bernad, M. Spray drying-microencapsulation of cinnamon infusions (Cinnamomum zeylanicum) with maltodextrin. LWT 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.; González-Laredo, R.; Guzmán, N.E.R.; Núñez-Ramírez, D.; Bernad-Bernad, M.; Manero, O. Microencapsulation by spray drying of laurel infusions (Litsea glaucescens) with maltodextrin. Ind. Crop. Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; Santana, A.S.; Monterrey-Quintero, E.S.; Trindade, M.A.; Netto, F.M. The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocoll. 2010, 24, 336–340. [Google Scholar] [CrossRef]
- Souza, A.L.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.; Tonon, R.V. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Tan, C.P.; Yazid, A.M.; Alhelli, A.M.; Hussin, A. Process conditions of spray drying microencap-sulation of Nigella sativa oil. Powder Technol. 2017, 315, 1–14. [Google Scholar] [CrossRef]
- Reinoso, Z.S.; Osorio, C.; Herrera, A. Effect of microencapsulation by spray drying on cocoa aroma compounds and physicochemical characterisation of microencapsulates. Powder Technol. 2017, 318, 110–119. [Google Scholar] [CrossRef]
- Shamaei, S.; Seiiedlou, S.S.; Aghbashlo, M.; Tsotsas, E.; Kharaghani, A. Microencapsulation of walnut oil by spray drying: Effects of wall material and drying conditions on physicochemical properties of microcapsules. Innov. Food Sci. Emerg. Technol. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Pavón-García, L.M.A.; César-Pérez, A.; Orozco-Villafuerte, J.; Pimentel-González, D.J.; Rodríguez-Huezo, M.A.; Vernon-Carter, E.J. Storage stability of the natural colourant from Justicia spicigera microencapsulated in protective colloids blends by spray-drying. Int. J. Food Sci. Technol. 2011, 46, 1428–1437. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepúlveda, E.; Chavez, J.; Saenz, C. Encapsulation of poluphenols and an-thocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1389. [Google Scholar] [CrossRef]
- Singlenton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosohomolybdic-phosphotugtic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Rufino, S.; Alves, R.; Brito, E.; Morais, S.; Sampaio, C.; Pérez-Jiménez, J.; Saura-Calixto, F. Metodologia Científica: De-terminação da atividade antioxidante total em frutas pela captura do radical livre ABTS. Comun. Téc. 2007, 128, 1–4. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Spranger, I.; Da-Silva, R.; Sun, B. Critical factora of vainillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar]
- Ḉam, M.; Gisil, Y. Pressurised water extraction of polyphenols from pomegranate peles. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Wrolstad, R. Characterization and measurement of anthocyanin pigments in plums. J. Agric. Food Chem. 2001, 57, 339–403. [Google Scholar]
- Acevedo, P.I.; García, O.; Contreras, J.; Acevedo, I. Elaboración y evaluación de las características sensoriales de un yogurt de leche de caprina con jalea semifluida de piña. Rev. UDO Agríc. 2009, 9, 442–448. [Google Scholar]
- Alcausa, S. 4 Formas de Preparar Gelatina. 2018. Available online: https://mejorconsalud.com/4-formas-deliciosas-de-preparar-la-gelatina/ (accessed on 10 January 2020).
- Nesterenko, A.; Alric, I.; Silverstre, F.; Durrieu, V. Influence of soy protein’s structural modifications on their microencapsulation properties: α-Tocopherol microparticle preparation. Food Res. Int. 2012, 48, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhou, W. Characterization of spray-dried soy sauce powders using maltodextrins as carrier. J. Food Eng. 2012, 109, 399–405. [Google Scholar] [CrossRef]
- Moreno, T.; Cocero, M.; Rodríguez-Rojo, S. Storage stability and simulated gastrointestinal release of spray dried grape marc phenolics. Food Bioprod. Process. 2018, 112, 96–107. [Google Scholar] [CrossRef]
- Molina-Ortiz, S.E.; Mauri, A.; Monterrey-Quintero, E.S.; Trindade, M.A.; Santana, A.S.; Favaro-Trindade, C.S. Production and properties of casein hydrolysate microencapsulated by spray drying with soybean protein isolate. Food Sci. Technol. 2009, 42, 919–923. [Google Scholar] [CrossRef]
- Tang, C.-H.; Li, X.-R. Microencapsulation properties of soy protein isolate and storage stability of the correspondingly spray-dried emulsions. Food Res. Int. 2013, 52, 419–428. [Google Scholar]

| Determination | Content |
|---|---|
| Phenolic compounds (PC) | 27.05 ± 0.020 |
| Flavonoids (FL) | 13.88 ± 0.20 |
| Antioxidant Capacity ABTS (AC) | 2.7 ± 0.35 |
| Antioxidant Capacity DPPH (AC) | 0.55 ± 0.39 |
| Hydrolyzable tannins (HT) | 322.31 ± 1.99 |
| Condensed tannins (CT) | 12.85 ± 0.33 |
| Monomeric anthocyanins (MA) | Not detectable |
| Determination | Optimized Muicle Extract | Concentrated Muicle Extract |
|---|---|---|
| Phenolic compounds (PC) | 0.33 ± 0.00 | 2.00 ± 0.03 |
| Flavonoids (FL) | 0.27 ± 0.00 | 1.76 ± 0.00 |
| Antioxidant Capacity ABTS(AC) | 4.10 ± 0.14 | 18.63 ± 0.16 |
| Antioxidant Capacity DPPH (AC) | 1.19 ± 0.09 | 2.11 ± 0.09 |
| Hydrolyzable tannins (HT) | 8.92 ± 0.04 | 41.67 ± 0.38 |
| Condensed tannins (HC) | 0.23 ± 0.04 | 1.24 ± 0.01 |
| Response Variables BC | Predicted Values Experimental Values % Retention Efficiency | |
|---|---|---|
| PC | 92.02 | 86.82 ± 0.87 |
| FL | 97.07 | 90.76 ± 5.72 |
| AA (ABTS) | 89.83 | 85.22 ± 3.63 |
| AA (DPPH) | 82.67 | 82.18 ± 3.62 |
| HT | 90.23 | 91.31 ± 1.99 |
| CT | 92.28 | 95.75 ± 3.37 |
| Response Variables BC % Retention Efficiency | Predicted Values Experimental Values % Retention Efficiency | |
|---|---|---|
| PC | 77.48 | 82.95 ± 1.57 |
| FL | 73.38 | 74.08 ± 1.99 |
| AA (ABTS) | 68.42 | 70.11 ± 2.10 |
| AA (DPPH) | 69.95 | 70.98 ± 0.91 |
| HT | 72.44 | 72.88 ± 1.49 |
| CT | 60 | 65 ± 1.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Alatorre, N.C.; Gallardo-Velázquez, T.; Boyano-Orozco, L.C.; Téllez-Medina, D.I.; Meza-Márquez, O.G.; Osorio-Revilla, G. Extraction and Microencapsulation of Bioactive Compounds from Muicle (Justicia spicigera) and Their Use in the Formulation of Functional Foods. Foods 2021, 10, 1747. https://doi.org/10.3390/foods10081747
Castro-Alatorre NC, Gallardo-Velázquez T, Boyano-Orozco LC, Téllez-Medina DI, Meza-Márquez OG, Osorio-Revilla G. Extraction and Microencapsulation of Bioactive Compounds from Muicle (Justicia spicigera) and Their Use in the Formulation of Functional Foods. Foods. 2021; 10(8):1747. https://doi.org/10.3390/foods10081747
Chicago/Turabian StyleCastro-Alatorre, Norma Cristina, Tzayhrí Gallardo-Velázquez, Luis Carlos Boyano-Orozco, Darío Iker Téllez-Medina, Ofelia Gabriela Meza-Márquez, and Guillermo Osorio-Revilla. 2021. "Extraction and Microencapsulation of Bioactive Compounds from Muicle (Justicia spicigera) and Their Use in the Formulation of Functional Foods" Foods 10, no. 8: 1747. https://doi.org/10.3390/foods10081747
APA StyleCastro-Alatorre, N. C., Gallardo-Velázquez, T., Boyano-Orozco, L. C., Téllez-Medina, D. I., Meza-Márquez, O. G., & Osorio-Revilla, G. (2021). Extraction and Microencapsulation of Bioactive Compounds from Muicle (Justicia spicigera) and Their Use in the Formulation of Functional Foods. Foods, 10(8), 1747. https://doi.org/10.3390/foods10081747






