Multiple Light Scattering Measurements for Online Monitoring of Milk Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
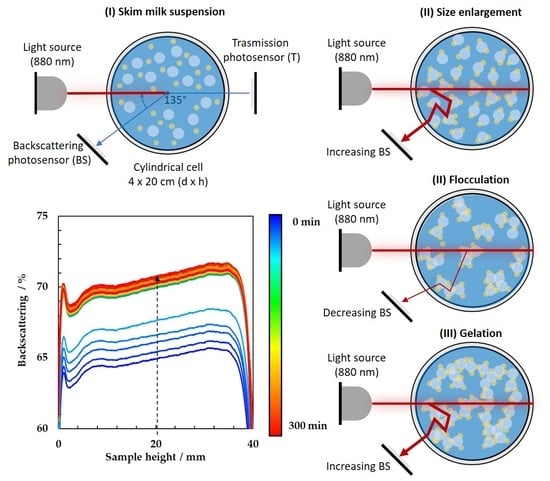
2.3. Multiple Light Scattering Monitoring
2.4. Texture Monitoring
2.5. pH Monitoring
2.6. Monitoring of Rheological Parameters
2.7. Statistical Analysis
3. Results
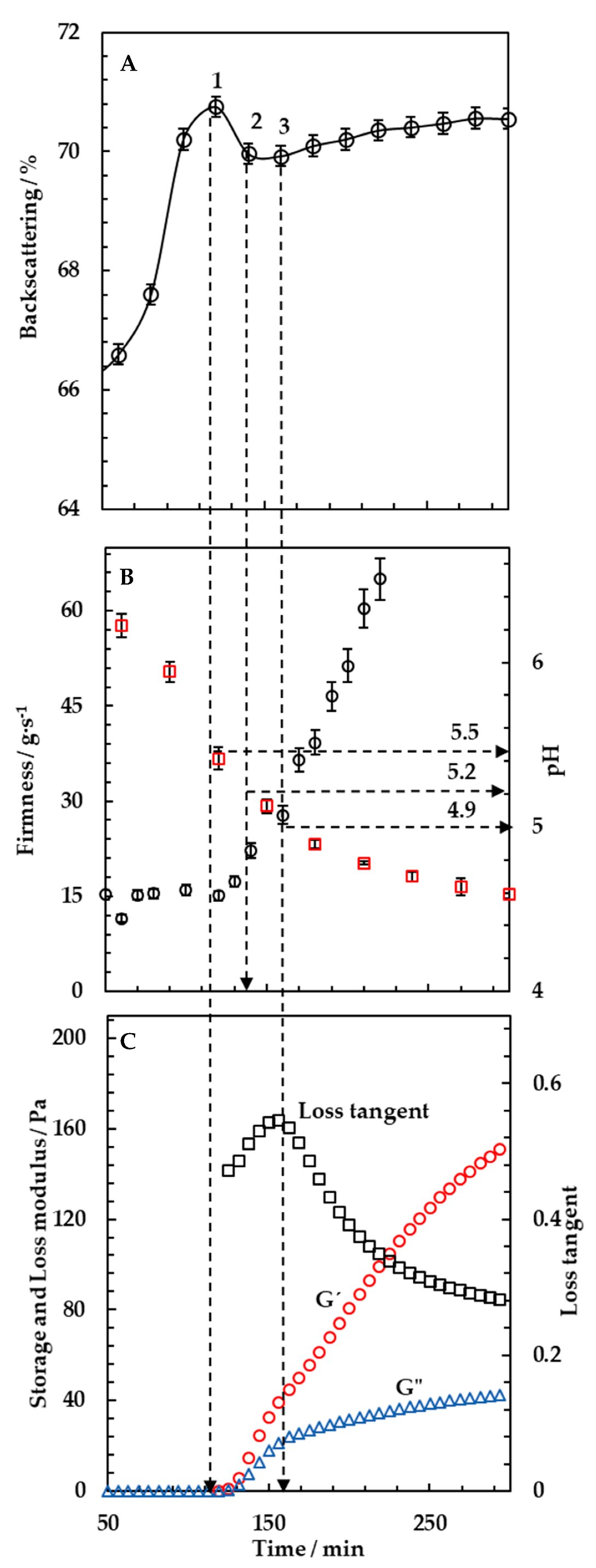
3.1. Monitoring of Milk Fermentation by Multiple Light Scattering
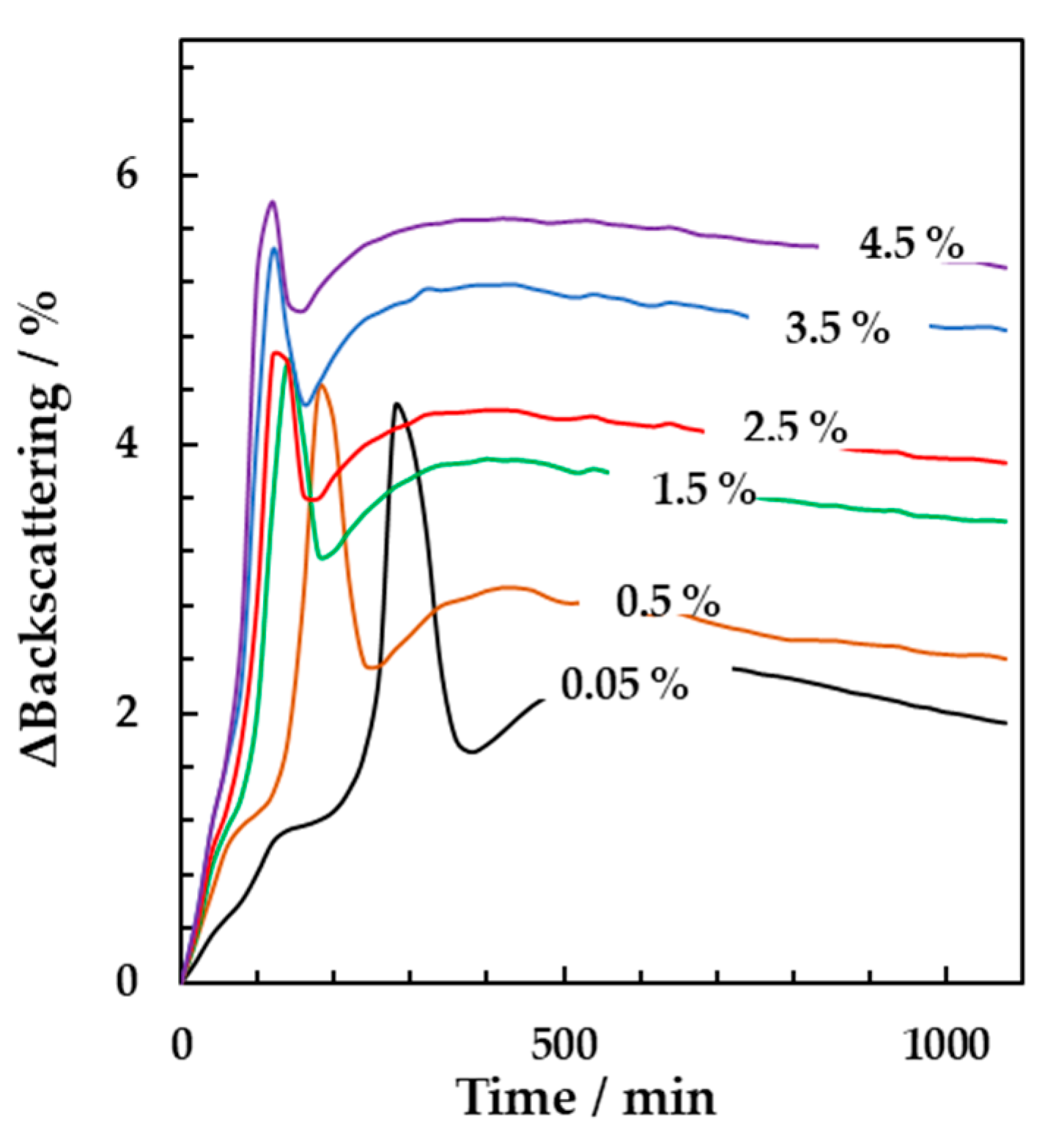
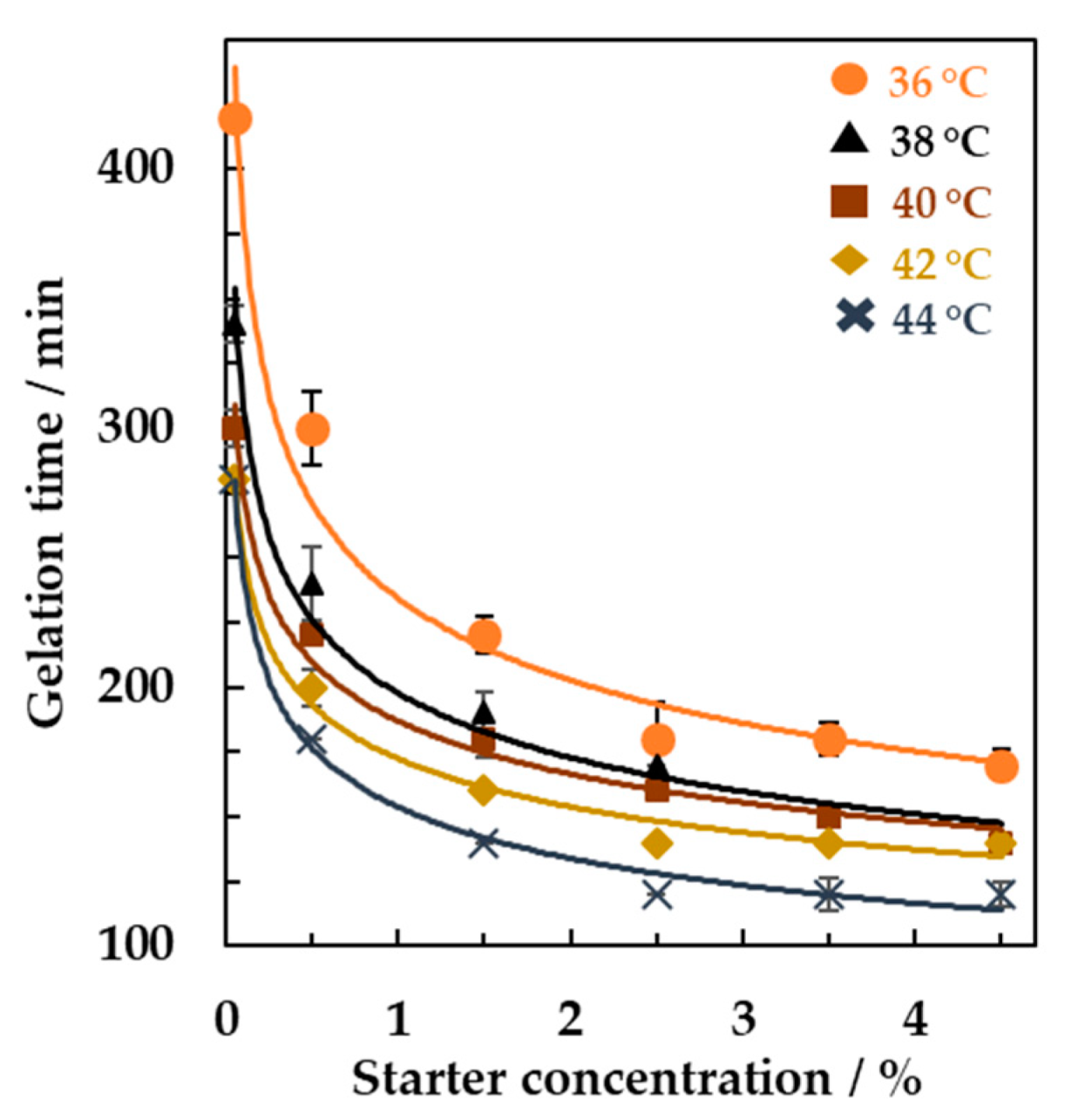
3.2. Online Monitoring of the Effect of Starter Concentrations and Incubation Temperature on Milk Fermentation
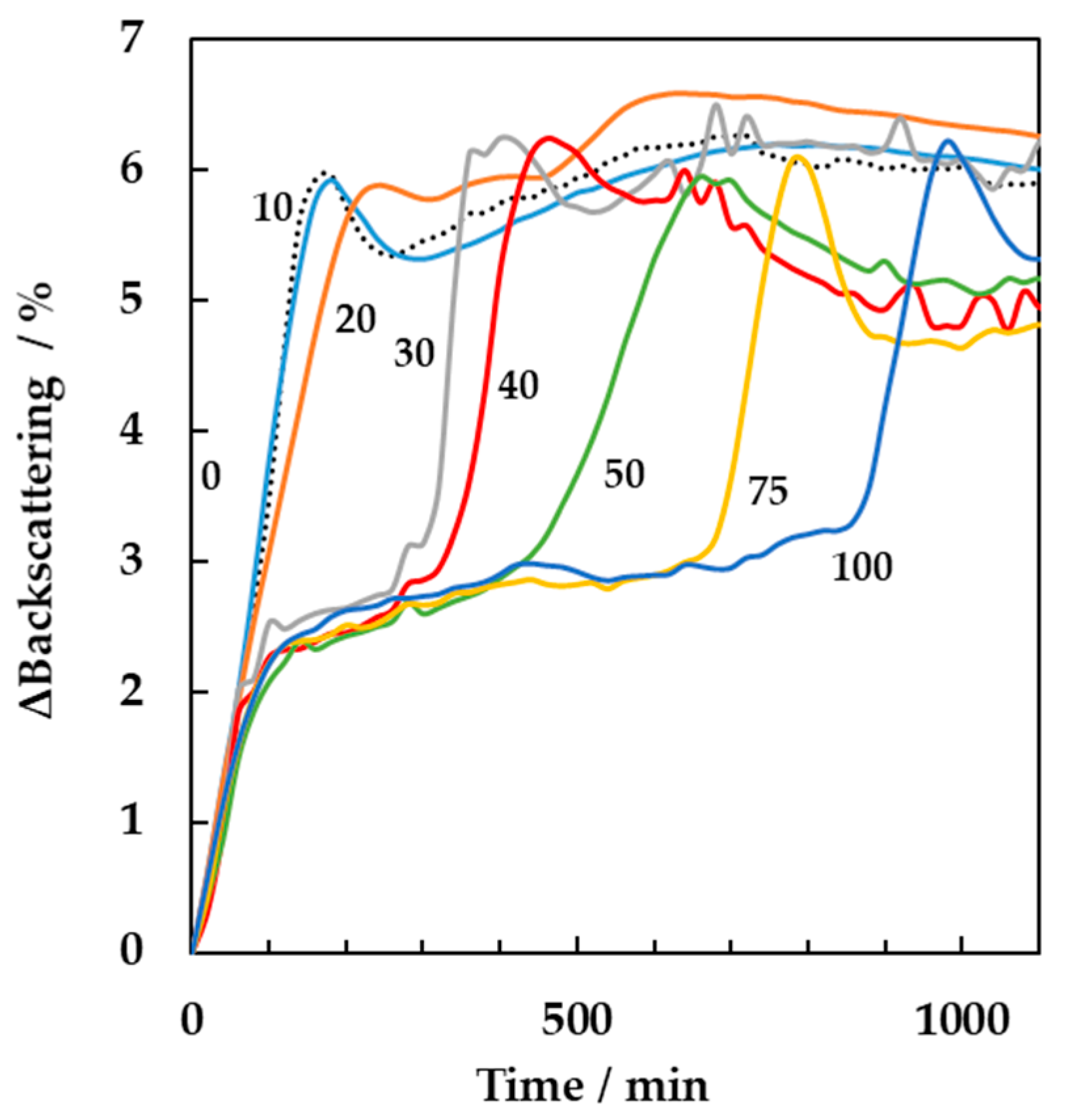
3.3. Online Monitoring of the Effect of Antibiotic on Milk Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandan, R.; O’Rell, K. Starter cultures for yogurt and fermented milks. In Manufacturing Yogurt and Fermented Milks; Chandan, R., Ed.; Blackwell Publishing: Oxford, UK, 2013; pp. 89–116. [Google Scholar]
- Bansal, S.; Mangal, M.; Sharma, S.K.; Yadav, D.N.; Gupta, R.K. Optimization of process conditions for developing yoghurt like probiotic product from peanut. LWT Food Sci. Technol. 2016, 73, 6–12. [Google Scholar] [CrossRef]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New perspectives in fermented dairy products and their health relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Structure and physical properties of yogurt gels: Effect of inoculation rate and incubation temperature. J. Dairy Sci. 2004, 87, 3153–3164. [Google Scholar] [CrossRef]
- Lucey, J.A.; Tamehana, M.; Singh, H.; Munro, P.A. Effect of interactions between denatured whey proteins and casein micelles on the formation and rheological properties of acid skim milk gels. J. Dairy Res. 1998, 65, 555–567. [Google Scholar] [CrossRef]
- Mousavi, M.; Heshmati, A.; Daraei Garmakhany, A.; Vahidinia, A.; Taheri, M. Texture and sensory characterization of functional yogurt supplemented with flaxseed during cold storage. Food Sci. Nutr. 2019, 7, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial yogurt manufacture: Monitoring of fermentation process and improvement of final product quality. J. Dairy Sci. 2007, 90, 2641–2654. [Google Scholar] [CrossRef]
- Vasbinder, A.J.; Van Mil, P.J.J.M.; Bot, A.; De Kruif, K.G. Acid-induced gelation of heat-treated milk studied by diffusing wave spectroscopy. Colloids Surfaces B Biointerfaces 2001, 21, 245–250. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Comparison of gelation profile of yoghurts during fermentation measured by RVA and ultrasonic spectroscopy. Int. J. Food Prop. 2005, 8, 193–198. [Google Scholar] [CrossRef]
- Guo, C.; Xin, L.; Dong, Y.; Zhang, X.; Wang, X.; Fu, H.; Wang, Y. Dielectric properties of yogurt for online monitoring of fermentation process. Food Bioprocess Technol. 2018, 11, 1096–1100. [Google Scholar] [CrossRef]
- Muncan, J.; Tei, K.; Tsenkova, R. Real-Time Monitoring of Yogurt Fermentation Process by Aquaphotomics Near-Infrared Spectroscopy. Sensors 2021, 21, 177. [Google Scholar] [CrossRef]
- Cimander, C.; Carlsson, M.; Mandenius, C.F. Sensor fusion for on-line monitoring of yoghurt fermentation. J. Biotechnol. 2002, 99, 237–248. [Google Scholar] [CrossRef]
- Navrátil, M.; Cimander, C.; Mandenius, C.F. On-line multisensor monitoring of yogurt and filmjölk fermentations on production scale. J. Agric. Food Chem. 2004, 52, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Arango, O.; Trujillo, A.J.; Castillo, M. Inline control of yoghurt fermentation process using a near infrared light backscatter sensor. J. Food Eng. 2020, 277, 109885. [Google Scholar] [CrossRef]
- Lemarchand, C.; Couvreur, P.; Vauthier, C.; Costantini, D.; Gref, R. Study of emulsion stabilization by graft copolymers using the optical analyzer Turbiscan. Int. J. Pharm. 2003, 254, 77–82. [Google Scholar] [CrossRef]
- Roland, I.; Piel, G.; Delattre, L.; Evrard, B. Systematic characterization of oil-in-water emulsions for formulation design. Int. J. Pharm. 2003, 263, 85–94. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surfaces B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Olatunji, O.N.; Du, J.; Hintz, W.; Tomas, J. Application of particle sedimentation analysis in sterically-stabilized TiO2 particles stability assessment. Adv. Powder Technol. 2016, 27, 1325–1336. [Google Scholar] [CrossRef]
- Ferrentino, G.; Ramezani, M.; Morozova, K.; Hafner, D.; Pedri, U.; Pixner, K.; Scampicchio, M. Fining of red wine monitored by multiple light scattering. J. Agric. Food Chem. 2017, 65, 5523–5530. [Google Scholar] [CrossRef]
- Raikos, V.; Grant, S.B.; Hayes, H.; Ranawana, V. Use of β-glucan from spent brewer’s yeast as a thickener in skimmed yogurt: Physicochemical, textural, and structural properties related to sensory perception. J. Dairy Sci. 2018, 101, 5821–5831. [Google Scholar] [CrossRef] [Green Version]
- De Brabandere, A.G.; De Baerdemaeker, J.G. Effects of process conditions on the pH development during yogurt fermentation. J. Food Eng. 1999, 41, 221–227. [Google Scholar] [CrossRef]
- Barroso, J.M. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, 1, 1–72. [Google Scholar]
- Alexander, M.; Dalgleish, D.G. Application of transmission diffusing wave spectroscopy to the study of gelation of milk by acidification and rennet. Colloids Surfaces B Biointerfaces 2004, 38, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lucey, J.A. Formation and physical properties of yogurt. Asian Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Lucey, J.A. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef]
- Donato, L.; Guyomarc’h, F.; Amiot, S.; Dalgleish, D.G. The mechanism of interaction of κ–casein and whey protein in heated milks. Int. Dairy J. 2007, 17, 1161–1167. [Google Scholar] [CrossRef]
- Guyomarc’h, F.; Queguiner, C.; Law, A.J.R.; Horne, D.; Dalgleish, D.G. Role of the soluble and micelle–bound heat–induced protein aggregates on network formation in acid skim milk gels. J. Agric. Food Chem. 2003, 51, 7743–7750. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, D.G.; Alexander, M.; Corredig, M. Studies of the acid gelation of milk using ultrasonic spectroscopy and diffusing wave spectroscopy. Food Hydrocoll. 2004, 18, 747–755. [Google Scholar] [CrossRef]
- Kristo, E.; Biliaderis, C.G.; Tzanetakis, N. Modelling of the acidification process and rheological properties of milk fermented with a yogurt starter culture using response surface methodology. Food Chem. 2003, 83, 437–446. [Google Scholar] [CrossRef]
- Suhren, G. Influence of residues of antimicrobials in milk on commercially applied cultures-model trials. Kiel. Milchwirtsch. Forsch. 1996, 49, 131–149. [Google Scholar]
- Berruga, M.I.; Noves, B.; Molina, M.P.; Roman, M.; Molina, A. Influence of cephalosporins on the coagulation time of yogurt made from ewes’ milk. Int. J. Dairy Technol. 2008, 61, 372–378. [Google Scholar] [CrossRef]
- Durel, L.; Gallina, G.; Pellet, T. Assessment of ceftiofur residues in cow milk using commercial screening test kits. Veterinary Record Open 2019, 6, e000329. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramezani, M.; Ferrentino, G.; Morozova, K.; Scampicchio, M. Multiple Light Scattering Measurements for Online Monitoring of Milk Fermentation. Foods 2021, 10, 1582. https://doi.org/10.3390/foods10071582
Ramezani M, Ferrentino G, Morozova K, Scampicchio M. Multiple Light Scattering Measurements for Online Monitoring of Milk Fermentation. Foods. 2021; 10(7):1582. https://doi.org/10.3390/foods10071582
Chicago/Turabian StyleRamezani, Mohsen, Giovanna Ferrentino, Ksenia Morozova, and Matteo Scampicchio. 2021. "Multiple Light Scattering Measurements for Online Monitoring of Milk Fermentation" Foods 10, no. 7: 1582. https://doi.org/10.3390/foods10071582
APA StyleRamezani, M., Ferrentino, G., Morozova, K., & Scampicchio, M. (2021). Multiple Light Scattering Measurements for Online Monitoring of Milk Fermentation. Foods, 10(7), 1582. https://doi.org/10.3390/foods10071582