Influence of the Initial Cell Number on the Growth Fitness of Salmonella Enteritidis in Raw and Pasteurized Liquid Whole Egg, Egg White, and Egg Yolk
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Growth Curves
2.3. Thermal Treatments
2.4. Growth Curve Fitting and Statistical Analysis
3. Results
3.1. Influence of the Initial Concentration on the Growth Fitness of Salmonella Enteritidis STCC 4300 in Raw Liquid Whole Egg, Egg White, and Egg Yolk
3.2. Growth Fitness of Salmonella Enteritidis STCC 4300 Cells in Raw and Pasteurized Egg Products
3.3. Variability in Growth Fitness in Raw Liquid Whole Egg, Egg White, and Egg Yolk among S. Enteritidis Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EFSA (European Food Safety Authority). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of Egg Contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.H.; Benson, C.E.; Krotec, K.; Eckroade, R.J. Salmonella enteritidis Colonization of the Reproductive Tract and Forming and Freshly Laid Eggs of Chickens. Infect. Immun. 1995, 63, 2443–2449. [Google Scholar] [CrossRef]
- Timoney, J.F.; Shivaprasad, H.L.; Baker, R.C.; Rowe, B. Egg Transmission after Infection of Hens with Salmonella enteritidis Phage Type 4. Vet. Rec. 1989, 125, 600–601. [Google Scholar] [PubMed]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell Factors Influencing Eggshell Penetration and Whole Egg Contamination by Different Bacteria, Including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef]
- Messens, W.; Grijspeerdt, K.; Herman, L. Eggshell Penetration by Salmonella: A Review. Worlds Poult. Sci. J. 2005, 61, 71–86. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Conner, D.E. Survival and Growth of Salmonella Enteritidis in Liquid Egg Products Varying by Temperature, Product Composition, and Carbon Dioxide Concentration. Foodborne Pathog. Dis. 2009, 6, 561–567. [Google Scholar] [CrossRef] [PubMed]
- McQuestin, O.J.; Musgrove, M.T.; Tamplin, M.L. Kinetics of Growth and Inactivation of Salmonella Enterica Serotype Typhimurium DT104 in Pasteurised Liquid Egg Products. Food Microbiol. 2010, 27, 396–402. [Google Scholar] [CrossRef]
- Sakha, M.Z.; Fujikawa, H. Growth Characteristics of Salmonella Enteritidis in Pasteurized and Unpasteurized Liquid Egg Products. Biocontrol Sci. 2012, 17, 183–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moon, H.J.; Lim, J.G.; Yoon, K.S. Comparative Study of Change in Salmonella Enteritidis and Salmonella Typhimurium Populations in Egg white and Yolk. J. Food Hyg. Saf. 2016, 31, 342–348. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Moon, H.-J.; Lee, S.-K.; Song, B.-R.; Lim, J.-S.; Heo, E.-J.; Park, H.-J.; Wee, S.-H.; Moon, J.-S. Development and Validation of Predictive Model for Salmonella Growth in Unpasteurized Liquid Eggs. Korean J. Food Sci. Anim. Resour. 2018, 38, 442–450. [Google Scholar] [CrossRef]
- Messens, W.; Duboccage, L.; Grijspeerdt, K.; Heyndrickx, M.; Herman, L. Growth of Salmonella Serovars in Hens’ Egg Albumen as Affected by Storage Prior to Inoculation. Food Microbiol. 2004, 21, 25–32. [Google Scholar] [CrossRef]
- Baron, F.; Gautier, M.; Brule, G. Factors Involved in the Inhibition of Growth of Salmonella Enteritidis in Liquid Egg White. J. Food Prot. 1997, 60, 1318–1323. [Google Scholar] [CrossRef]
- Baron, F.; Nau, F.; Guérin-Dubiard, C.; Bonnassie, S.; Gautier, M.; Andrews, S.C.; Jan, S. Egg White versus Salmonella Enteritidis! A Harsh Medium Meets a Resilient Pathogen. Food Microbiol. 2016, 53, 82–93. [Google Scholar] [CrossRef]
- Singh, A.; Korasapati, N.R.; Juneja, V.K.; Subbiah, J.; Froning, G.; Thippareddi, H. Dynamic Predictive Model for the Growth of Salmonella spp. in Liquid Whole Egg. J. Food Sci. 2011, 76, M225–M232. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cepeda, J.; Subbiah, J.; Froning, G.; Juneja, V.K.; Thippareddi, H. Dynamic Predictive Model for Growth of Salmonella spp. in Scrambled Egg Mix. Food Microbiol. 2017, 64, 39–46. [Google Scholar] [CrossRef]
- Carrasco, E.; del Rosal, S.; Racero, J.C.; García-Gimeno, R.M. A Review on Growth/No Growth Salmonella Models. Food Res. Int. 2012, 47, 90–99. [Google Scholar] [CrossRef]
- Guillén, S.; Marcén, M.; Álvarez, I.; Mañas, P.; Cebrián, G. Influence of the Initial Cell Number on the Growth Fitness of Salmonella Enteritidis in Raw and Pasteurized Liquid Whole Egg. Proceedings 2020, 70, 21. [Google Scholar] [CrossRef]
- Kang, M.S.; Park, J.H.; Kim, H.J. Predictive Modeling for the Growth of Salmonella Spp. in Liquid Egg White and Application of Scenario-Based Risk Estimation. Microorganisms 2021, 9, 486. [Google Scholar] [CrossRef]
- Guillén, S.; Marcén, M.; Mañas, P.; Cebrián, G. Differences in Resistance to Different Environmental Stresses and Non-Thermal Food Preservation Technologies among Salmonella enterica subsp. Enterica Strains. Food Res. Int. 2020, 132, 109042. [Google Scholar] [CrossRef]
- Conesa, R.; Periago, P.M.; Esnoz, A.; López, A.; Palop, A. Prediction of Bacillus subtilis Spore Survival after a Combined Non-Isothermal-Isothermal Heat Treatment. Eur. Food Res. Technol. 2003, 217, 319–324. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A Dynamic Approach to Predicting Bacterial Growth in Food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Kang, G.; Soohyun, C.; Pilnam, S.; Beomyoung, P.; Junsang, H.; Seokgeun, J.; Donghun, K.; Hyunseok, C. Microbial and Physicochemical Properties of Liquid Egg during Cold Storage. Korean J. Food Sci. Anim. 2011, 31, 557–562. [Google Scholar] [CrossRef][Green Version]
- Zaher, S.M.; Fujikawa, H. Effect of Native Microflora on the Growth Kinetics of Salmonella Enteritidis Strain 04-137 in Raw Ground Chicken. J. Food Prot. 2011, 74, 735–742. [Google Scholar] [CrossRef]
- Kang, H.; Loui, C.; Clavijo, R.I.; Riley, L.W.; Lu, S. Survival Characteristics of Salmonella Enterica Serovar Enteritidis in Chicken Egg Albumen. Epidemiol. Infect. 2006, 134, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Cogan, T.A.; Domingue, G.; Lappin-Scott, H.M.; Benson, C.E.; Woodward, M.J.; Humphrey, T.J. Growth of Salmonella Enteritidis in Artificially Contaminated Eggs: The Effects of Inoculum Size and Suspending Media. Int. J. Food Microbiol. 2001, 70, 131–141. [Google Scholar] [CrossRef]
- Guillén, S.; Marcén, M.; Fau, E.; Mañas, P.; Cebrián, G. Relationship between growth fitness, virulence, and resistance to food processing-related stresses in non-typhoidal Salmonellae. Int. J. Food Microbiol. Submitted for publication.
- Lechevalier, V.; Guérin-Dubiard, C.; Anton, M.; Beaumal, V.; David Briand, E.; Gillard, A.; Le Gouar, Y.; Musikaphun, N.; Tanguy, G.; Pasco, M.; et al. Pasteurisation of Liquid Whole Egg: Optimal Heat Treatments in Relation to Its Functional, Nutritional and Allergenic Properties. J. Food Eng. 2017, 195, 137–149. [Google Scholar] [CrossRef]
- Baron, F.; Cochet, M.-F.; Alabdeh, M.; Guérin-Dubiard, C.; Gautier, M.; Nau, F.; Andrews, S.C.; Bonnassie, S.; Jan, S. Egg-White Proteins Have a Minor Impact on the Bactericidal Action of Egg White Toward Salmonella Enteritidis at 45 °C. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- De Vylder, J.; Raspoet, R.; Dewulf, J.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Salmonella Enteritidis Is Superior in Egg White Survival Compared with Other Salmonella Serotypes. Poult. Sci. 2013, 92, 842–845. [Google Scholar] [CrossRef]
- Clavijo, R.I.; Loui, C.; Andersen, G.L.; Riley, L.W.; Lu, S. Identification of Genes Associated with Survival of Salmonella Enterica Serovar Enteritidis in Chicken Egg Albumen. Appl. Environ. Microbiol. 2006, 72, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.H.; Casavant, C.; Hawley, Q.; Addwebi, T.; Call, D.R.; Guard, J. Salmonella Enteritidis Strains from Poultry Exhibit Differential Responses to Acid Stress, Oxidative Stress, and Survival in the Egg Albumen. Foodborne Pathog. Dis. 2012, 9, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, B.; Xu, X.; Zhang, L.; Wei, C.; Ou, H.; Cui, Y.; Shi, C.; Shi, X. Comparative Genomic Analysis and Characterization of Two Salmonella Enterica Serovar Enteritidis Isolates from Poultry with Notably Different Survival Abilities in Egg Whites. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, X.; Jia, B.; Li, N.; Jia, J.; He, M.; He, Y.; Qin, X.; Cui, Y.; Shi, C.; et al. Transcriptional Sequencing Uncovers Survival Mechanisms of Salmonella Enterica Serovar Enteritidis in Antibacterial Egg White. mSphere 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Van Immerseel, F. Salmonella Enterica Serovar Enteritidis Genes Induced during Oviduct Colonization and Egg Contamination in Laying Hens. Appl. Environ. Microbiol. 2008, 74, 6616–6622. [Google Scholar] [CrossRef]
- Julien, L.A.; Baron, F.; Bonnassie, S.; Nau, F.; Guérin, C.; Jan, S.; Andrews, S.C. The Anti-Bacterial Iron-Restriction Defence Mechanisms of Egg White; the Potential Role of Three Lipocalin-like Proteins in Resistance against Salmonella. BioMetals 2019, 32, 453–467. [Google Scholar] [CrossRef]
- Legros, J.; Jan, S.; Bonnassie, S.; Gautier, M.; Croguennec, T.; Pezennec, S.; Cochet, M.-F.; Nau, F.; Andrews, S.C.; Baron, F. The Role of Ovotransferrin in Egg-White Antimicrobial Activity: A Review. Foods 2021, 10, 823. [Google Scholar] [CrossRef]
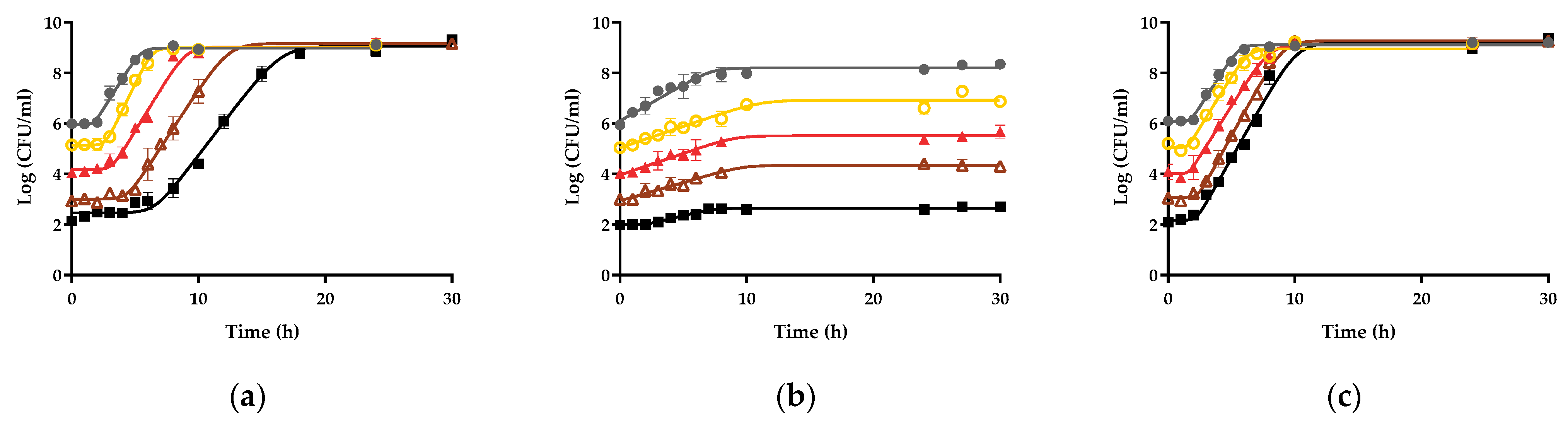

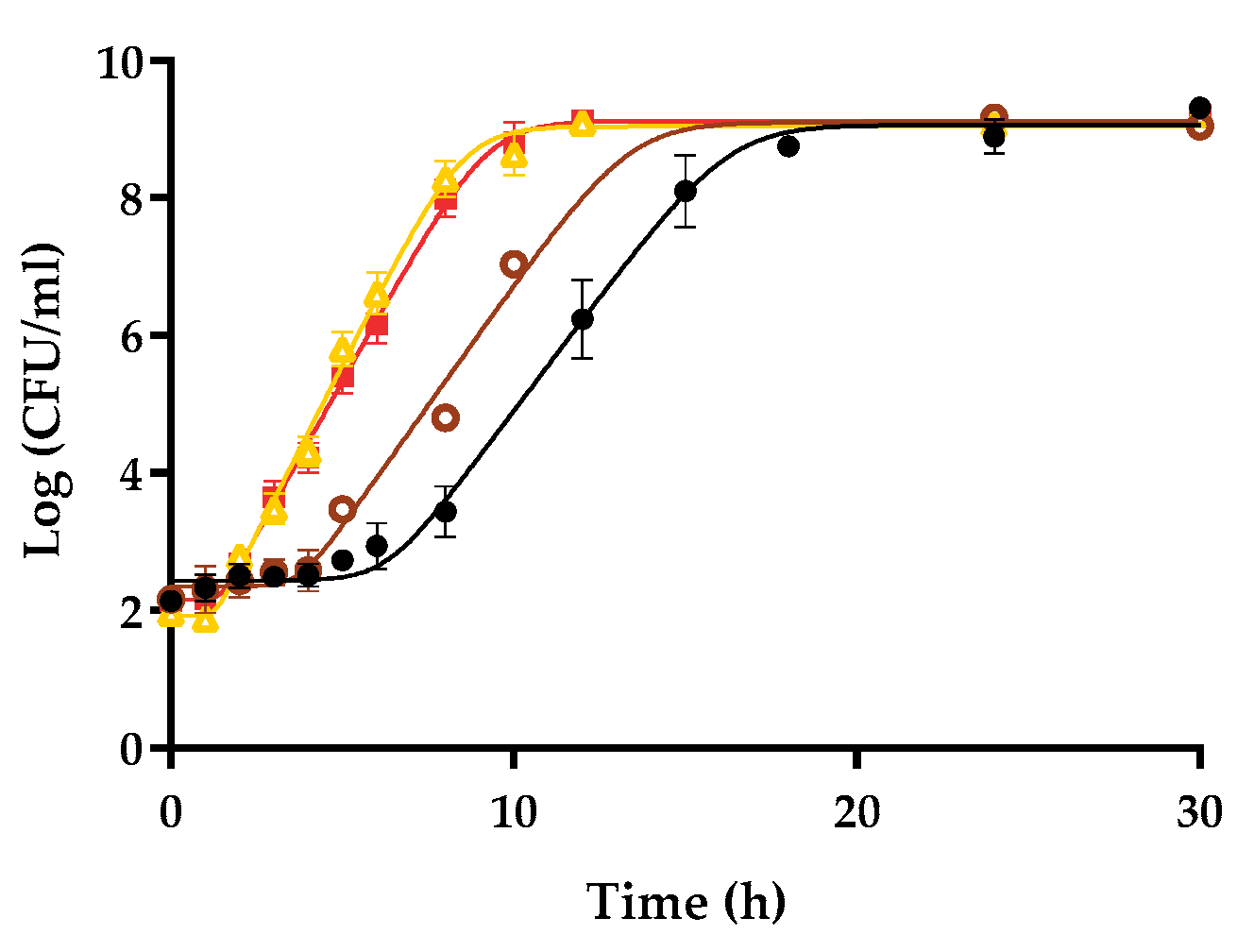



| Y0 (CFU/mL) | µmax (h−1) | λ (h) | Ymax (CFU/mL) | R2 | RMSE |
|---|---|---|---|---|---|
| Raw whole egg | |||||
| 6.00 (0.061) | 0.845 (0.038) a | 1.97 (0.038) a | 8.96 (0.093) | 0.99–1.00 | 0.115–0.166 |
| 5.15 (0.066) | 1.056 (0.142) a,b | 2.71 (0.230) b | 8.99 (0.013) | 0.99–1.00 | 0.127–0.201 |
| 4.21 (0.086) | 0.982 (0.039) b | 3.49 (0.128) c | 9.01 (0.079) | 0.99–0.99 | 0.287–0.316 |
| 3.01 (0.035) | 0.836 (0.018) a | 4.54 (0.505) d | 9.16 (0.038) | 0.99–1.00 | 0.133–0.284 |
| 2.22 (0.070) | 0.663 (0.015) c | 6.32 (0.549) e | 9.05 (0.030) | 0.99–1.00 | 0.225–0.274 |
| Raw egg white | |||||
| 6.06 (0.116) | 0.292 (0.066) a | - a | 8.24 (0.084) | 0.97–0.99 | 0.131–0.172 |
| 5.03 (0.026) | 0.193 (0.022) a,b | 0.29 (0.082) b | 6.97 (0.051) | 0.96–0.97 | 0.172–0.187 |
| 4.02 (0.023) | 0.205 (0.014) a | 0.43 (0.094) b | 5.45 (0.045) | 0.96–0.98 | 0.097–0.144 |
| 2.98 (0.059) | 0.158 (0.010) b | 0.40 (0.211) b | 4.42 (0.120) | 0.98 -0.99 | 0.055–0.096 |
| 2.03 (0.013) | 0.104 (0.012) c | 1.83 (0.106) c | 2.64 (0.037) | 0.95–0.99 | 0.067–0.138 |
| Raw egg yolk | |||||
| 6.10 (0.028) | 0.837 (0.057) a | 1.94 (0.147) a | 9.07 (0.038) | 0.99–0.99 | 0.121–0.181 |
| 5.07 (0.073) | 0.829 (0.027) a | 1.60 (0.140) a | 8.93 (0.139) | 0.98–0.99 | 0.249–0.257 |
| 4.05 (0.381) | 0.838 (0.018) a | 1.89 (0.403) a | 9.09 (0.071) | 0.99–1.00 | 0.163–0.264 |
| 3.09 (0.067) | 0.882 (0.047) a | 2.21 (0.240) a | 9.27 (0.106) | 0.99–1.00 | 0.134–0.235 |
| 2.15 (0.081) | 0.857 (0.016) a | 2.01 (0.195) a | 9.23 (0.056) | 0.98–0.99 | 0.271–0.414 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillén, S.; Marcén, M.; Álvarez, I.; Mañas, P.; Cebrián, G. Influence of the Initial Cell Number on the Growth Fitness of Salmonella Enteritidis in Raw and Pasteurized Liquid Whole Egg, Egg White, and Egg Yolk. Foods 2021, 10, 1621. https://doi.org/10.3390/foods10071621
Guillén S, Marcén M, Álvarez I, Mañas P, Cebrián G. Influence of the Initial Cell Number on the Growth Fitness of Salmonella Enteritidis in Raw and Pasteurized Liquid Whole Egg, Egg White, and Egg Yolk. Foods. 2021; 10(7):1621. https://doi.org/10.3390/foods10071621
Chicago/Turabian StyleGuillén, Silvia, María Marcén, Ignacio Álvarez, Pilar Mañas, and Guillermo Cebrián. 2021. "Influence of the Initial Cell Number on the Growth Fitness of Salmonella Enteritidis in Raw and Pasteurized Liquid Whole Egg, Egg White, and Egg Yolk" Foods 10, no. 7: 1621. https://doi.org/10.3390/foods10071621
APA StyleGuillén, S., Marcén, M., Álvarez, I., Mañas, P., & Cebrián, G. (2021). Influence of the Initial Cell Number on the Growth Fitness of Salmonella Enteritidis in Raw and Pasteurized Liquid Whole Egg, Egg White, and Egg Yolk. Foods, 10(7), 1621. https://doi.org/10.3390/foods10071621






