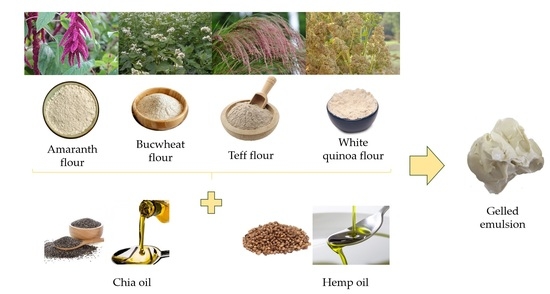
Assessment of Chemical, Physicochemical, and Lipid Stability Properties of Gelled Emulsions Elaborated with Different Oils Chia (Salvia hispanica L.) or Hemp (Cannabis sativa L.) and Pseudocereals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Lipid Profile of Vegetable Oils
2.3. Gelled Emulsions Preparation
2.4. Gelled Emulsion Analysis
2.4.1. Proximate Composition
2.4.2. Physicochemical Properties
2.5. Stability of Gelled Emulsion during Frozen Storage
2.5.1. Emulsion Stability
2.5.2. Instrumental Color Analysis
2.5.3. Oxidative Stability
2.6. Statistical Assay
3. Results and Discussion
3.1. Fatty Acid Profile of Oils Used for Gelled Emulsions Preparation
3.2. Gelled Emulsions
3.2.1. Proximal Composition of Gelled Emulsions
3.2.2. Physicochemical Properties of Gelled Emulsions
3.3. Stability of Gelled Emulsion during Frozen Storage
3.3.1. Emulsion Stability of Gelled Emulsions
3.3.2. Lipid Oxidation of GE
3.3.3. Color Properties of GE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda Martos, M. Anthocyanins and cardiovascular diseases. In Anthocyanins: Antioxidant Properties, Sources and Health Benefits; Lorenzo, J.M., Barba, J., Munekata, P.E.S., Eds.; Nova Publisher: New York, NY, USA, 2020; pp. 205–230. ISBN 978-1-53617-816-6. [Google Scholar]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, A.M.; Claudia Ruiz-Capillas, C. Novel lipid materials based on gelling procedures as fat analogues in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 39, 1–6. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Schoon, K.; Rudman, M.; Brand, T.S.; Dalle Zotte, A.; Cullere, M. Profile of cabanossi made with exotic meats and olive oil. Meat Sci. 2019, 147, 20–27. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Jung, S.; Kim, Y.B.; Choi, Y.S. Effects of replacing pork fat with grape seed oil and gelatine/alginate for meat emulsions. Meat Sci. 2020, 163, 108079. [Google Scholar] [CrossRef]
- Bolger, Z.; Brunton, N.P.; Monahan, F.J. Impact of inclusion of flaxseed oil (pre-emulsified or encapsulated) on the physical characteristics of chicken sausages. J. Food Eng. 2018, 230, 39–48. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Barba, F.J.; Franco, D.; Campagnol, P.C.B.; Munekata, P.E.S.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Microencapsulation of healthier oils to enhance the physicochemical and nutritional properties of deer pâté. LWT 2020, 125, 109223. [Google Scholar] [CrossRef]
- Franco, D.; Martins, A.J.; López-Pedrouso, M.; Purriños, L.; Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M.; Zapata, C.; Lorenzo, J.M. Strategy towards replacing pork backfat with a linseed oleogel in frankfurter sausages and its evaluation on physicochemical, nutritional, and sensory characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Ferro, A.C.; de Souza Paglarini, C.; Rodrigues Pollonio, M.A.; Lopes Cunha, R. Glyceryl monostearate-based oleogels as a new fat substitute in meat emulsion. Meat Sci. 2021, 174, 108424. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Roldán-Verdu, A.; Sayas-Barberá, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of emulsion gels formulated with chestnut (Castanea sativa M.) flour and chia (Salvia hispanica L.) oil as partial fat replacers in pork burger formulation. J. Sci. Food Agric. 2020, 100, 1265–1273. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; de Carvalho, F.A.L.; Domínguez, R.; Trindade, M.A.; Pateiro, M.; Lorenzo, J.M. Healthy beef burgers: Effect of animal fat replacement by algal and wheat germ oil emulsions. Meat Sci. 2021, 173, 108396. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Glisic, M.; Baltic, M.; Glisic, M.; Trbovic, D.; Jokanovic, M.; Suvajdzic, B.; Boscoviv, M.; Vasilev, D. Inulin-based emulsion-filled gel as a fat replacer in prebiotic-and PUFA-enriched dry fermented sausages. Int. J. Food Sci. Technol. 2019, 54, 787–797. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.S.; de Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of tiger nut (Cyperus esculentus L.) oil emulsion as animal fat replacement in beef burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Ospina, E.J.C.; Sierra-C, A.; Ochoa, O.; Pérez-Álvarez, J.A.; Fernández-López, J. Substitution of saturated fat in processed meat products: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Bohrer, B.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Recent discoveries in the field of lipid bio-based ingredients for meat processing. Molecules 2021, 26, 190. [Google Scholar] [CrossRef]
- de Souza-Paglarini, C.; de Figueiredo Furtado, G.; Honório, A.R.; Mokarzel, L.; da Silva Vidal, V.A.; Ribeiro, A.P.B.; Cunha, R.L.; Pollonio, M.A.R. Functional emulsion gels as pork back fat replacers in Bologna sausage. Food Struct. 2019, 20, 100105. [Google Scholar] [CrossRef]
- Villanueva-Bermejo, D.; Calvo, M.V.; Castro-Gómez, P.; Fornari, T.; Fontecha, J. Production of omega 3-rich oils from underutilized chia seeds. Comparison between supercritical fluid and pressurized liquid extraction methods. Food Res. Int. 2019, 115, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, M.F. A comparative study of seed yield and oil composition of four cultivars of hemp (Cannabis sativa L.) grown from three regions in northern Iran. Ind. Crops Prod. 2020, 152, 112397. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-Martos, M.; Pérez-Álvarez, J.A. Quinoa and chia products as ingredients for healthier process meat products: Technological strategies for their application and effects on the final product. Curr. Opin. Food Sci. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Oleogeles, ingredientes para desarrollar productos cárnicos más sanos. Tecnifood 2021, 133, 100–103. Available online: https://techpress.es/oleogeles-ingredientes-para-desarrollar-productos-carnicos-mas-sanos/# (accessed on 19 April 2021).
- Haros, C.M.; Schoenlechner, R. Peudocereals: Chemistry and Technology; John Wiley & Sons Ltd.: West Sussex, UK, 2017; ISBN 978-1-118-93828-7. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- Moskowitz, H.R. Food Texture: Instrumental and Sensory Measurement; Dekker, M., Ed.; Wiley: Hoboken, NJ, USA, 1987; ISBN 08-247-75856. [Google Scholar]
- Warner, K.; Eskin, N.A.M. Methods to Assess Quality and Stability of Oils and Fat-Containing Foods; AOCS Press: Champaign, IL, USA, 1994. [Google Scholar] [CrossRef]
- Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Carmona, P.; Herrero, A.M. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: Technological and infrared spectroscopic characterization. Food Chem. 2015, 185, 470–478. [Google Scholar] [CrossRef]
- Rosmini, M.; Perlo, F.; Pérez-Alvarez, J.; Pagán-Moreno, M.; Gago-Gago, M.; López-Santoveña, F.; Aranda-Catalá, V. TBA test by an extractive method applied to pate. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- Gómez-Candela, C.; Bermejo-López, L.M.; Loria-Kohen, V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutr. Hosp. 2011, 26, 323–329. [Google Scholar]
- Harris, W.S.; Mozaffarian, D.; Rimm, E.; Kris-Etherton, P.; Rudel, L.L.; Appel, L.J.; Engler, M.M.; Engler, M.B.; Sacks, F. Omega-6 fatty acids and risk for cardiovascular disease: A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009, 119, 902–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafoor, K.; Ahmed, I.A.M.; Özcan, M.M.; Al-Juhaimi, F.Y.; Babiker, E.E.; Azmi, I.U. An evaluation of bioactive compounds, fatty acid composition and oil quality of chia (Salvia hispanica L.) seed roasted at different temperatures. Food Chem. 2020, 333, 127531. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al-Juhaimi, F.Y.; Ahmed, I.A.M.; Osman, M.A.; Gassem, M.A. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chem. 2019, 278, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Giménez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) Seed Oil: Analytical and Phytochemical Characterization of the Unsaponifiable Fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef]
- Ghouila, Z.; Sehailia, M.; Chemat, S. Vegetable oils and fats: Extraction, composition and applications. In Plant Based “Green Chemistry 2.0”; Li, Y., Chemat, F., Eds.; Springer: Singapore, 2019; ISBN 978-981-13-3810-6. [Google Scholar]
- Mottram, H.R.; Crossman, Z.M.; Evershed, R.P. Regiospecific characterization of the triacylglycerols in animal fats using high performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Analyst 2001, 126, 1018–1024. [Google Scholar] [CrossRef]
- Alm, M. Edible oil processing. In AOCS Lipid Library; The American Oil Chemists’ Society: Urbana, IL, USA, 2019. [Google Scholar]
- Daly, C.C.; young, O.A.; Graafhuis, A.E.; Moorhead, S.M.; Easton, H.S. Some effects on diet on beef meat and fat attributes. N. Z. J. Agric. Res. 1999, 42, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Ospina-E, J.C.; Cruz-S, A.; Pérez-Álvarez, J.A.; Fernández-López, J. Development of combinations of chemically modified vegetable oils as pork backfat substitutes in sausages formulation. Meat Sci. 2010, 84, 491–497. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Fernández-Martín, F.; Rodríguez-Salas, L.; Ruiz-Capillas, C. Konjac gel fat analogue for use in meat products: Comparison with pork fats. Food Hydrocoll. 2012, 26, 63–72. [Google Scholar] [CrossRef]
- Méndez-Cid, F.J. Estudio del Enranciamiento Autooxidativo de Algunas Grasas Animales: Correlación y Representatividad de los Parámetros Indicadores. Ph.D. Thesis, Universidad de Vigo, Vigo, Spain, 2019. [Google Scholar]
- Sheu, K.S.; Chen, T.C. Yield and quality characteristics of edible broiler skin fat as obtained from five rendering methods. J. Food Eng. 2002, 55, 263–269. [Google Scholar] [CrossRef]
- Feddern, V.; Kupski, L.; Cipolatti, E.P.; Giacobbo, G.; Mendes, G.L.; Badiale-Furlong, E.; de Souza-Soares, L.A. 2010. Physico-chemical composition, fractionated glycerides and fatty acid profile of chicken skin fat. Eur. J. Lipid Sci. Technol. 2010, 112, 1277–1284. [Google Scholar] [CrossRef]
- Peña-Saldarriaga, L.M.; Fernández-López, J.; Pérez-Alvarez, J.A. Quality of chicken fat by-products: Lipid profile and colour properties. Foods 2020, 9, 1046. [Google Scholar] [CrossRef]
- Herrero, A.M.; Ruiz-Capillas, C.; Pintado, T.; Carmona, P.; Jiménez-Colmenero, F. Elucidation of lipid structural characteristics of chia oil emulsion gels by Raman spectroscopy and their relationship with technological properties. Food Hydrocoll. 2018, 77, 212–219. [Google Scholar] [CrossRef]
- Nie, S.P.; Wang, C.; Cui, S.W.; Wang, Q.; Xie, M.Y.; Phillips, G.O. The core carbohydrate structure of Acacia seyal var. seyal (Gum arabic). Food Hydrocoll. 2013, 32, 221–227. [Google Scholar] [CrossRef]
- Li, Y.F.; Yue, P.P.; Hao, X.; Bian, J.; Ren, J.L.; Peng, F.; Sun, R.C. Comparison of emulsifying capacity of two hemicelluloses from moso bamboo in soy oil-in-water emulsions. RSC Adv. 2020, 10, 4657–4663. [Google Scholar] [CrossRef]
- Öztürk-Kerimoğlu, B.; Kavuşan, H.S.; Gürel, D.B.; Çağınd, O.; Serdaroğlu, M. Cold-set or hot-set emulsion gels consisted of a healthy oil blend to replace beef fat in heat-treated fermented sausages. Meat Sci. 2021, 176, 108461. [Google Scholar] [CrossRef]
- Verheyen, D.; Baka, M.; Glorieux, S.; Duquenne, B.; Fraeye, I.; Skara, T.; Van Impe, J.F. Development of fish-based model systems with various microstructures. Food Res. Int. 2018, 106, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Alemayeu, F.R.; Bendevis, M.A.; Jacobsen, S.E. The potential for utilizing the seed crop amaranth (Amaranthus spp.) in East Africa as an alternative crop to support food security and climate change mitigation. J. Agron. Crop Sci. 2015, 201, 321–329. [Google Scholar] [CrossRef]
- Pellegrini, M.; Lucas-Gonzales, R.; Ricci, A.; Fontecha, J.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, fatty acid, polyphenolic profile, techno-functional and antioxidant properties of flours obtained from quinoa (Chenopodium quinoa Willd) seeds. Ind. Crops Prod. 2018, 111, 38–46. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and food uses of teff (Eragrostis tef). Food Chem. 2018, 239, 402–415. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. The breakdown properties of heat-set whey protein emulsion gels in the human mouth. Food Hydrocoll. 2013, 33, 215–224. [Google Scholar] [CrossRef]
- McClements, D.J.; Monahan, F.J.; Kinsella, J.E. Effect of emulsion droplets on the rheology of whey protein isolate gels. J. Texture Stud. 1993, 24, 411–422. [Google Scholar] [CrossRef]
- Martín, D.; Ruiz, J.; Kivikari, R.; Puolanne, E. Partial replacement of pork fat by conjugated linoleic acid and/or olive oil in liver pâtés: Effect on physicochemical characteristics and oxidative stability. Meat Sci. 2008, 80, 496–504. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Giarnetti, M.; Summo, C.; Pasqualone, A.; Minervini, F.; Caponio, F. Production and characterization of emulsion filled gels based on inulin and extra virgin olive oil. Food Hydrocoll. 2015, 45, 30–40. [Google Scholar] [CrossRef]
- Fidantsi, A.; Doxastakis, G. Emulsifying and foaming properties of amaranth seed protein isolates. Colloids Surf. B Biointerfaces 2001, 21, 119–124. [Google Scholar] [CrossRef]
- Tang, C.H. Thermal properties of buckwheat proteins as related to their lipid contents. Food Res Int. 2007, 40, 381–387. [Google Scholar] [CrossRef]
- Janssen, F.; Pauly, A.; Rombouts, I.; Jansens, K.J.A.; Deleu, L.J.; Delcou, J.A. Proteins of amaranth (Amaranthus spp.), buckwheat (Fagopyrum spp.), and quinoa (Chenopodium spp.): A food science and technology perspective. Comp. Rev. Food Sci. Food Saf. 2007, 16, 39–58. [Google Scholar] [CrossRef]
- Cramp, G.L.; Docking, A.M.; Ghosh, S.; Coupland, J.N. On the stability of oil-in-water emulsions to freezing. Food Hydrocoll. 2004, 18, 899–905. [Google Scholar] [CrossRef]
- Chizawa, Y.; Miyagawa, Y.; Yoshida, M.; Adachi, S. Effect of crystallization of oil phase on the destabilization of O/W emulsions containing vegetable oils with low melting points. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123824. [Google Scholar] [CrossRef]
- Ghosh, S.; Coupland, J.N. Factors affecting the freeze-thaw stability of emulsions. Food Hydrocoll. 2008, 22, 105–111. [Google Scholar] [CrossRef]
- Degner, B.M.; Chung, C.; Schlegel, V.; Hutkins, R.; McClements, D.J. Factors influencing the freeze-thaw stability of emulsion-based foods. Comp. Rev. Food Sci. Food Saf. 2014, 13, 98–113. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Sulewska, H.; Kreft, I.; Gujska, E.; Walkowjak, J. The content of dietary fibre and polyphenols in morphological parts of buckwheat (Fagopyrum tataricum). Plant Foods Hum. Nutr. 2018, 73, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Jubete, L.; Holse, M.; Hansen, Å.; Arendt, E.K.; Gallagher, E. Impact of baking on vitamin e content of pseudocereals amaranth, quinoa, and buckwheat. Cereal Chem. 2009, 86, 511–515. [Google Scholar] [CrossRef]
- Antoniewska, A.; Rutkowska, J.; Martinez Pineda, M.; Adamska, A. Antioxidative, nutritional and sensory properties of muffins with buckwheat flakes and amaranth flour blend partially substituting for wheat flour. LWT Food Sci. Techol. 2018, 89, 217–223. [Google Scholar] [CrossRef]
- Jiménez, D.; Lobo, M.; Irigaray, B.; Grompone, M.A.; Sammán, N. Oxidative stability of baby dehydrated purees formulated with different oils and germinated grain flours of quinoa and amaranth. LWT Food Sci. Technol. 2020, 127, 109229. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Lorenzo Rodriguez, J.M.; Barba, F.J.; Giuberti, G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, J.A.; Fernández-López, J. Color Measurements on Muscle-Based Foods. In Book Sensory Analysis of Foods of Animal Origin, 1st ed.; Nollet, L.M.N., Toldra, F., Eds.; Imprint CRC Press: Boca Raton, FL, USA, 2011; ISBN 9780429104732. [Google Scholar]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Aguilar-Rico, M.; Blanca-Giménez, V. Iluminación y Color; Servicio de Publicaciones de la Universidad Politécnica de Valencia: Valencia, Spain, 1995. [Google Scholar]


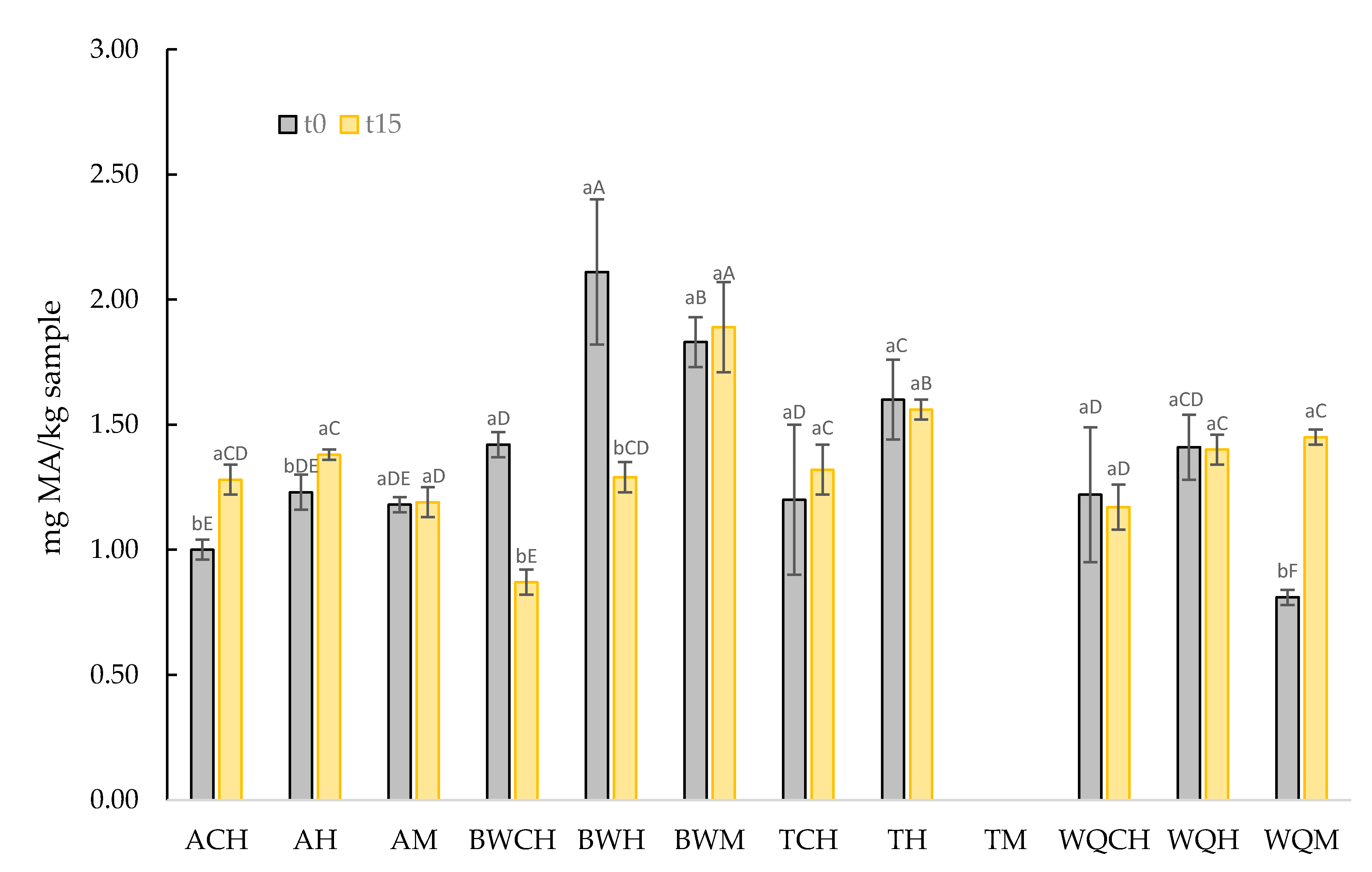
| Samples | Water | Instant Gel | Gellan Gum | Amaranth Flour | Buckwheat Flour | Teff Flour | Quinoa Flour | Chia Oil | Hemp Oil |
|---|---|---|---|---|---|---|---|---|---|
| ACH | 47 | 1.5 | 1.5 | 10 | - | - | - | 40 | - |
| AH | 47 | 1.5 | 1.5 | 10 | - | - | - | - | 40 |
| AM | 47 | 1.5 | 1.5 | 10 | - | - | - | 20 | 20 |
| BWCH | 47 | 1.5 | 1.5 | - | 10 | - | - | 40 | - |
| BWH | 47 | 1.5 | 1.5 | - | 10 | - | - | - | 40 |
| BWM | 47 | 1.5 | 1.5 | - | 10 | - | - | 20 | 20 |
| TCH | 47 | 1.5 | 1.5 | - | - | 10 | - | 40 | - |
| TH | 47 | 1.5 | 1.5 | - | - | 10 | - | - | 40 |
| TM | 47 | 1.5 | 1.5 | - | - | 10 | - | 20 | 20 |
| WQCH | 47 | 1.5 | 1.5 | - | - | - | 10 | 40 | - |
| WQH | 47 | 1.5 | 1.5 | - | - | - | 10 | - | 40 |
| WQM | 47 | 1.5 | 1.5 | - | - | - | 10 | 20 | 20 |
| Fatty Acid | Hemp Oil | Chia Oil | Chia/Hemp Oils Mix |
|---|---|---|---|
| C14:0 | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a |
| C16:0 | 6.17 ± 0.08 a | 5.84 ± 0.04 b | 6.03 ± 0.01 a |
| C17:0 | 0.06 ± 0.01 b | 0.05 ± 0.01 b | 0.10 ± 0.03 a |
| C18:0 | 2.3 ± 0.01 c | 3.63 ± 0.09 a | 2.98 ± 0.01 b |
| C20:0 | 0.94 ± 0.03 a | 0.19 ± 0.02 c | 0.57 ± 0.02 b |
| C22:0 | 0.41 ± 0.01 a | 0.1 ± 0.01 c | 0.27 ± 0.03 b |
| C24:0 | 0.32 ± 0.01 a | 0.15 ± 0.01 b | 0.14 ± 0.01 b |
| C16:1 cis | 0.1 ± 0.01 c | 0.05 ± 0.01 b | 0.08 ± 0.01 a |
| C16:1 trans | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0.02 ± 0.01 a |
| C17:1 | 0.03 ± 0.01 a | 0.03 ± 0.01 a | 0.04 ± 0.01 a |
| C18:1 cis | 8.23 ± 0.01 c | 15.05 ± 0.07 a | 11.60 ± 0.04 b |
| C18:1 trans | 0.56 ± 0.01 b | 0.62 ± 0.02 ab | 0.68 ± 0.03 a |
| C18:2 (ω-6) | 54.44 ± 0.01 a | 17.43 ± 0.09 c | 36.11 ± 0.14 b |
| C18:2 (ω-3) | 4.26 ± 0.01 a | 0.01 ± 0.00 c | 2.16 ± 0.01 b |
| C18:3 (ω-3) | 19.95 ± 0.01 c | 56.61 ± 0.12 a | 38.04 ± 0.06 b |
| C18:3 (ω-6) | 1.62 ± 0.01 a | 0.02 ± 0.01 c | 0.81 ± 0.03 b |
| C20:1 | 0.45 ± 0.01 a | 0.13 ± 0.02 c | 0.29 ± 0.02 b |
| C20:2 | 0.09 ± 0.01 a | 0.05 ± 0.01 ab | 0.08 ± 0.02 a |
| C20:3 (ω-11) | 0.02 ± 0.01 | ND | ND |
| SFA | 10.24 ± 0.08 a | 10.00 ± 0.03 a | 10.12 ± 0.04 a |
| UFA | 89.77 ± 0.06 a | 90.01 ± 0.02 a | 89.83 ± 0.09 a |
| MUFA | 9.39 ± 0.02 c | 15.89 ± 0.09 a | 12.70 ± 0.03 b |
| PUFA | 80.38 ± 0.07 a | 74.12 ± 0.08 c | 77.20 ± 0.05 b |
| SFA/UFA | 0.11 ± 0.01 a | 0.11 ± 0.01 a | 0.11 ± 0.01 a |
| ω-3/ω-6 ratio | 0.43 ± 0.03 c | 3.24 ± 0.01 a | 1.09 ± 0.02 b |
| Parameters | Beef Tallow (1) | Pork Back Fat (2) | Poultry Skin (3) |
|---|---|---|---|
| Lipid profile (% of total lipids) | |||
| C14:0 | 1–1.5 | 1–1.5 | - |
| C16:0 | 24–28 | 24–28 | 20–24 |
| C16:1 | 2–3 | 2–3 | 5–9 |
| C18:0 | 20–24 | 13–14 | 4–6 |
| C18:1 (ω-9) | 40–43 | 43–47 | 33–44 |
| C18:2 (ω-6) | 2–4 | 8–11 | 18–20 |
| C18:3 (ω-3) | <1 | <1 | 1–2 |
| SFA | 46–55 | 38–43.5 | 25–31.5 |
| MUFA | 42–46 | 45–50 | 38–53 |
| PUFA | 2–4 | 8–11 | 19–22 |
| SFA/UFA | 1.0 | 0.7 | 0.4 |
| Color parameters | |||
| L* | 71.4 | 71.9 | 64.6 |
| a* | 1.2 | 3.3 | 2.6 |
| b* | 24.5 | 7.8 | 9.9 |
| pH | 5.3 | 6.3 | 6.0 |
| Sample | Moisture | Fats | Proteins | Ash |
|---|---|---|---|---|
| ACH | 45.76 ± 0.30 cd | 42.82 ± 0.30 ab | 2.50 ± 0.03 c | 0.43 ± 0.01 c |
| AH | 46.07 ± 0.14 c | 42.56 ± 0.10 b | 2.52 ± 0.01 c | 0.44 ± 0.01 bc |
| AM | 46.10 ± 0.30 c | 42.24 ± 0.13 b | 2.52 ± 0.02 c | 0.48 ± 0.09 bc |
| BWCH | 45.24 ± 0.36 cd | 42.69 ± 0.32 ab | 2.61 ± 0.01 b | 0.49 ± 0.08 bc |
| BWH | 46.18 ± 0.28 c | 41.69 ± 0.54 c | 2.63 ± 0.02 ab | 0.41 ± 0.01 c |
| BWM | 47.59 ± 0.30 b | 40.41 ± 0.37 d | 2.61 ± 0.01 b | 0.45 ± 0.04 bc |
| TCH | 48.28 ± 1.94 ab | 37.89 ± 1.23 e | 2.47 ± 0.01 c | 0.68 ± 0.06 a |
| TH | 49.91 ± 2.57 a | 35.55 ± 1.52 f | 2.50 ± 0.02 c | 0.73 ± 0.05 a |
| TM | ND | ND | ND | ND |
| WQCH | 44.73 ± 0.06 d | 43.86 ± 2.31 a | 2.62 ± 0.06 ab | 0.46 ± 0.03 bc |
| WQH | 45.33 ± 0.21 cd | 43.16 ± 1.97 a | 2.68 ± 0.01 a | 0.40 ± 0.03 c |
| WQM | 47.15 ± 0.15 b | 41.53 ± 0.30 c | 2.69 ± 0.02 a | 0.26 ± 0.02 d |
| Sample | pH | Work of Shear (N·s) | Firmness (N) |
|---|---|---|---|
| ACH | 6.38 ± 0.01 a | 5.78 ± 0.63 b | 6.64 ± 0.64 b |
| AH | 6.41 ± 0.02 a | 4.51 ± 0.08 c | 5.26 ± 0.60 c |
| AM | 6.35 ± 0.01 a | 5.22 ± 0.20 b | 11.69 ± 0.52 a |
| BWCH | 6.03 ± 0.02 c | 0.82 ± 0.02 f | 0.83 ± 0.02 f |
| BWH | 6.06 ± 0.01 c | 0.89 ± 0.12 f | 0.94 ± 0.08 f |
| BWM | 6.21 ± 0.01 b | 11.49 ± 1.18 a | 14.70 ± 2.25 a |
| TCH | 6.14 ± 0.01 b | 5.34 ± 0.20 b | 6.76 ± 1.94 b |
| TH | 6.16 ± 0.01 b | 3.56 ± 0.18 d | 4.08 ± 0.16 d |
| TM | ND | ND | ND |
| WQCH | 5.94 ± 0.01 d | 4.15 ± 0.12 cd | 3.82 ± 0.14 d |
| WQH | 5.98 ± 0.01 d | 2.77 ± 0.05 e | 2.71 ± 0.92 e |
| WQM | 5.53 ± 0.02 e | 3.82 ± 0.03 d | 7.22 ± 0.26 b |
| Samples | t0 | t15 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h | L* | a* | b* | C* | h | |
| ACH | 74.58 ± 1.52 Ba | 0.33 ± 0.06 Ba | 10.77 ± 0.28 Fb | 10.77 ± 0.28 Fb | 88.22 ± 0.35 Da | 70.77 ± 0.60 Ab | 0.34 ± 0.08 Ca | 11.82 ± 0.30 Da | 11.83 ± 0.30 Da | 88.35 ± 0.34 Ca |
| AH | 69.45 ± 1.91 Ca | −1.03 ± 0.22 Fb | 23.52 ± 0.54 BCa | 23.54 ± 0.54 BCa | 92.52 ± 0.54 Aa | 61.83 ± 2.29 CDb | −0.53 ± 0.28 Ga | 23.22 ± 1.62 Ba | 23.23 ± 1.62 Ba | 91.27 ± 0.65 Aa |
| AM | 64.68 ± 2.86 Da | −1.04 ± 0.08 Fb | 25.15 ± 2.69 Ba | 25.17 ± 2.69 Ba | 92.41 ± 0.46 Aa | 61.74 ± 0.79 Da | −0.12 ± 0.27 Fa | 25.54 ± 1.31 Aa | 25.55 ± 1.31 Aa | 90.25 ± 0.59 Bb |
| BWCH | 74.27 ± 1.98 Ba | 1.23 ± 0.20 Ab | 8.78 ± 0.47 Ga | 8.87 ± 0.49 Ga | 82.57 ± 0.86 Fa | 65.28 ± 2.35 Cb | 1.51 ± 0.4 Aa | 8.85 ± 0.27 Fa | 8.98 ± 0.26 Fa | 80.28 ± 1.02 Eb |
| BWH | 64.35 ± 0.52 Da | 0.56 ± 0.18 Eb | 23.20 ± 0.57 BCb | 23.21 ± 0.57 BCb | 91.37 ± 0.42 Ba | 60.09 ± 0.87 DEb | −0.15 ± 0.24 Fa | 24.98 ± 2.31 ABa | 24.98 ± 2.31 ABa | 90.32 ± 0.50 Bb |
| BWM | 78.07 ± 1.73 Aa | 0.45 ± 0.14 Db | 17.56 ± 0.53 Da | 17.57 ± 0.53 Da | 91.48 ± 0.47 Ba | 65.65 ± 1.30 Cb | 0.05 ± 0.18 Ea | 16.95 ± 0.94 Cb | 16.95 ± 0.94 Cb | 89.81 ± 0.62 Cb |
| TCH | 64.50 ± 1.77 Da | 1.23 ± 0.12 Aa | 11.75 ± 0.17 Ea | 11.81 ± 0.16 Ea | 84.03 ± 0.64 Ea | 59.56 ± 1.39 Eb | 0.81 ± 0.10 Bb | 11.67 ± 0.36 Da | 11.69 ± 0.37 Da | 84.50 ± 0.36 Da |
| TH | 58.78 ± 0.80 Fa | 0.19 ± 0.09 Ca | 22.25 ± 1.01 Cb | 22.25 ± 1.01 Ca | 89.50 ± 0.22 Ca | 54.63 ± 1.76 Fb | 0.10 ± 0.13 EDb | 22.98 ± 1.35 Ba | 22.98 ± 1.35 Ba | 90.25 ± 0.33 Ba |
| TM | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| WQCH | 72.38 ± 2.09 BCa | 0.34 ± 0.08 Da | 11.70 ± 0.56 Ea | 11.71 ± 0.56 Ea | 91.69 ± 0.41 Bb | 68.98 ± 0.50 Bb | −0.51 ± 0.07 Gb | 10.80 ± 0.29 Eb | 10.82 ± 0.29 Eb | 92.74 ± 0.43 Aa |
| WQH | 62.00 ± 1.02 DEa | −1.16 ± 0.20 Fb | 24.67 ± 0.58 Bb | 24.70 ± 0.58 Bb | 92.70 ± 0.44 Aa | 54.12 ± 2.82 Fb | −0.89 ± 0.28 Ha | 26.17 ± 1.40 Aa | 26.19 ± 1.39 Aa | 91.95 ± 0.62 Ab |
| WQM | 60.68 ± 0.50 Ea | 1.48 ± 0.14 Hb | 29.73 ± 0.77 Aa | 29.77 ± 0.77 Aa | 92.85 ± 0.23 Aa | 58.89 ± 2.25 Eb | −1.09 ± 0.15 Ia | 23.41 ± 1.15 Bb | 23.44 ± 1.15 Bb | 92.66 ± 0.30 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botella-Martínez, C.; Pérez-Álvarez, J.Á.; Sayas-Barberá, E.; Fernández-López, J.; Viuda-Martos, M. Assessment of Chemical, Physicochemical, and Lipid Stability Properties of Gelled Emulsions Elaborated with Different Oils Chia (Salvia hispanica L.) or Hemp (Cannabis sativa L.) and Pseudocereals. Foods 2021, 10, 1463. https://doi.org/10.3390/foods10071463
Botella-Martínez C, Pérez-Álvarez JÁ, Sayas-Barberá E, Fernández-López J, Viuda-Martos M. Assessment of Chemical, Physicochemical, and Lipid Stability Properties of Gelled Emulsions Elaborated with Different Oils Chia (Salvia hispanica L.) or Hemp (Cannabis sativa L.) and Pseudocereals. Foods. 2021; 10(7):1463. https://doi.org/10.3390/foods10071463
Chicago/Turabian StyleBotella-Martínez, Carmen, José Ángel Pérez-Álvarez, Estrella Sayas-Barberá, Juana Fernández-López, and Manuel Viuda-Martos. 2021. "Assessment of Chemical, Physicochemical, and Lipid Stability Properties of Gelled Emulsions Elaborated with Different Oils Chia (Salvia hispanica L.) or Hemp (Cannabis sativa L.) and Pseudocereals" Foods 10, no. 7: 1463. https://doi.org/10.3390/foods10071463
APA StyleBotella-Martínez, C., Pérez-Álvarez, J. Á., Sayas-Barberá, E., Fernández-López, J., & Viuda-Martos, M. (2021). Assessment of Chemical, Physicochemical, and Lipid Stability Properties of Gelled Emulsions Elaborated with Different Oils Chia (Salvia hispanica L.) or Hemp (Cannabis sativa L.) and Pseudocereals. Foods, 10(7), 1463. https://doi.org/10.3390/foods10071463