Cellulose Nanofibers from Olive Tree Pruning as Food Packaging Additive of a Biodegradable Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pulping Process and Isolation of Nanocellulose
2.3. Preparation of PVA/(Ligno)nanocellulose Films
2.4. Characterization
2.4.1. Optical Properties
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. X-ray Diffraction (XRD) Analysis
2.4.6. Antioxidant Activity
2.4.7. Mechanical Properties
2.4.8. Barrier Properties: Water Vapor Permeability and Oxygen Transmission Rate (WVP and OTR)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Structure Characterization
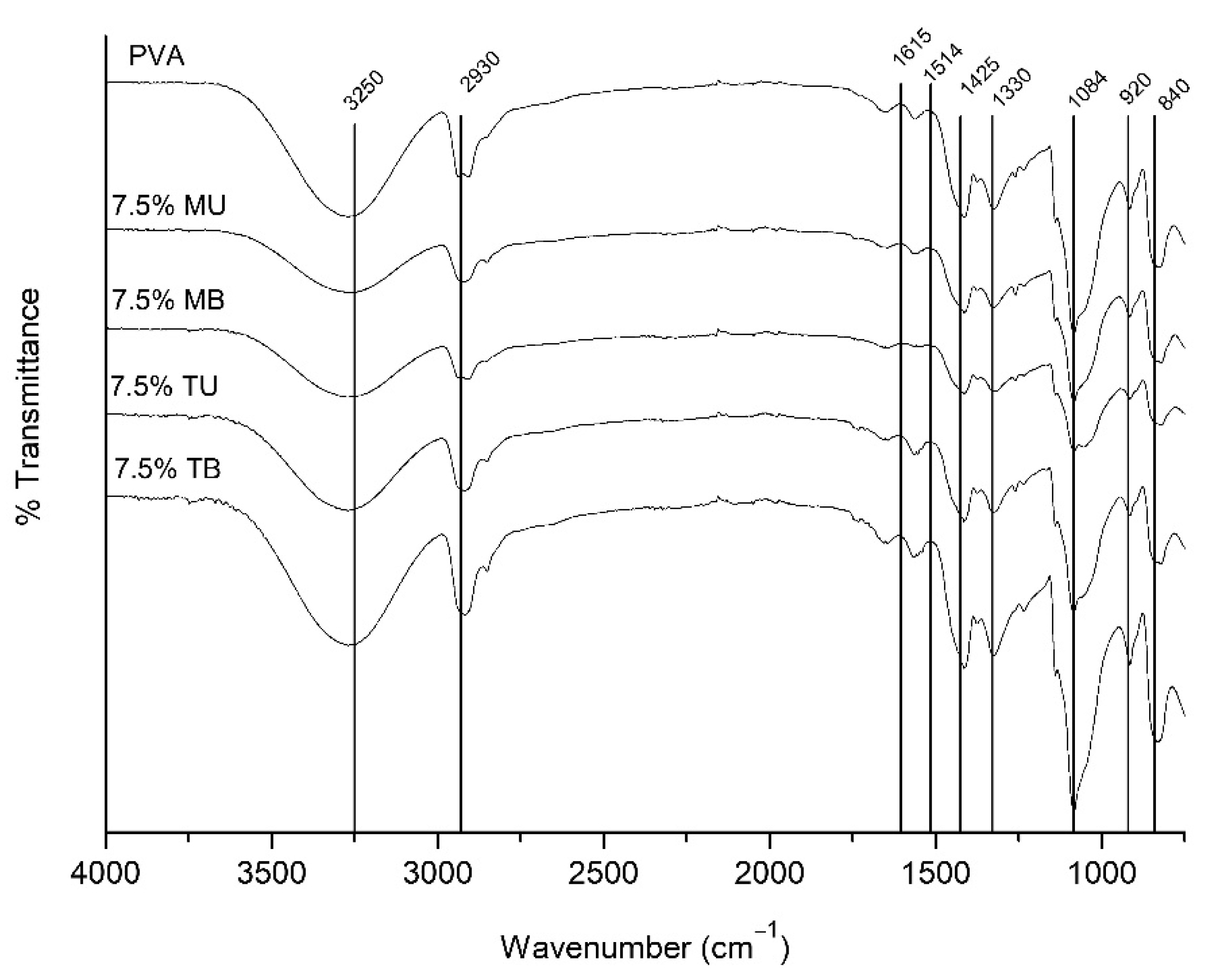
3.2. Chemical Structure
3.3. Crystalline Structure
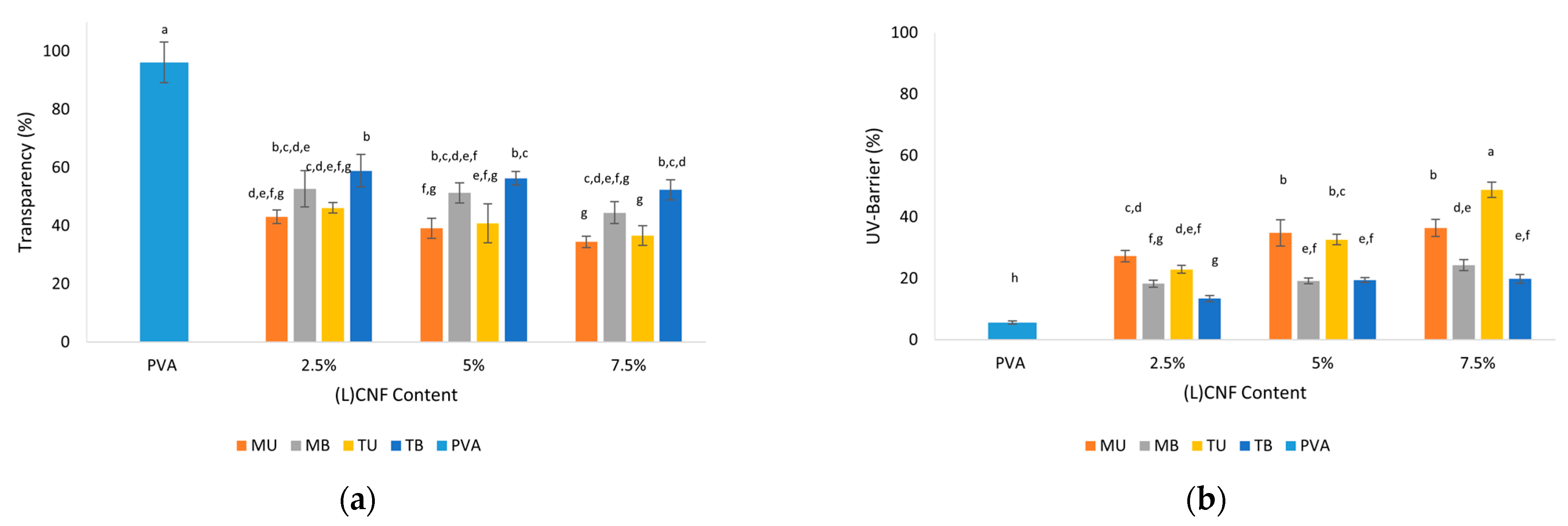
3.4. Optical Properties
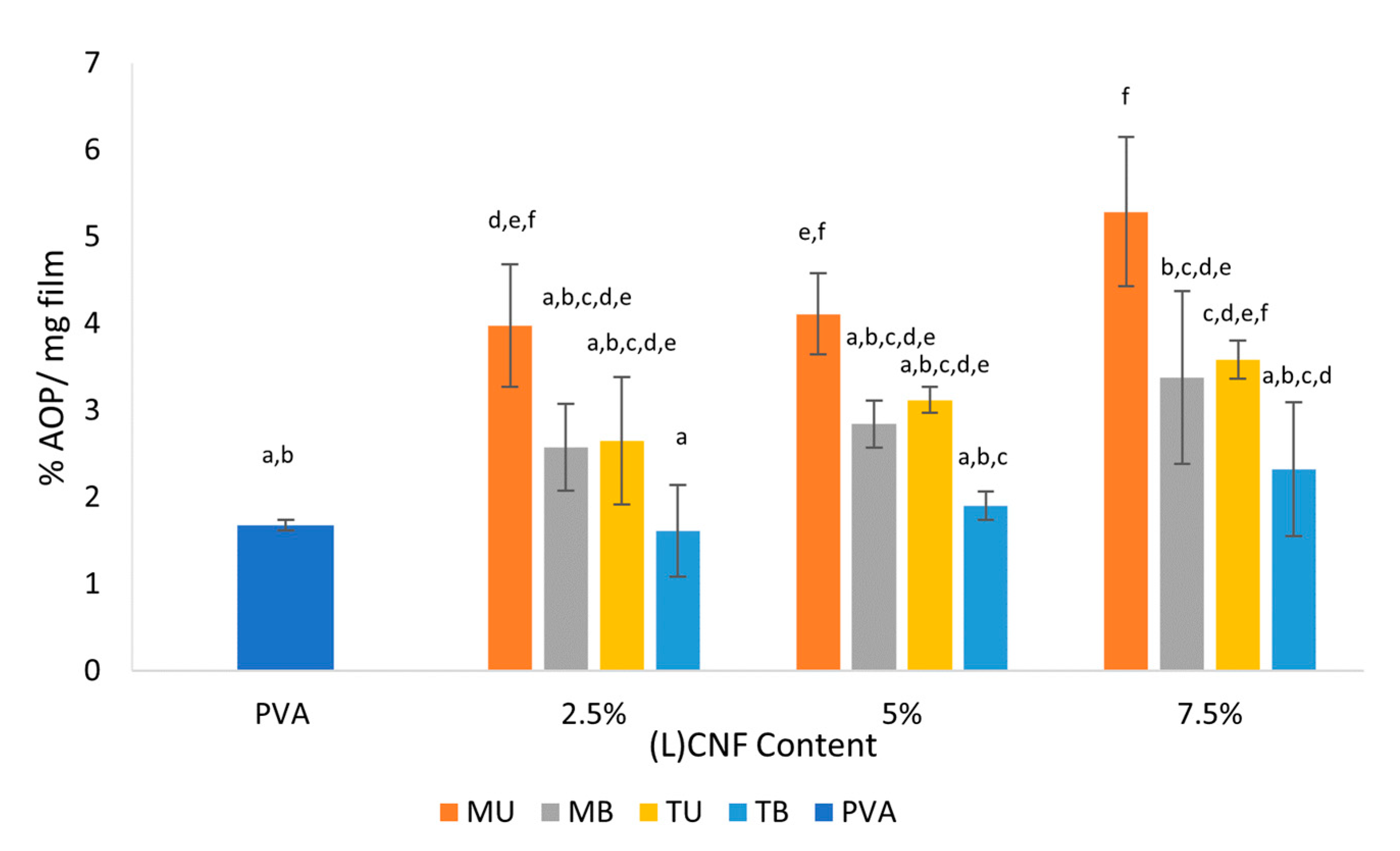
3.5. Antioxidant Activity
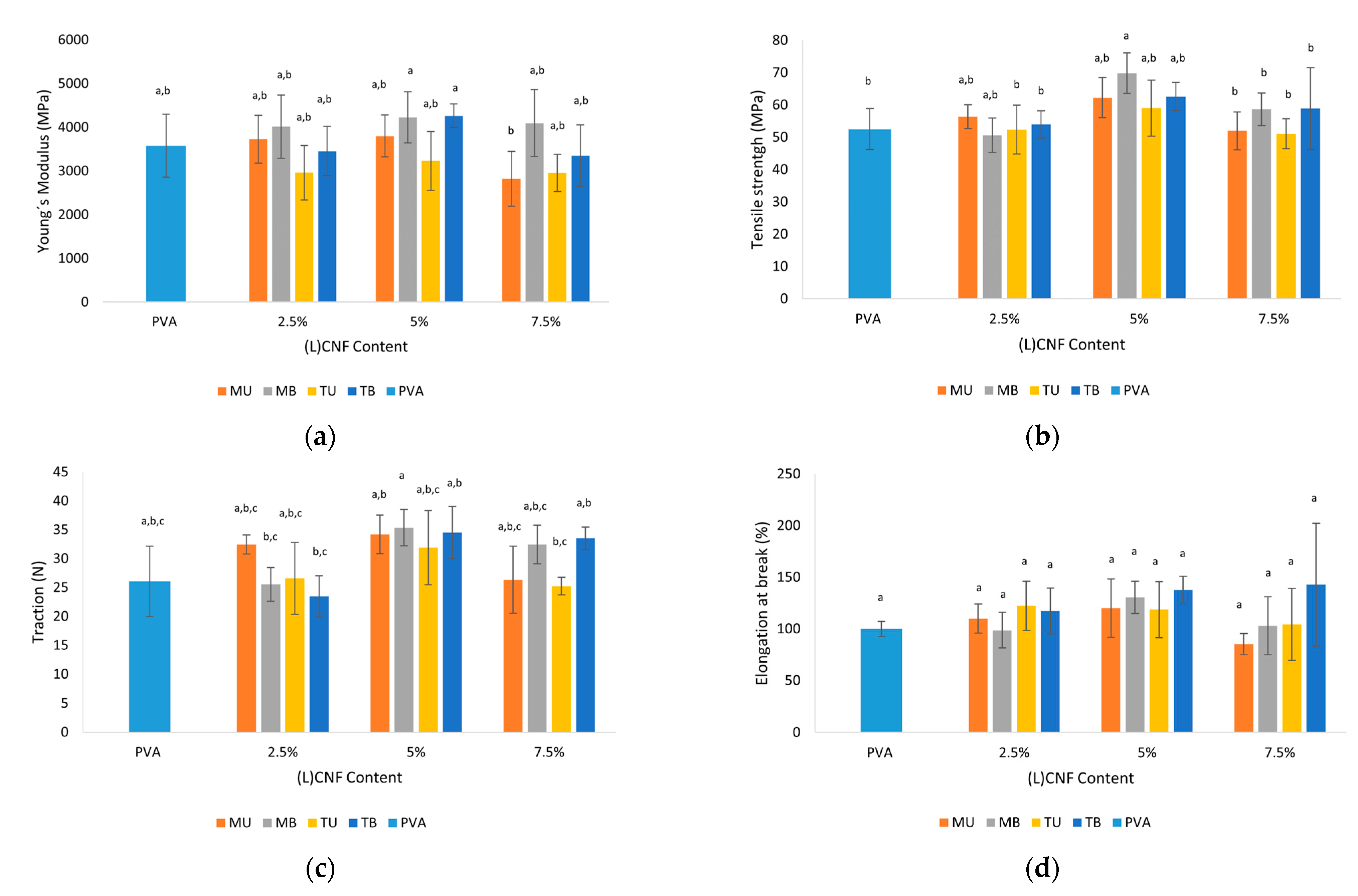
3.6. Mechanical Properties
3.7. Barrier Properties
3.8. Thermal Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Chakori, S.; Aziz, A.A.; Smith, C.; Dargusch, P. Untangling the underlying drivers of the use of single-use food packaging. Ecol. Econ. 2021, 185, 107063. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer hybrid materials: Development, characterization, and food packaging applications. Food Packag. Shelf Life 2021, 28, 100676. [Google Scholar] [CrossRef]
- Mendes, A.C.; Pedersen, G.A. Perspectives on sustainable food packaging:– is bio-based plastics a solution? Trends Food Sci. Technol. 2021, 112, 839–846. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist-Andberg, H.; Åkerman, M. Sustainability governance and contested plastic food packaging e An integrative review. J. Clean. Prod. 2021, 306, 127111. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef]
- de Carvalho, S.M.; Noronha, C.M.; da Rosa, C.G.; Sganzerla, W.G.; Bellettini, I.C.; Nunes, M.R.; Bertoldi, F.C.; Manique Barreto, P.L. PVA antioxidant nanocomposite films functionalized with alpha-tocopherol loaded solid lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123793. [Google Scholar] [CrossRef]
- Lee, H.; You, J.; Jin, H.J.; Kwak, H.W. Chemical and physical reinforcement behavior of dialdehyde nanocellulose in PVA composite film: A comparison of nanofiber and nanocrystal. Carbohydr. Polym. 2020, 232, 115771. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.R.; Dixit, S.; Pal, D.B.; Mishra, P.K.; Srivastava, P.; Srivastava, N.; Hashem, A.; Alqarawi, A.A.; Abd_Allah, E.F. Effect of nanocellulose on mechanical and barrier properties of PVA–banana pseudostem fiber composite films. Environ. Technol. Innov. 2021, 21, 101312. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Li, C.; Xu, P.; Zheng, T.; Zhu, X.; Kenny, J.M.; Puglia, D.; Ma, P. Highly transparent PVA/nanolignin composite films with excellent UV shielding, antibacterial and antioxidant performance. React. Funct. Polym. 2021, 162, 104873. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly(vinyl alcohol) and nanocellulose from sugarcane bagasse. J. Ind. Eng. Chem. 2014, 20, 462–473. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Antimicrobial and antioxidant properties of polyvinyl alcohol bio composite films containing seaweed extracted cellulose nano-crystal and basil leaves extract. Int. J. Biol. Macromol. 2018, 107, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(ligno)nanocellulose biocomposite films. Effect of residual lignin content on structural, mechanical, barrier and antioxidant properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Amara, C.; El Mahdi, A.; Medimagh, R.; Khwaldia, K. Nanocellulose-based composites for packging applications. Curr. Opin. Green Sustain. Chem. 2021, 31, 100512. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Karimi, A.; Kazemi, M.; Samani, S.A.; Simal-Gandara, J. Bioactive compounds from by-products of eggplant: Functional properties, potential applications and advances in valorization methods. Trends Food Sci. Technol. 2021, 112, 518–531. [Google Scholar] [CrossRef]
- Bascón-Villegas, I.; Espinosa, E.; Sánchez, R.; Tarrés, Q.; Pérez-Rodríguez, F.; Rodríguez, A. Horticultural plant residues as new source for lignocellulose nanofibers isolation: Application on the recycling paperboard process. Molecules 2020, 25, 3275. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M. Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renew. Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- FAOSTAT. 2021. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 18 May 2021).
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of cellulose nanofibers from olive tree harvest—A residue with wide applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Eibes, G.; Cara, C.; De Torres, A.; López-Linares, J.C.; Ruiz, E.; Castro, E. Valorisation of olive agro-industrial by-products as a source of bioactive compounds. Sci. Total Environ. 2018, 645, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Rincón, E.; Serrano, L.; Balu, A.M.; Aguilar, J.J.; Luque, R.; García, A. Effect of bay leaves essential oil concentration on the properties of biodegradable carboxymethyl cellulose-based edible films. Materials 2019, 12, 2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM D638-14 Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014; pp. 1–15. [CrossRef]
- ASTM-E96/E96M-10 Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2010; p. 14.
- ASTM D3985—17 Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor; ASTM International: West Conshohocken, PA, USA, 2017; p. 7.
- Shankar, S.; Reddy, J.P.; Rhim, J.W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biol. Macromol. 2015, 81, 267–273. [Google Scholar] [CrossRef]
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica oleraceae (Red Cabbage) as Time-Temperature Indicators for application in intelligent food packaging. Food Hydrocoll. 2015, 43, 180–188. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Yuan, Q.; Huang, F. Characterization of alkaline lignins for use in phenol-formaldehyde and epoxy resins. BioResources 2011, 6, 2647–2662. [Google Scholar] [CrossRef]
- Guo, J.; Ge, L.; Li, X.; Mu, C.; Li, D. Periodate oxidation of xanthan gum and its crosslinking effects on gelatin-based edible films. Food Hydrocoll. 2014, 39, 243–250. [Google Scholar] [CrossRef]
- Chaochanchaikul, K.; Jayaraman, K.; Rosarpitak, V.; Sombatsompop, N. Influence of Lignin Content on Photodegradation in Wood/HDPE Composites Under UV Weathering. BioResources 2012, 7, 38–55. [Google Scholar]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the performance of edible food packaging films by using nanocellulose as an additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Lundgren, E.; Matos, M.; Avelino, F.; Lomonaco, D.; Rodrigues-souza, I.; Stephanie, V.; Gagosian, C.; Margarete, M.; Luiz, W.; Magalhães, E.; et al. Safety aspects of kraft lignin fractions: Discussions on the in chemico antioxidant activity and the induction of oxidative stress on a cell-based in vitro model. Int. J. Biol. Macromol. 2021, 182, 977–986. [Google Scholar] [CrossRef]
- Mujtaba, M.; Akyuz, L.; Koc, B.; Kaya, M.; Ilk, S.; Cansaran-Duman, D.; Martinez, A.S.; Cakmak, Y.S.; Labidi, J.; Boufi, S. Novel, multifunctional mucilage composite films incorporated with cellulose nanofibers. Food Hydrocoll. 2019, 89, 20–28. [Google Scholar] [CrossRef]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López de Dicastillo, C.; Garrido, L.; Roa, K.; Galotto, M.J. Electrospun PVA fibers loaded with antioxidant fillers extracted from Durvillaea antarctica algae and their effect on plasticized PLA bionanocomposites. Eur. Polym. J. 2018, 103, 145–157. [Google Scholar] [CrossRef]
- da Silva, J.B.A.; Nascimento, T.; Costa, L.A.S.; Pereira, F.V.; Machado, B.A.; Gomes, G.V.P.; Assis, D.J.; Druzian, J.I. Effect of Source and Interaction with Nanocellulose Cassava Starch, Glycerol and the Properties of Films Bionanocomposites. Mater. Today Proc. 2015, 2, 200–207. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Struller, C.; Kelly, P.; Copeland, N. Conversion of aluminium oxide coated films for food packaging applications—From a single layer material to a complete pouch. Food Packag. Shelf Life 2019, 20, 100309. [Google Scholar] [CrossRef]
- Lange, J.; Wyser, Y. Recent Innovations in Barrier Technologies for Plastic Packaging—A Review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 2015, 22, 3137–3150. [Google Scholar] [CrossRef]


| Films | WVP (10−7.g/s·m·Pa) | OTR (cc/m2⋅Day) |
|---|---|---|
| PVA | 6.97 ± 0.07 a | 3.75 |
| 5% MU | 4.31 ± 0.31 b | 0.08 |
| 5% MB | 4.16 ± 0.02 b | 0.64 |
| 5% TU | 3.88 ± 0.07 b | 0.02 |
| 5% TB | 2.82 ± 0.17 c | 0.06 |
| Films | Tmax (°C) | Residue Mass at 600 °C (%) |
|---|---|---|
| PVA | 250.32 | 12 |
| 5% MU | 264.99 | 8 |
| 5% MB | 259.12 | 1 |
| 5% TU | 263.39 | 5 |
| 5% TB | 257.52 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Espinosa, E.; Carrasco, E.; Pérez-Rodríguez, F.; Rodríguez, A. Cellulose Nanofibers from Olive Tree Pruning as Food Packaging Additive of a Biodegradable Film. Foods 2021, 10, 1584. https://doi.org/10.3390/foods10071584
Sánchez-Gutiérrez M, Bascón-Villegas I, Espinosa E, Carrasco E, Pérez-Rodríguez F, Rodríguez A. Cellulose Nanofibers from Olive Tree Pruning as Food Packaging Additive of a Biodegradable Film. Foods. 2021; 10(7):1584. https://doi.org/10.3390/foods10071584
Chicago/Turabian StyleSánchez-Gutiérrez, Mónica, Isabel Bascón-Villegas, Eduardo Espinosa, Elena Carrasco, Fernando Pérez-Rodríguez, and Alejandro Rodríguez. 2021. "Cellulose Nanofibers from Olive Tree Pruning as Food Packaging Additive of a Biodegradable Film" Foods 10, no. 7: 1584. https://doi.org/10.3390/foods10071584
APA StyleSánchez-Gutiérrez, M., Bascón-Villegas, I., Espinosa, E., Carrasco, E., Pérez-Rodríguez, F., & Rodríguez, A. (2021). Cellulose Nanofibers from Olive Tree Pruning as Food Packaging Additive of a Biodegradable Film. Foods, 10(7), 1584. https://doi.org/10.3390/foods10071584