Native Yeasts and Lactic Acid Bacteria Isolated from Spontaneous Fermentation of Seven Grape Cultivars from the Maule Region (Chile)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Berries Samples and Grape Juice Preparation
2.2. Spontaneous Fermentation
2.2.1. Chemical Analysis
2.2.2. Culture-Dependent Analysis
Microbiological Characterization
Isolation and Identification of Microorganisms
2.2.3. Culture-Independent Analysis
Preparation and Sequencing of the Grape Juices’ Fermentation rDNA Libraries
Processing of the 16S rDNA Amplicon Sequences Data Corresponding to Spontaneous Fermentation Samples
ITS Amplicon Sequences Data Processing, Corresponding to Grape Juice Fermentation Samples
2.3. Alcohol Production by the Selected Non-Saccharomyces Isolates
2.4. Non-Saccharomyces Isolates Sequential Fermentations with S. cerevisiae in Carmenere Grape Juice
2.5. Statistical Analysis
3. Results and Discussion
3.1. Spontaneous Fermentation
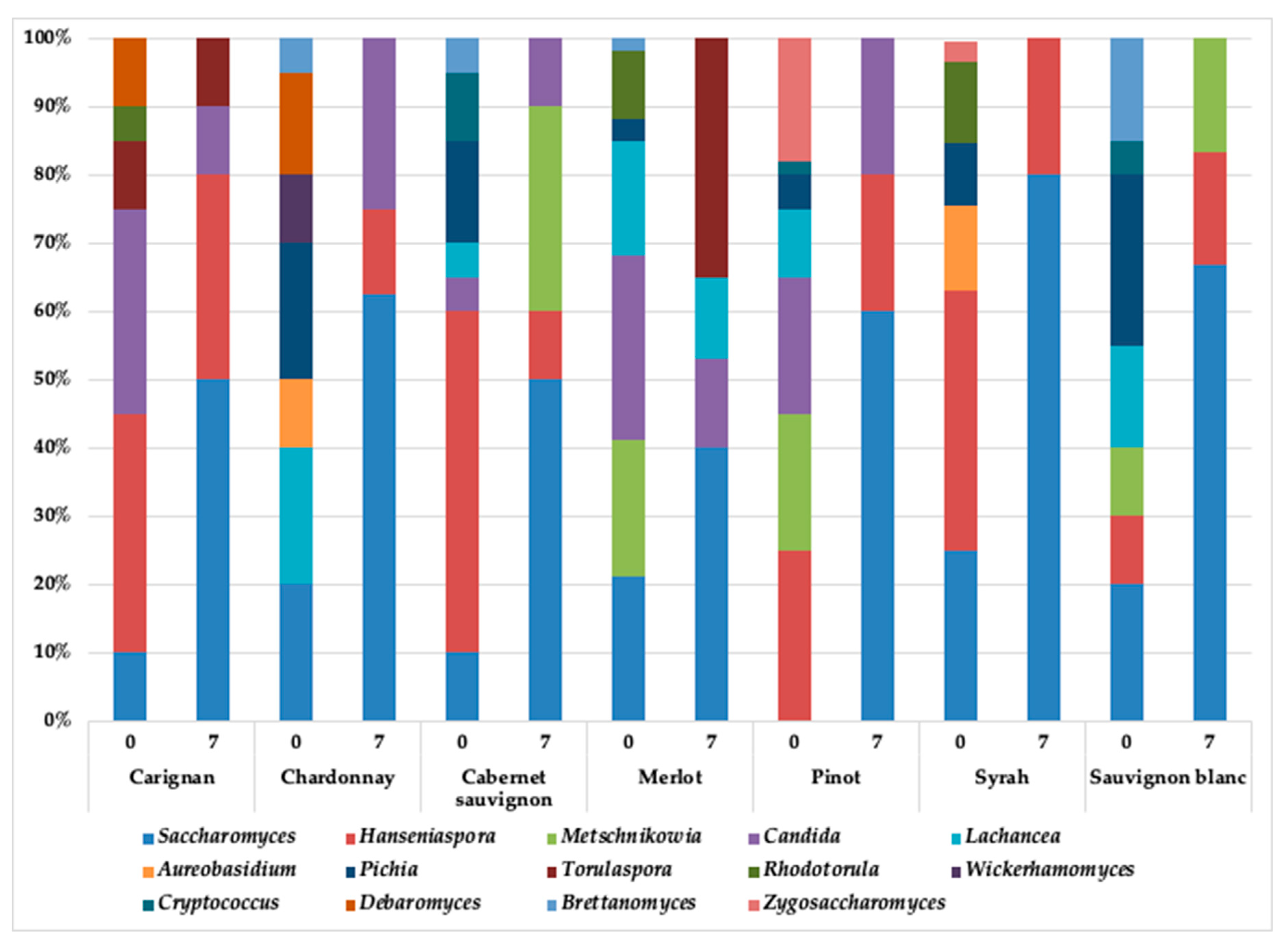
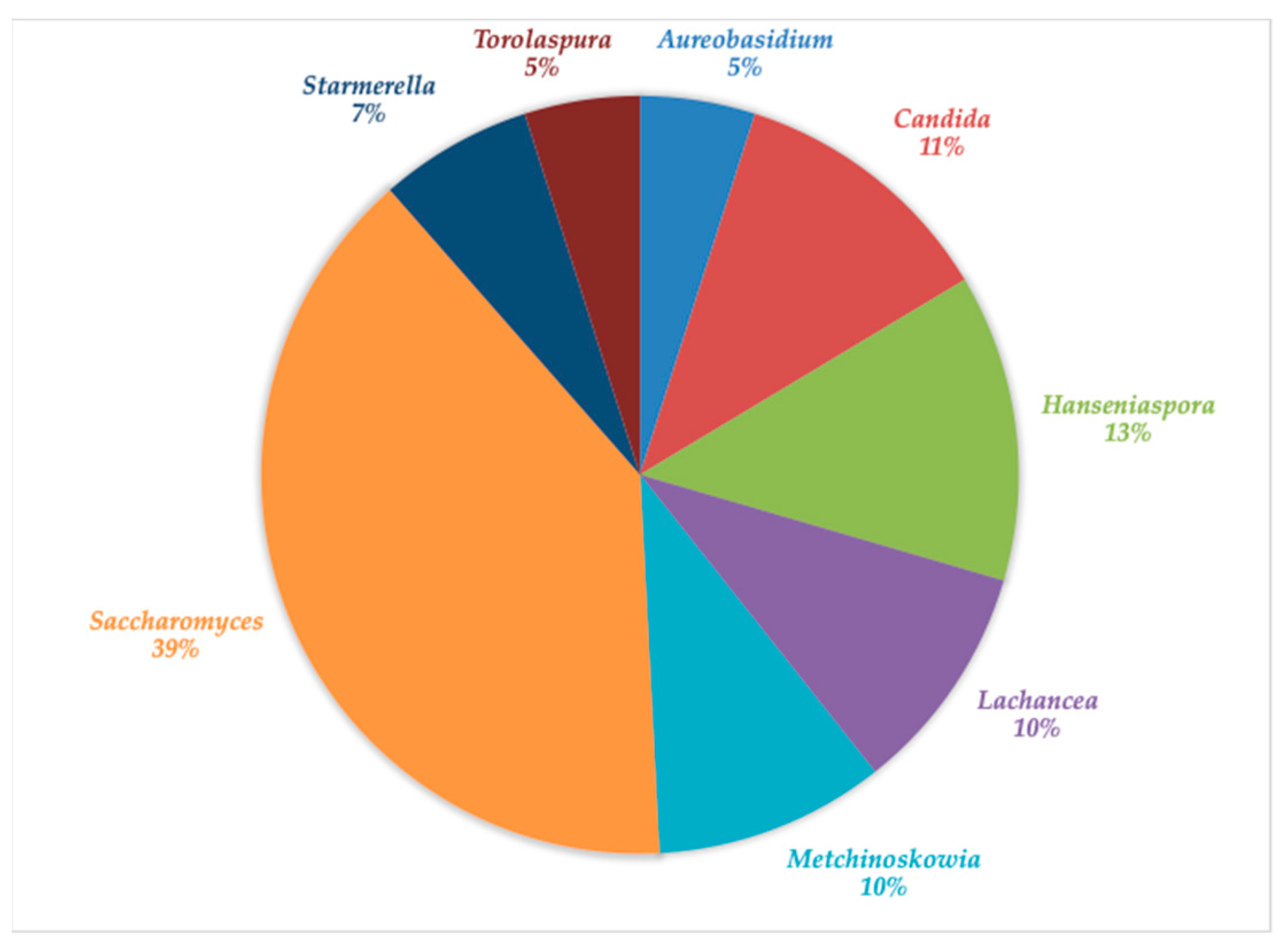
3.2. Yeasts Diversity during the Spontaneous Fermentation
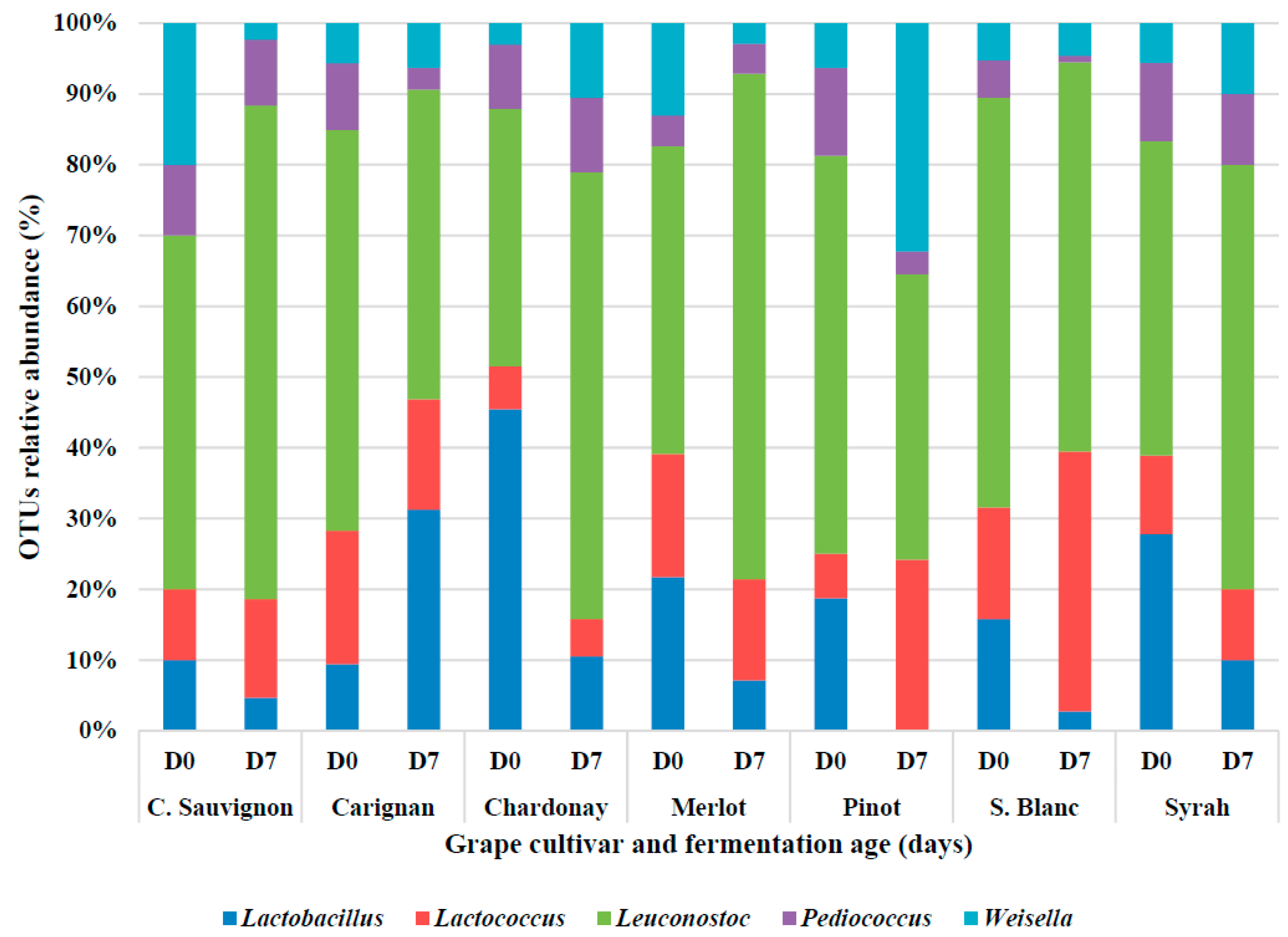
3.3. Lactic Acid Bacteria Diversity during the Alcoholic Fermentation
3.4. Fermentative Profile of Selected Yeast Isolates
3.5. Sequential Fermentations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Han-Seniaspora Vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Liu, Y.; Rousseaux, S.; Tourdot-Maréchal, R.; Sadoudi, M.; Gougeon, R.; Schmitt-Kopplin, P.; Alexandre, H. Wine Microbiome: A Dynamic World of Microbial Interactions. Crit. Rev. Food Sci. Nutr. 2015, 57, 856–873. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Ohta, M.; Richardson, P.M.; Mills, D.A. Monitoring Seasonal Changes in Winery-Resident Microbiota. PLoS ONE 2013, 8, e66437. [Google Scholar]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.A.; van der Lelie, D.; Zarraonaindia, I. Microbial Terroir for Wine Grapes. Proc. Natl. Acad. Sci. USA 2014, 111, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of Indigenous Yeast Populations during Spontaneous Fermentation of Wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef]
- Garofalo, C.; Russo, P.; Beneduce, L.; Massa, S.; Spano, G.; Capozzi, V. Non-Saccharomyces Biodiversity in Wine and the ‘microbial Terroir’: A Survey on Nero di Troia Wine from the Apulian Region, Italy. Ann. Microbiol. 2015, 66, 143–150. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional Microbial Signatures Positively Correlate with Differential Wine Phenotypes: Evidence for a Microbial Aspect to Terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef] [Green Version]
- Vilela, A. Use of Nonconventional Yeasts for Modulating Wine Acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef] [Green Version]
- Cordero-Bueso, G.; Esteve-Zarzoso, B.; Cabellos, J.M.; Gil-Díaz, M.; Arroyo, T. Biotechnological Potential of Non-Saccharomyces Yeasts Isolated During Spontaneous Fermentations of Malvar (Vitis Vinifera cv. L.). Eur. Food Res. Technol. 2012, 236, 193–207. [Google Scholar] [CrossRef]
- Nikolaou, E.; Soufleros, E.H.; Bouloumpasi, E.; Tzanetakis, N. Selection of Indigenous Saccharomyces Cerevisiae Strains According to Their Oenological Characteristics and Vinification Results. Food Microbiol. 2006, 23, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-T.; Lu, L.; Duan, C.-Q.; Yan, G.-L. The Contribution of Indigenous Non-Saccharomyces Wine Yeast to Improved Aromatic Quality of Cabernet Sauvignon Wines by Spontaneous Fermentation. LWT Food Sci. Technol. 2016, 71, 356–363. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosac-Charomyces Pombe and Torulaspora Delbrueckii Strains in Mixed and Sequential Fermentations to Improve Red Wine Sensory Quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological Consequences of Sequential Inoculation with Non-Saccharomyces Yeasts (Torulaspora Del-Brueckii or Metschnikowia Pulcherrima) and Saccharomyces Cerevisiae in Base Wine for Sparkling Wine Production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Berbegal, C.; Khomenko, I.; Russo, P.; Spano, G.; Fragasso, M.; Biasioli, F.; Capozzi, V. PTR-ToF-MS for the Online Monitoring of Alcoholic Fermentation in Wine: Assessment of VOCs Variability Associated with Different Combinations of Saccharomyces/Non-Saccharomyces as a Case-Study. Fermentation 2020, 6, 55. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Steensels, J.; Verstrepen, K.J. Taming Wild Yeast: Potential of Conventional and Nonconventional Yeasts in Industrial Fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; García-Fernández, D.; González, B.; Izidoro, I.; Esteve-Zarzoso, B.; Beltran, G.; Mas, A. Yeast Biodiversity from DOQ Priorat Uninoculated Fermentations. Front. Microbiol. 2016, 7, 930. [Google Scholar] [CrossRef] [Green Version]
- Portillo M del, C.; Mas, A. Analysis of Microbial Diversity and Dynamics During Wine Fermentation of Grenache Grape Variety by High-Throughput Barcoding Sequencing. LWT Food Sci. Technol. 2016, 72, 317–321. [Google Scholar] [CrossRef]
- Díaz, C.; Molina, A.M.; Nähring, J.; Fischer, R. Characterization and Dynamic Behavior of Wild Yeast during Spontaneous Wine Fermentation in Steel Tanks and Amphorae. BioMed Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular Characterization and Oenological Properties of Wine Yeasts Isolated During Spontaneous Fermentation of Six Varieties of Grape Must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Rementeria, A.; Rodriguez, J.A.; Cadaval, A.; Amenabar, R.; Muguruza, J.R.; Hernando, F.L.; Sevilla, M.J. Yeast Associated with Spontaneous Fermentations of White Wines from the “Txakoli de Bizkaia” Region (Basque Country, North Spain). Int. J. Food Microbiol. 2003, 86, 201–207. [Google Scholar] [CrossRef]
- Li, E.; Liu, A.; Xue, B.; Liu, Y. Yeast Species Associated with Spontaneous Wine Fermentation of Cabernet Sauvignon from Ningxia, China. World J. Microbiol. Bioetchnol. 2011, 27, 2475–2482. [Google Scholar] [CrossRef]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the Wine Metabolome by Combining Untargeted SPME–GCxGC-TOF-MS and Sensory Analysis to Profile Sauvignon Blanc Cofermented with Seven Different Yeasts. Metabolomics 2016, 12, 1–25. [Google Scholar] [CrossRef]
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation 2020, 6, 77. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y. Investigating of Yeast Species in Wine Fermentation Using Terminal Restriction Fragment Length Polymorphism Method. Food Microbiol. 2014, 38, 201–207. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. Interaction between Hanseniaspora Uvarum and Saccharomyces Cerevisiae during Alcoholic Fermentation. Int. J. Food Microbiol. 2015, 206, 67–74. [Google Scholar] [CrossRef]
- Grangeteau, C.; Gerhards, D.; Rousseaux, S.; von Wallbrunn, C.; Alexandre, H.; Guilloux-Benatier, M. Diversity of Yeast Strains of the Genus Hanseniaspora in the Winery Environment: What is Their Involvement in Grape Must Fermentation? Food Microbiol. 2015, 50, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Esteve-Zarzoso, B.; Mas, A. Monitoring of Saccharomyces Cerevisiae, Hanseniaspora Uvarum, and Starmerella Bacillaris (Synonym Candida Zemplinina) Populations during Alcoholic Fermentation by Fluorescence In Situ Hybridization. Int. J. Food Microbiol. 2014, 191, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora Delbrueckii in the Brewing Process: A New Approach to Enhance Bioflavour and to Reduce Ethanol Content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the Aroma of White Wines by Controlled Torulaspora Delbrueckii Cultures in Association with Saccharomyces Cerevisiae. World J. Microbiol. Biotechnol. 2014, 31, 277–293. [Google Scholar] [CrossRef]
- Contreras, A.; Curtin, C.; Varela, C. Yeast Population Dynamics Reveal a Potential ‘Collaboration’ between Metschnikowia Pulcherrima and Saccharomyces Uvarum for the Production of Reduced Alcohol Wines during Shiraz Fermentation. Appl. Microbiol. Biotechnol. 2014, 99, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Hatziloukas, E.; Drainas, C.; Perisynakis, A. The Effect of Debina Grapevine Indigenous Yeast Strains of Metschnikowia and Saccharomyces on Wine Flavour. J. Ind. Microbiol. Biotechnol. 2010, 37, 85–93. [Google Scholar] [CrossRef]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The Impact of Oxygen on the Final Alcohol Content of Wine Fermented by a Mixed Starter Culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef] [Green Version]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of Non-Saccharomyces Wine Yeasts as Novel Sources of Mannoproteins in Wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea Thermotolerans and Saccharomyces Cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the Overall Quality of Wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine Use of Selected Schizosaccharomyces Pombe and Lachancea Thermotolerans Yeast Strains as an Alternative to the Traditional Malolactic Fermentation in Red Wine Production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [Green Version]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological Traits of Lachancea Thermotolerans Show Signs of Domestication and Allopatric Differentiation. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces Cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The Interaction between Saccharomyces Cerevisiae and Non-Saccharomyces Yeast during Alcoholic Fermentation Is Species and Strain Specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [Green Version]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential Fermentation with Selected Immobilized Non-Saccharomyces Yeast for Reduction of Ethanol Content in Wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef] [Green Version]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile Flavour Profile of Reduced Alcohol Wines Fermented with the Non-Conventional Yeast Species Metschnikowia Pulcherrima and Saccharomyces Uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V. A Meta-Genomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int. J. Mol. Sci. 2019, 20, 3980. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, M.J.; Barrajón, N.; Baffi, M.A.; Arévalo-Villena, M.; Briones, A. Spontaneous Must Fermentation: Identification and Biotechnological Properties of Wine Yeasts. LWT Food Sci. Technol. 2013, 50, 371–377. [Google Scholar] [CrossRef]
- Brysch-Herzberg, M.; Seidel, M. Yeast Diversity on Grapes in Two German Wine Growing Regions. Int. J. Food Microbiol. 2015, 214, 137–144. [Google Scholar] [CrossRef]
- Jara, C.; Laurie, V.F.; Mas, A.; Romero, J. Microbial Terroir in Chilean Valleys: Diversity of Non-Conventional Yeast. Front. Microbiol. 2016, 7, 663. [Google Scholar] [CrossRef]
- McFeeters, R.F.; Barish, A.O. Sulfite Analysis of Fruits and Vegetables by High-Performance Liquid Chromatography (HPLC) with Ultraviolet Spectrophotometric Detection. J. Agric. Food Chem. 2003, 51, 1513–1517. [Google Scholar] [CrossRef]
- Iland, P.; Grodin, P.; Grinbergs, M.; Schmidtke, L.; Soden, A. Microbiologycal Analysis of Grapes and Wine: Techniques and Concepts; Wine Promotions Pty Ltd.: Sydney, Australia, 2001. [Google Scholar]
- Franco, W.; Pérez-Díaz, I.M.; Johanningsmeier, S.D.; McFeeters, R.F. Characteristics of Spoilage-Associated Secondary Cucumber Fermentation. Appl. Environ. Microbiol. 2012, 78, 1273–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrangou, R.; Yoon, S.-S.; Breidt, F.; Fleming, H.P.; Klaenhammer, T.R. Identification and Characterization of Leuconostoc Fallax Strains Isolated from an Industrial Sauerkraut Fermentation Identification and Characterization of Leuconostoc Fallax Strains Isolated from an Industrial Sauerkraut Fermentation. Appl. Environ. Microbiol. 2002, 68, 2877–2884. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.H.; Blitchington, R.B.; Greene, R.C. Amplification of Bacterial 16S Ribosomal DNA with Polymerase Chain Reaction. J. Clin. Microbiol. 1990, 28, 1942–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 35, 1216. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Mizrachi, I.K.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2012, 41, D36–D42. [Google Scholar] [CrossRef] [Green Version]
- Franco, W.; Pérez-Díaz, I.M.; Connelly, L.; Diaz, J. Isolation of Exopolysaccharide-Producing Yeast and Lactic Acid Bacteria from Quinoa (Chenopodium Quinoa) Sourdough Fermentation. Foods 2020, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Breidt, F. Enumeration of Viable Listeria monocytogenes Cells by Real-Time PCR with Propidium Monoazide and Ethidium Monoazide in the Presence of Dead Cells. Appl. Environ. Microbiol. 2007, 73, 8028–8031. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, L.; Osman, O.A.; Bertilsson, S.; Eiler, A. Microbial Community Composition and Diversity via 16S rRNA Gene Amplicons: Evaluating the Illumina Platform. PLoS ONE 2015, 10, e0116955. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Aronesty, E. Eautils: Command-Line Tools for Processing Biological Sequencing Data; Expression Analysis: Durham, NC, USA, 2011. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. Vsearch: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucl. Acids 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-Species Living Tree Project (LTP)” Taxonomic Frameworks. Nucl. Acids 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A Fexible Tool for Aligning Sequences to a Template Alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.; Curtin, C.; Varela, C. The Application of Non-Saccharomyces Yeast in Fermentations with Limited Aeration as a Strategy for the Production of Wine with Reduced Alcohol Content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wine of Chile. Carmenère. Available online: https://www.winesofchile.org/chile-vitivinicola/diversidad-vitivinicola/carmenere/ (accessed on 2 January 2021).
- Comitini, F.; Ciani, M. Influence of Fungicide Treatments on the Occurrence of Yeast Flora Associated with Wine Grapes. Ann. Microbiol. 2008, 58, 489–493. [Google Scholar] [CrossRef]
- Martins, G.; Vallance, J.; Mercier, A.; Albertin, W.; Stamatopoulos, P.; Rey, P.; Lonvaud, A.; Masneuf-Pomarede, I. Influence of the Farming System on the Epiphytic Yeasts and Yeast-Like Fungi Colonizing Grape Berries during the Ripening Process. Int. J. Food Microbiol. 2014, 177, 21–28. [Google Scholar] [CrossRef]
- Renouf, V.; Claisse, O.; Lonvaud-Funel, A. Understanding the Microbial Ecosystem on the Grape Berry Surface through Numeration and Identification of Yeast and Bacteria. Aust. J. Grape Wine Res. 2005, 11, 316–327. [Google Scholar] [CrossRef]
- Ministerio del Medio Ambiente. Región del MAULE Normales Climatológicas. Available online: http://basedigitaldelclima.mma.gob.cl/pdf_estudio_dos/9Maule.pdf (accessed on 17 February 2021).
- Alexandre, H. Wine Yeast Terroir: Separating the Wheat from the Chaff—for an Open Debate. Microorganisms 2020, 8, 787. [Google Scholar] [CrossRef]
- Gamboa, G.G.; Moreno-Simunovic, Y. Location Effects on Ripening and Grape Phenolic Composition of Eight ‘Carignan’ Vineyards from Maule Valley (Chile). Chil. J. Agric. Res. 2018, 78, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-S.; Cheng, C.; Li, Z.; Chen, J.; Yan, B.; Han, B.-Z.; Reeves, M. Yeast Species Associated with Wine Grapes in China. Int. J. Food Microbiol. 2010, 138, 85–90. [Google Scholar] [CrossRef]
- Catrileo, D.; Acuña-Fontecilla, A.; Godoy, L. Adaptive Laboratory Evolution of Native Torulaspora Delbrueckii YCPUC10 with Enhanced Ethanol Resistance and Evaluation in Co-Inoculated Fermentation. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Maccarelli, F. Oenological Properties of Non-Saccharomyces Yeasts Associated with Wine-Making. World J. Microbiol. Biotechnol. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Binati, R.L.; Junior, W.J.L.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of Non-Saccharomyces Yeasts to Wine Volatile and Sensory Diversity: A Study on Lachancea Thermotolerans, Metschnikowia spp. and Starmerella Bacillaris Strains Isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Jeffery, D.W.; Grbin, P.R.; Jiranek, V. Lower-Alcohol Wines Produced by Metschnikowia Pulcherrima and Saccharomyces Cerevisiae Co-Fermentations: The Effect of Sequential Inoculation Timing. Int. J. Food Microbiol. 2020, 329, 108651. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora Delbrueckii in Varietal Thiol (3-SH and 4-MSP) Release in Wine Sequential Fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic Analysis of Physiological Properties of Torulaspora Delbrueckii in Wine Fermentations and Its Incidence on Wine Quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Onetto, C.A.; Borneman, A.R.; Schmidt, S.A. Investigating the Effects of Aureobasidium Pullulans on Grape Juice Composition and Fermentation. Food Microbiol. 2020, 90, 103451. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Unravelling the Diversity of Grapevine Microbiome. PLoS ONE 2014, 9, e85622. [Google Scholar] [CrossRef] [Green Version]
- Vaudano, E.; Quinterno, G.; Costantini, A.; Pulcini, L.; Pessione, E.; Garcia-Moruno, E. Yeast Distribution in Grignolino Grapes Growing in a New Vineyard in Piedmont and the Technological Characterization of Indigenous Saccharomyces spp. Strains. Int. J. Food Microbiol. 2019, 289, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Cravero, M.C. Organic and Biodynamic Wines Quality and Characteristics: A review. Food Chem. 2019, 295, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Wine. In Food Microbiology Fundamentals and Fronteirs; Doyle, M.P., Beuchat, L.R., Monteville, J.T., Eds.; ASM Press: Washington, DC, USA, 2001; pp. 747–772. [Google Scholar]
- Kačániová, M.; Kunová, S.; Sabo, J.; Ivanišová, E.; Žiarovská, J.; Felšöciová, S.; Fatrcová-Šramková, K.; Terentjeva, M. Isolation and Identification of Lactic Acid Bacteria in Wine Production by Maldi-Tof MS Biotyper. Acta Hortic. Regiotect. 2020, 23, 21–24. [Google Scholar] [CrossRef]
- Pittet, V.; Morrow, K.; Ziola, B. Ethanol Tolerance of Lactic Acid Bacteria, Including Relevance of the Exopolysaccharide GeneGtf. J. Am. Soc. Brew. Chem. 2011, 69, 57–61. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Moreno-Arribas, M.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative Study of the Inhibitory Effects of Wine Polyphenols on the Growth of Enological Lactic Acid Bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef] [PubMed]
- López-Seijas, J.; García-Fraga, B.; Da Silva, A.F.; Zas-García, X.; Lois, L.C.; Gago-Martínez, A.; Leão-Martins, J.M.; Sieiro, C. Evaluation of Malolactic Bacteria Associated with Wines from Albariño Variety as Potential Starters: Screening for Quality and Safety. Foods 2020, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, L.M.; de Nadra, M.C.M.; Farías, M.E. Antagonistic Interaction between Yeasts and Lactic Acid Bacteria of Oenological Relevance: Partial Characterization of Inhibitory Compounds Produced by Yeasts. Food Res. Int. 2010, 43, 1990–1998. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.A.; Fernández-Cruz, E.; Garcia-Parrilla, M.C.; Troncoso, A.M.; Mattivi, F.; Vrhovsek, U.; Arapitsas, P. Saccharomyces Cerevisiae and Torulaspora Delbrueckii Intra- and Extra-Cellular Aromatic Amino Acids Metabolism. J. Agric. Food Chem. 2019, 67, 7942–7953. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Advances in the Study of Candida Stellata. Fermentation 2018, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Ciani, M.; Ferraro, L. Combined Use of Immobilized Candida Stellata Cells and Saccharomyces Cerevisiae to Improve the Quality of Wines. J. Appl. Microbiol. 1998, 85, 247–254. [Google Scholar] [CrossRef]
- Beltran, G.; Torija, M.J.; Novo, M.; Ferrer, N.; Poblet, M.; Guillamón, J.M.; Rozès, N.; Mas, A. Analysis of Yeast Populations during Alcoholic Fermentation: A Six Year Follow-Up Study. Syst. Appl. Microbiol. 2002, 25, 287–293. [Google Scholar] [CrossRef]
- Hong, Y.-A.; Park, H.-D. Role of Non-Saccharomyces Yeasts in Korean Wines Produced from Campbell Early Grapes: Potential Use of Hanseniaspora Uvarum as a Starter Culture. Food Microbiol. 2013, 34, 207–214. [Google Scholar] [CrossRef]
- Benito, S. The impact Of Torulaspora Delbrueckii Yeast in Winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Domizio, P.; Lencioni, L.; Ciani, M.; Di Blasi, S.; Pontremolesi, C.; Sabatelli, M. Spontaneous and Inoculated Yeast Populations Dynamics and Their Effect on Organoleptic Characters of Vinsanto Wine under Different Process Conditions. Int. J. Food Microbiol. 2007, 115, 281–289. [Google Scholar] [CrossRef]
- UC DAVIS. Wine Microbiology; The University of California: Davis, CA, USA, 2011; Available online: https://wineserver.ucdavis.edu/industry-info/enology/wine-microbiology (accessed on 2 July 2020).
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [Green Version]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-Conventional Yeast Species for Lowering Ethanol Content of Wines. Front. Microbiol. 2016, 7, 642. [Google Scholar] [CrossRef] [Green Version]
- Englezos, V.; Torchio, F.; Cravero, F.; Marengo, F.; Giacosa, S.; Gerbi, V.; Rantsiou, K.; Rolle, L.; Cocolin, L. Aroma prfile and comosition of Barbera by of Starmerella bacillaris (syonym Candida zemplinina) and Saccharomyces ceevisiae. LWT 2016, 73, 567–575. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the Non-Saccharomyces Yeast Starmerella Bacillaris (Synonym Candida zemplinina) in Wine Fermentation: Physiological and Molecular Characterizations. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Ferraro, L.; Fatichenti, F. Influence of Glycerol Production on the Aerobic and Anaerobic Growth of the Wine Yeast Candida Stellata. Enzym. Microb. Technol. 2000, 27, 698–703. [Google Scholar] [CrossRef]

| Grape Variety | LAB (log CFU/mL) | Yeast (log CFU/mL) | ||
|---|---|---|---|---|
| D0 | D7 | D0 | D7 | |
| C. Sauvignon | 2.13 ± 0.06 | 7.80 ± 0.07 | 4.81 ± 0.07 | 7.81 ± 0.11 |
| Carignan | 2.32 ± 0.05 | 6.33 ± 0.01 | 4.69 ± 0.08 | 7.52 ± 0.23 |
| Chardonnay | 2.38 ± 0.08 | 7.77 ± 0.16 | 5.08 ± 0.00 | 7.71 ± 0.05 |
| Merlot | 1.81 ± 0.90 | 7.50 ± 0.06 | 5.19 ± 0.02 | 7.71 ± 0.05 |
| Pinot | 1.69 ± 0.08 | 7.59 ± 0.30 | 4.63 ± 0.03 | 7.39 ± 0.31 |
| S. Blanc | 1.72 ± 0.11 | 7.35 ± 0.03 | 4.75 ± 0.12 | 7.67 ± 0.11 |
| Syrah | 1.82 ± 0.17 | 7.70 ± 0.03 | 4.89 ± 0.14 | 7.60 ± 0.12 |
| Grape Variety | Yeast Identification (% Abundance) |
|---|---|
| Carignan | Hanseniaspora uvarum (40) |
| Saccharomyces cerevisiae (20) | |
| Starmerella bacillaris (20) | |
| Lachancea thermotolerans (20) | |
| Chardonnay | Aureobasidium pullulans (16) |
| Candida oleophila (12) | |
| Candida stellate (12) | |
| Starmerella bacillaris (12) | |
| Hanseniospora uvarum (12) | |
| Lachancea thermotolerans (12) | |
| Saccharomyces cerevisiae (24) | |
| Cabernet Sauvignon | Candida stellata (24) |
| Hanseniaspora uvarum (20) | |
| Torolaspura delbrueckii (7) | |
| Metchinoskowia pulcherrima (21) | |
| Saccharmyces cerevisiae (28) | |
| Merlot | Candida stellata (15) |
| Starmerella bacillaris (15) | |
| Candida oleophila (15) | |
| Lachancea thermotolerans (25) | |
| Saccharomyces cervisiae (15) | |
| Torulaspora delbrueckii (15) | |
| Pinot | Hanseniospora uvarum (20) |
| Metschnikowia pulecherrima (20) | |
| Saccharomyces cerevisiae (60) | |
| Syrah | Aureobasidium pullulans (17) |
| Hanseniospora uvarum (17) | |
| Saccharomyces cerevisiae (66) | |
| Sauvignon blanc | Lachancea thermotolerans (14) |
| Metschnikowia pulcherrima (28) | |
| Saccharomyces cerevisiae (58) |
| Grape Variety (No. of Colonies) | Isolation Time Point (Day) | LAB Identification |
|---|---|---|
| C. Sauvignon (6) | 0, 7 | Leuconostoc mesenteroides |
| Carignan (4) | 0, 7 | |
| Chardonnay (3) | 0, 7 | |
| Merlot (6) | 0, 7 | |
| Pinot (11) | 0, 3, 7 | |
| S. Blanc (17) | 0, 3, 7 | |
| Syrah (3) | 0, 7 | |
| Carignan (3) | 0 | Fructilactobacillus fructivorans |
| Pinot (5) | 0 | Lactobacillus delbrueckii ssp delbrueckii |
| Grape | Lactic Acid (g/L) | Acetic Acid (g/L) | Malic Acid (g/L) | |||
|---|---|---|---|---|---|---|
| D0 | D7 | D0 | D7 | D0 | D7 | |
| C. Sauvignon | ND | 1.15 ± 0.01 a | ND | 1.07 ± 0.03 a | 2.50 ± 0.01 a | 0.04 ± 0.01 a |
| Carignan | ND | 1.18 ± 0.04 a | 0.03 ± 0.01 | 1.10 ± 0.05 b | 2.61 ± 0.01 b | 0.04 ± 0.01 c |
| Chardonnay | ND | 1.12 ± 0.01 a | ND | 1.06 ± 0.02 a | 2.32 ± 0.01 c | 0.08 ± 0.02 b |
| Merlot | ND | 1.14 ± 0.03 a | ND | 1.05 ± 0.01 a | 3.13 ± 0.01 e | 0.06 ± 0.02 d |
| Pinot | 0.08 ± 0.03 a | 1.17 ± 0.04 b | ND | 1.17 ± 0.04 b | 2.37 ± 0.03 c | 0.06 ± 0.01 c |
| S. Blanc | 0.03 ± 0.02 b | 1.13 ± 0.02 a | ND | 1.05 ± 0.03 a | 2.85 ± 0.01 d | 0.07 ± 0.02 b |
| Syrah | ND | 1.12 ± 0.05 a | ND | 1.06 ± 0.03 a | 2.82 ± 0.01 d | 0.08 ± 0.01 b |
| Yeast Isolate | Grape Source | Ethanol Yield (g Ethanol/g Glucose) | % Glucose Consumed | Category |
|---|---|---|---|---|
| Candida olepohila–1 | Chardonnay | 0.18 | 63.50% | LOW |
| Candida oleophila–2 | Merlot | 0.23 | 50.90% | |
| Hanseniaspora uvarum–1 | Carignan | 0.3 | 23.40% | |
| Metschnikowia pulcherrima–1 | C. Sauvignon | 0.31 | 22.70% | |
| Hanseniaspora uvarum–2 | Chardonnay | 0.33 | 20.50% | |
| Hanseniaspora uvarum–3 | C. Sauvignon | 0.33 | 20.50% | |
| Metschnikowia pulcherrima–2 | Pinot | 0.33 | 21.20% | |
| Metschnikowia pulcherrima–3 | Pinot | 0.33 | 37.60% | |
| Hanseniaspora uvarum–4 | Pinot | 0.34 | 21.60% | |
| Metschnikowia pulcherrima–4 | S. blanc | 0.35 | 42.70% | |
| Metschnikowia pulcherrima–5 | S. blanc | 0.35 | 41.60% | |
| Starmerella bacillaris | Carignan | 0.39 | 35.00% | MEDIUM |
| Candida stellata | Chardonnay | 0.4 | 42.00% | |
| Hanseniaspora uvarum–5 | Syrah | 0.4 | 23.70% | |
| Torulaspora delbrueckii–1 | C. Sauvignon | 0.41 | 41.90% | |
| Lanchacea thermotolerans–2 | Merlot | 0.43 | 33.70% | |
| Saccharomyces cerevisiae | Control | 0.44 | 98.50% | |
| Aureobasidium pullulans–1 | Syrah | 0.45 | 26.40% | |
| Candida oleophila–3 | Chardonnay | 0.45 | 20.00% | |
| Lanchacea thermotolerants–2 | Carignan | 0.45 | 32.70% | |
| Torulaspora delbrueckii–2 | Merlot | 0.45 | 30.70% | |
| Aureobasidium pullulans–2 | Chardonnay | 0.47 | 27.00% | HIGH |
| Hanseniaspora uvarum–6 | C. Sauvignon | 0.48 | 14.90% | |
| Lanchacea thermotolerans–3 | Carignan | 0.48 | 31.70% | |
| Lanchacea thermotolerans–4 | Merlot | 0.48 | 29.70% | |
| Aureobasidium pullulans–3 | Chardonnay | 0.51 | 30.00% | |
| Lanchacea thermotolerans–5 | S. blanc | 0.51 | 35.30% |
| Yeast | Glucose (g/L) | Fructose (g/L) | Ethanol % (v/v) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fermentation Day * | |||||||||
| 0 | 3 | 14 | 0 | 3 | 14 | 0 | 3 | 14 | |
| C. stellata | 139.2 ± 0.08 a | 49.2 ± 0.01 a | 1.90 ± 0.05 a | 110.1 ± 0.01 a | 50.0 ± 0.01 a | 1.00 ± 0.03 a | ND | 7.70 ± 0.05 a | 10.8 ± 0.1 a |
| C. oleophila | 140.1 ± 0.03 a | 453.1 ± 0.12 b | 1.12 ± 0.01 b | 110.0 ± 0.02 a | 58.2 ± 0.08 b | 0.85 ± 0.07 a | ND | 4.55 ± 0.08 b | 12.5 ± 0.1 b |
| S. cerevisiae | 139.5 ± 0.03 a | 5.13 ± 0.03 b | ND | 109.4 ± 0.01 a | 15.31 ± 0.02 d | 1.46 ± 0.09 c | ND | 12.8 ± 0.03 d | 13.5 ± 0.67 c |
| Yeast | Acetic Acid | Malic Acid | Succinic Acid | Glycerol |
|---|---|---|---|---|
| (g/L) | ||||
| C. stellata | 0.52 ± 0.07 a | 0.82 ± 0.13 a | 1.99 ± 0.15 a | 10.3 ± 0.05 a |
| C. oleophila | 0.53 ± 0.11 a | 0.65 ± 0.15 b | 2.31 ± 0.01 b | 18.1 ± 0.05 b |
| S. cerevisiae | 0.26 ± 0.01 c | 1.03 ± 0.03 d | 1.21 ± 0.05 d | 10.4 ± 2.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, W.; Benavides, S.; Valencia, P.; Ramírez, C.; Urtubia, A. Native Yeasts and Lactic Acid Bacteria Isolated from Spontaneous Fermentation of Seven Grape Cultivars from the Maule Region (Chile). Foods 2021, 10, 1737. https://doi.org/10.3390/foods10081737
Franco W, Benavides S, Valencia P, Ramírez C, Urtubia A. Native Yeasts and Lactic Acid Bacteria Isolated from Spontaneous Fermentation of Seven Grape Cultivars from the Maule Region (Chile). Foods. 2021; 10(8):1737. https://doi.org/10.3390/foods10081737
Chicago/Turabian StyleFranco, Wendy, Sergio Benavides, Pedro Valencia, Cristian Ramírez, and Alejandra Urtubia. 2021. "Native Yeasts and Lactic Acid Bacteria Isolated from Spontaneous Fermentation of Seven Grape Cultivars from the Maule Region (Chile)" Foods 10, no. 8: 1737. https://doi.org/10.3390/foods10081737
APA StyleFranco, W., Benavides, S., Valencia, P., Ramírez, C., & Urtubia, A. (2021). Native Yeasts and Lactic Acid Bacteria Isolated from Spontaneous Fermentation of Seven Grape Cultivars from the Maule Region (Chile). Foods, 10(8), 1737. https://doi.org/10.3390/foods10081737










